New processing route has been developed for zircon based on powder injection moulding (PIM). Raw zircon powders, obtained from mineral sands, have been processed using a new water soluble binder system composed of PEG and CAB. Water solvent debinding stage has been studied in depth. On one hand, influence of some debinding parameters (temperature, debinding rate, additives and the use of climate chamber) has been tested. On the other hand, new binder systems were tested and compared with previous studied ones. The full PIM process has been carried out. Mixing, injection molding, solvent and thermal debinding and finally sintering, have been performed with the optimal binder system composition. Homogeneity along the process has been assessed by thermo-gravimetric analysis and by density measurements. After sintering, dimensional variation, density and fracture surface obtained after flexural strength test, have been analyzed. A competitive flexural strength has been achieved for injected zircon samples.
En este estudio se desarrolla con éxito una nueva vía de procesamiento para el circón basada en el moldeo por inyección de polvos. El polvo de circón, obtenido de arenas minerales, se mezcla con un nuevo ligante soluble en agua compuesto por PEG y CAB, el cual permite obtener piezas inyectadas de circón. Se ha estudiado con detalle la etapa de eliminación por disolución del PEG, en la que se ha evaluado la influencia de varios parámetros (temperatura, velocidad de disolución, el uso aditivos y de la cámara climática). Además, se han estudiado diferentes composiciones del ligante basadas en varios pesos moleculares y porcentajes en volumen de los polímeros hasta la etapa de eliminación del PEG. Se han completado con éxito todas las etapas del proceso (mezcla, inyección, eliminación del ligante, por disolución seguida de degradación térmica y sinterización) para la composición óptima del ligante. Se ha verificado la homogeneidad del sistema a lo largo del proceso mediante termogravimetría y medidas de densidad. Tras la sinterización se ha evaluado variación dimensional, la densidad y la superficie de fractura, obtenida tras el ensayo de flexión, mediante SEM. Las piezas de circón inyectado muestran un valor competitivo de resistencia a flexión.
Zirconium silicate (ZrSiO4) or “zircon” is one of the most abundant minerals in the earth crust. It is also the oldest known material on our planet due to its resistance to mechanical and chemical weathering [1]. Therefore, the interest in this ceramic material arises from its wide availability and its excellent and stable thermo-physical properties in a wide range of temperatures [2]. Zircon shows high chemical inertness and it does not undergo any structural transformation until its dissociation about 1676°C. However, this temperature can be reduced by the presence of impurities [3,4]. A very low and uniform thermal expansion coefficient compared with conventional structural ceramics (4.1×10−6°C from room temperature to 1400°C) and low thermal conductivity (5.1Wm−1°C−1 at room temperature and 3.5Wm−1°C−1 at 1000°C) derive in an outstanding thermal shock resistance. Furthermore, mechanical properties, especially at high temperatures, are also remarkable, it maintain a good strength up to 1400°C [5–7]. Zircon is generally used in refractory field, although its main application is in the ceramic industry as an opacifier, without taking advantage of some of its remarkable properties. This material could be considered as a potential structural ceramic material, especially in those fields where an abrupt change of temperature could happen.
The processing of structural ceramics is the main limitation in many applications due to its high cost and the difficulty of the technology. Most of the processes for zircon powder consolidation found in the literature are based on press and sintering [8] or slip casting [9]. The study of new processing methods may lead to interesting advantages for ceramic materials increasing the number of their applications.
Powder injection moulding (PIM) combines the traditional shape-making capability of the plastic injection molding and the material flexibility of the powder technology. PIM is a cost effective and competitive manufacturing technology for large production of small, complex shaped and high performance parts of metal or ceramic materials [10,11]. In particular, ceramic injection moulding (CIM) is the most efficient processing route for the high volume production of ceramic components with complex geometry, especially for materials that are difficult, costly or impossible to develop through traditional processes [12]. The process consists of the following four steps: mixing the powder and the organic binder to produce the initial product (named feedstock), injection moulding, debinding and sintering. In PIM technology each step has a critical role being the feedstock design and production decisive to obtain an effective PIM process. Among the desire properties of feedstocks, homogeneity, rheological properties and shape maintenance during debinding are the most important, and all of them depend on powder and binder properties [13]. Binder system design and debinding steps are crucial for the success of the process due to time consuming and defect appearance. Among debinding methods, two-step debinding (solvent followed by thermal degradation of polymers) brings about shortening debinding times and defect free parts. Therefore, multicomponent binder system should be composed by two different polymers at least. On one hand, a low molecular polymer (major binder) that can be chemically extracted by solvent debinding and, on the other hand, insoluble backbone polymer (minor binder) that must keep the strength and shape in the whole solvent debinding process [14].
After removing the soluble polymer, an open pore network is created in the sample through which gasses from thermal degradation of insoluble polymer can easily diffuse to the surface, avoiding common defects in thermal debinding such as blistering, cracking or swelling [15]. Although solvent extraction is probably the fastest debinding route, solvents are often flammable, toxic, carcinogenic and not environmentally acceptable [16,17]. In addition, several types of defects could still occur during the solvent debinding step because of large dimensional changes when parts are immersed in the solvent. The use of Polyethylene Glycol (PEG), a water-soluble polymer, has been reported with good results in the process. However, a polyolefin component is commonly used as backbone polymer [18,19]. Lately, several studies have proposed the use of natural derived Cellulose Acetate Butyrate (CAB) as a potential alternative to polyolefin binder systems, which also brings about zero balance of CO2 emissions from its thermal degradation [20,21].
CIM technology has been widely studied and applied in the industry for many ceramic and advanced ceramic materials [22]. However, zircon has never been processed by injection moulding. Moreover, development of new binders has always been at the most interest of researches, especially of environmentally acceptable binder systems. Therefore, the present work carries out a new processing route for raw zircon powders. Powder injection molding process has been developed using an eco-friendly binder composition, based on PEG and CAB. Debinding parameters and binder composition influences have been studied for this system based on PEG, CAB and zircon. Complete PIM process has been successfully carried out and free defects samples have been achieved after water solvent debinding. After sintering, samples with acceptable flexural strength and density have been obtained.
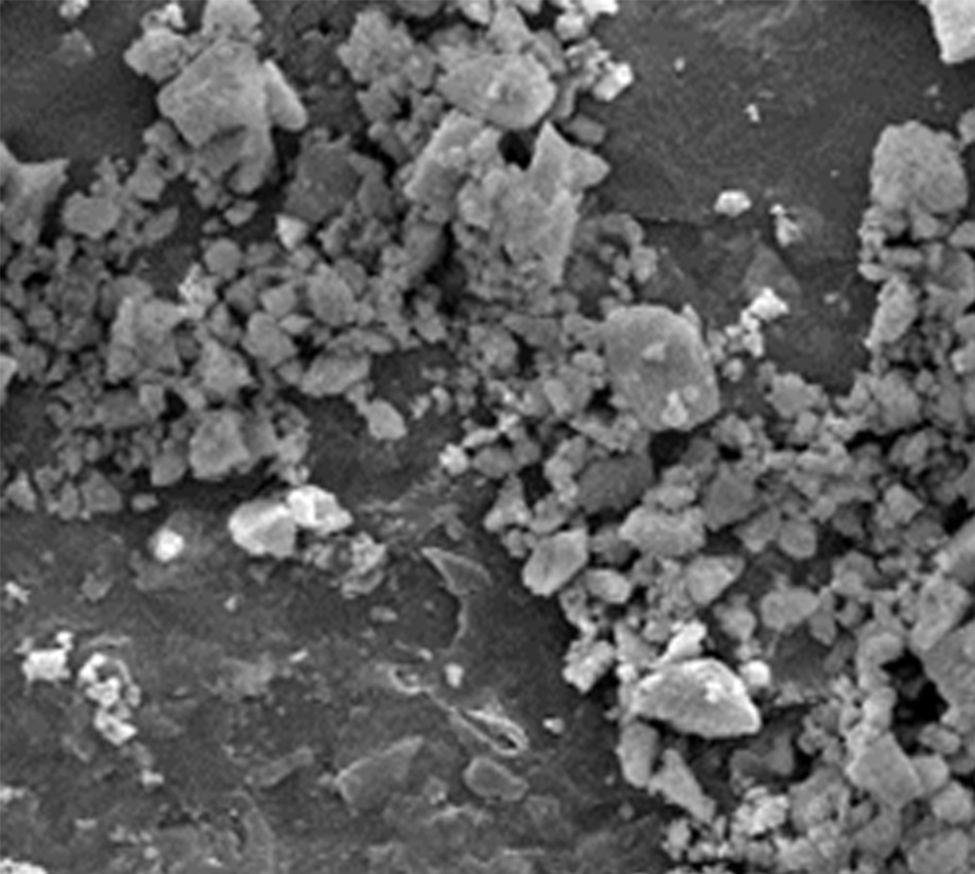
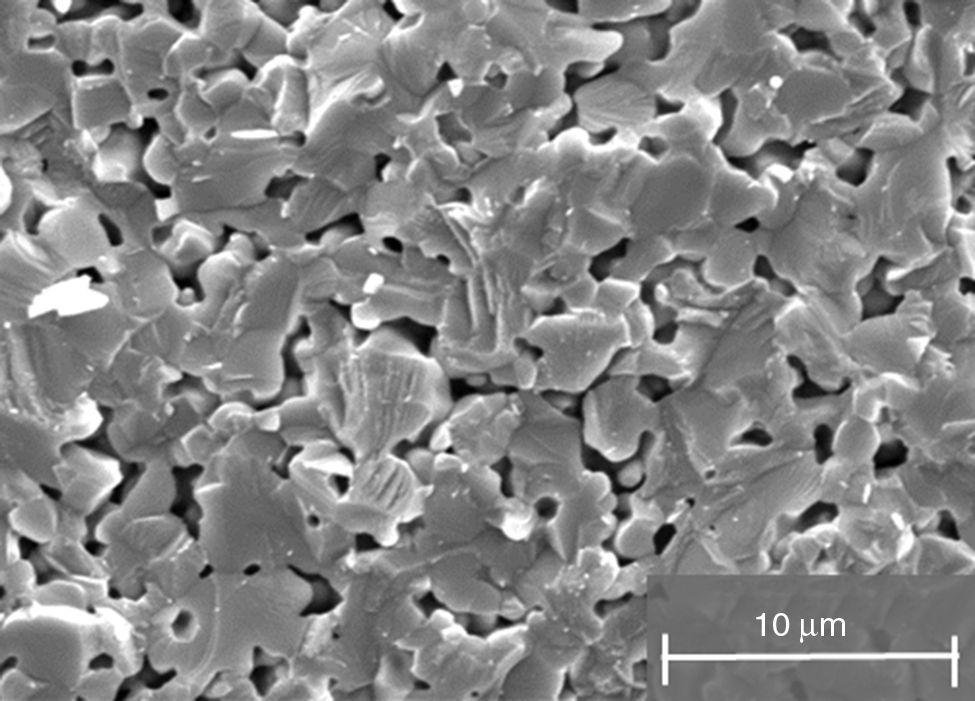
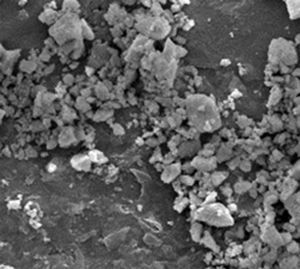
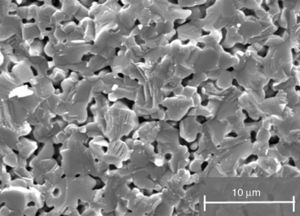
Experimental procedureMaterialsRaw zircon powders used in this study were supplied by Guzman Global S.L. (Spain). Particle size distribution for powders was determined by a Malvern 2000 laser scattering particle size analyzer, particle sizes parameters D50 and D90 are 1.94 and 5.49μm respectively. The specific surface area of the powders is 4.08m2g−1, which was determined by a Micrometrics Gemini VII BET measurement device. Density obtained by means of helium Picnometer is 4.599±0.001gcm−3 and the apparent density of raw powders is 19.05%. Powder morphology was analyzed by scanning electron microscopy (SEM) (Philips XL30 microscope). In Fig. 1, the irregular morphology of this material contrasts with the conventional one used in metal injection molding. Normally, in PIM, synthetic powders are employed with rounded morphology, a particle size between 0.5 and 20μm with a D50 in the range of 4–8μm, a tap density greater than 50% of the theoretical density and no agglomeration [10].
Binder system is composed of Cellulose Acetate Butyrate (Eastman) and polyethylene glycol (Sigma–Aldrich) also some additives have been added such as surfactant (stearic acid, Acros organics) and antioxidant (phenothiazine, Sigma–Aldrich). Different grades of molecular weights (Mw) have been employed in order to study the binder composition influence in the process, PEG between 1.5K and 20K Mw and CAB between 20K and 30K Mw.
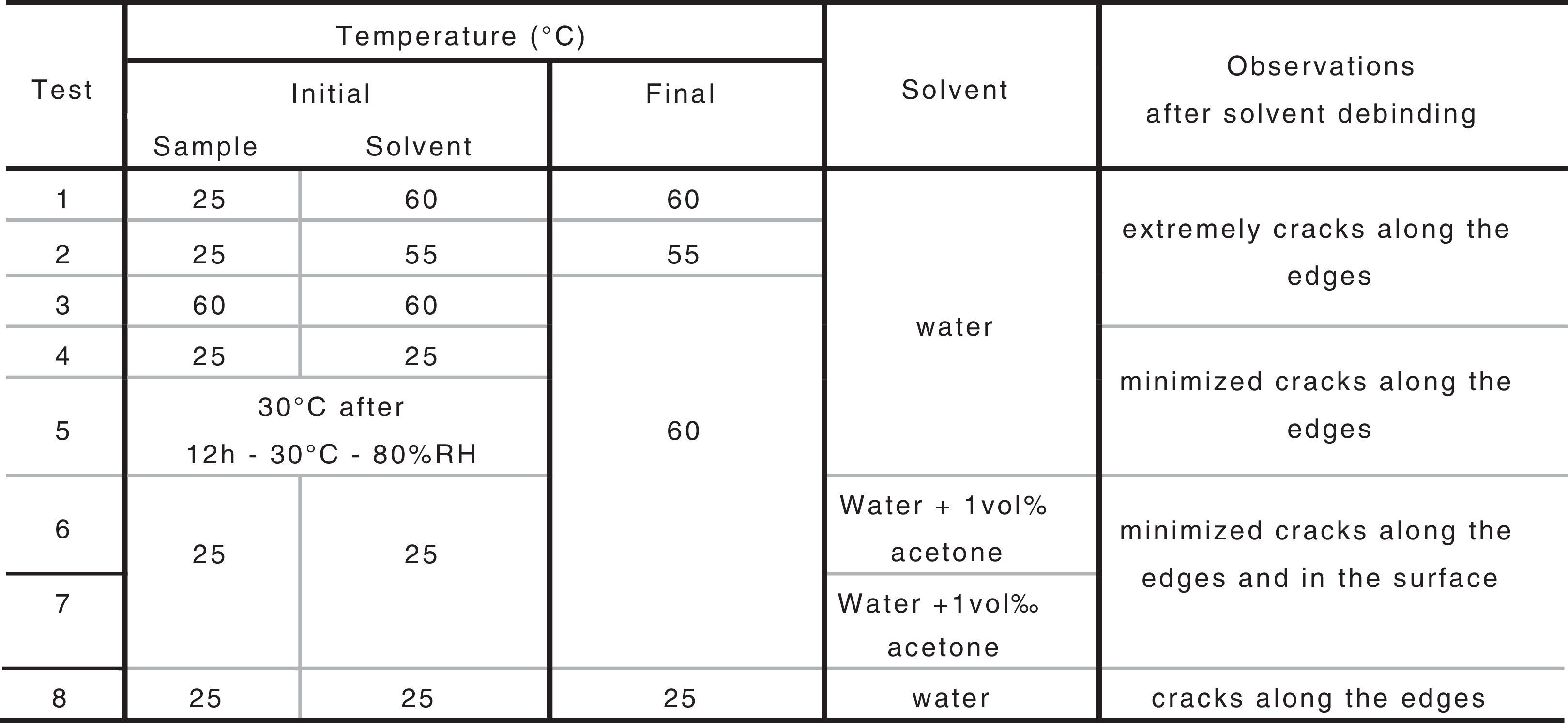
Feedstock development and characterizationThere are three different parts in this work. Firstly, it is studied the influence of debinding parameters in the solvent debinding stage, secondly it is analyzed the influence of the binder composition on solvent debinding stage and finally it is carried out the whole PIM process for the optimized binder composition.
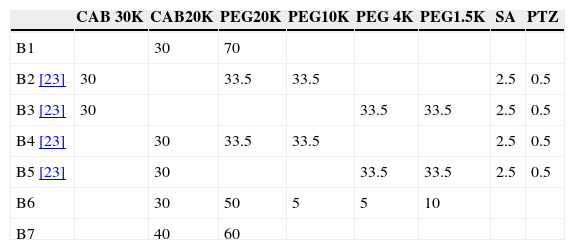
Different binder compositions have been studied along the process; they are summarized in Table 1. The solid volume percentage is the same for all of them, 57.5vol.%.
Previous study determined the parameters to obtain bars at low pressure for feedstock based on B1 [20]. Injected samples were obtained under that conditions and the debinding study were carried out for them. PEG was extracted by solvent elimination in distilled water and the influence of several parameters has been tested. Among these parameters, initial temperatures of the water and sample, final temperature of the process, the use of solvent additive and the use of a climate chamber before getting the samples into the water have been studied. Percentage of PEG removed along time during solvent was evaluated by weighing the parts before immersion in water and after drying them at 60°C during 3h in a furnace.
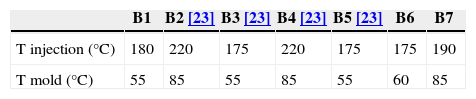
In the second part, new feedstock compositions (B6 and B7) were prepared in a Rheomix 600 Haake rheometer coupled with a Haake Rheocord 252p module. Roller blade-type rotors were used to perform the mixing stage. This process was carried out at 160°C and at a rotors speed of 50r.p.m. for 60min in all cases. After granulation, injection stage was performed in a Bimba AB transfer molding machine. Low pressure of 0.8MPa was employed at a holding time of 11s. These new compositions have been compared with others previously studied. The injection and mold temperatures were varied depending on each composition (Table 2). The dimensions of the green pieces obtained were 60mm×8mm×4mm. Solvent debinding was carried out from room temperature to 60°C in every case. These composition have been compared with B2–B5 previous studied.
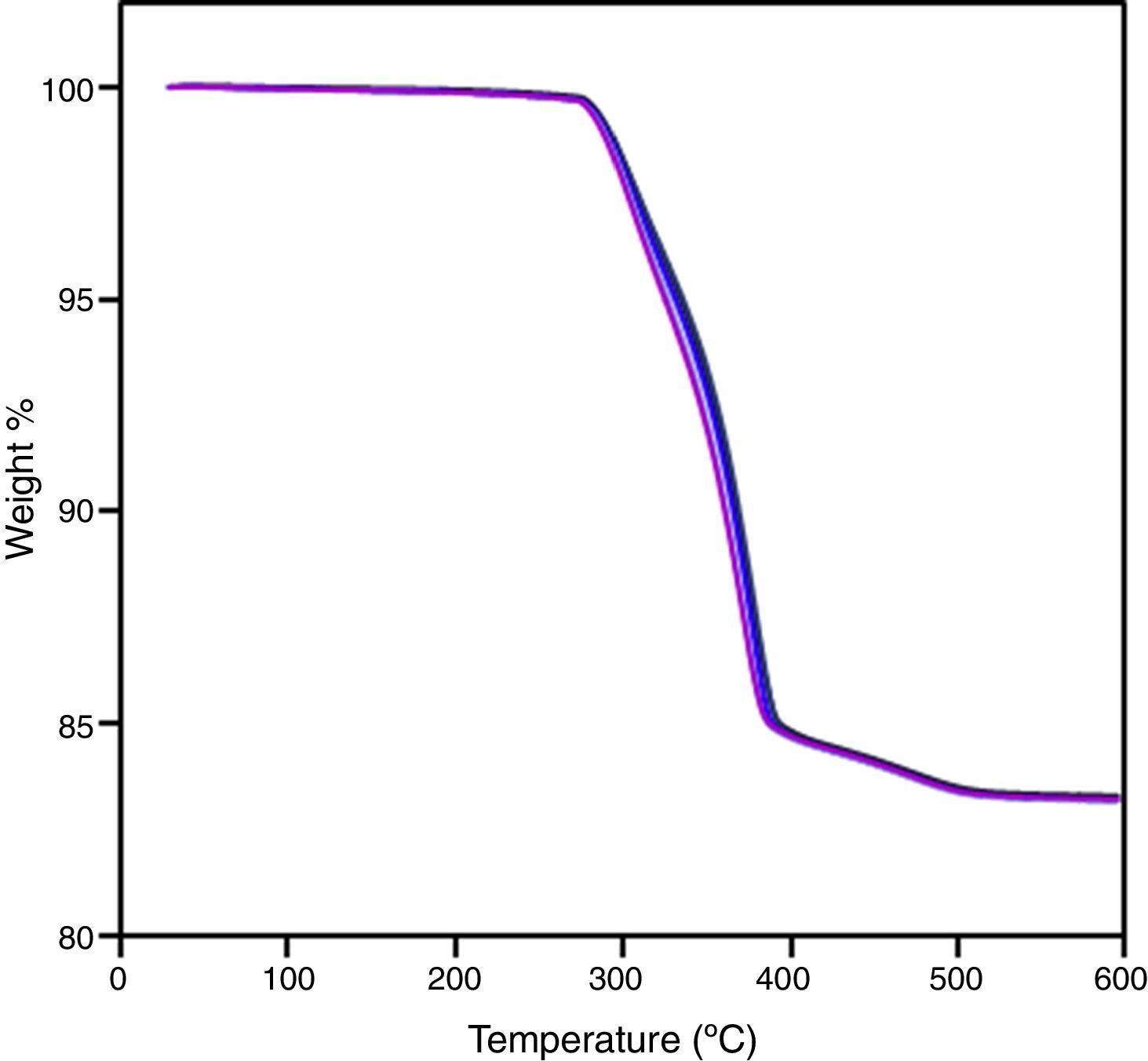
Finally, the whole PIM process has been carried out for system based on B3, since it is necessary to verify if the feedstock composition is suitable in every stage. The homogeneity of the feedstock has been assessed by burnt-out test by comparing the weight loss of five random portions of feedstock of different batches. Thermal analysis of the feedstocks was carried out in a simultaneous thermal analyser (STA) Perkin Elmer STA6000. Tests were carried out from 50 to 600°C at a heating rate of 10°Cmin−1 in air. The quality of injected samples was tested by measuring the density of five different parts per sample. Thermal debinding cycle was tailored after a thermo-gravimetrical analysis of one brown part (2°Cmin−1 up to 500°C in three steps). Samples were sintered at 1500°C during 3h with a heating rate of 5°Cmin−1. Dimensional change, relative density by water absorption method and flexural strength were tested for sintered parts. Flexural strength was obtained on a three-point bending machine (Shimadzu autograph AG-1 universal testing machine), equipped with a 1KN load cell. A crosshead speed of 0.15mmmin−1 was applied. After this test, fracture surface of sintered parts was analyzed by SEM.
Results and discussionSolvent debinding step can considerably reduce the time of the PIM process. However, many parameters must be controlled because the possible defect appearance. This defect appearance is extremely sensible to the solid loading content, particle size distribution and especially to the debinding parameters and binder system composition.
Influence of debinding parametersOptimal solid loading, mixing, capillary rheology and injection steps for B1 have been determined in a previous study [24]. However, samples with defects were obtained during solvent debinding when samples, at room temperature, were immersed in water at 60°C [20]. It is well known that the temperature difference between the samples and the water leads the sharp dimensional changes which can distort the samples [25]. Furthermore, temperature is one of the most important variables in solvent extraction because the highest temperature leads to the highest extraction rate, since molecular chain moves quickly improving the diffusion coefficient. Therefore, the higher is the temperature the higher is the dissolution of the PEG, and it is well-known that a too fast removal of polymer can cause distortion [15]. With the purpose of avoiding those defects during solvent debinding, several parameters have been tested.
Table 3 includes the studied parameters, procedures and the achieved results.
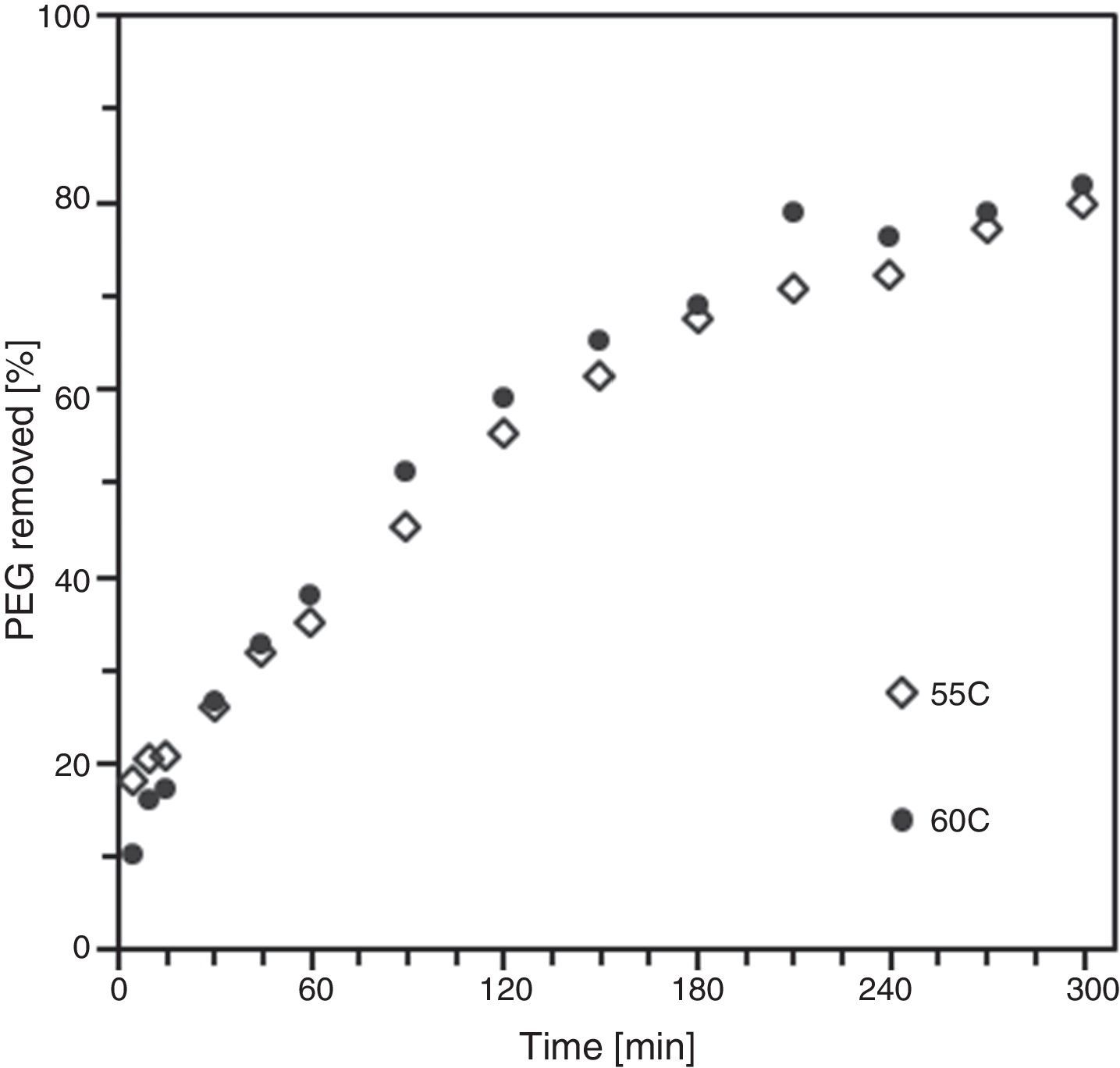
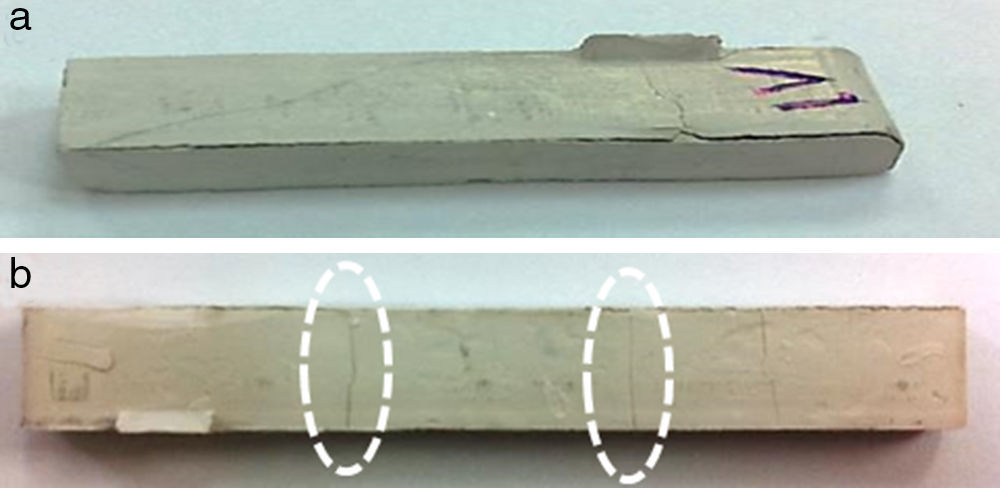
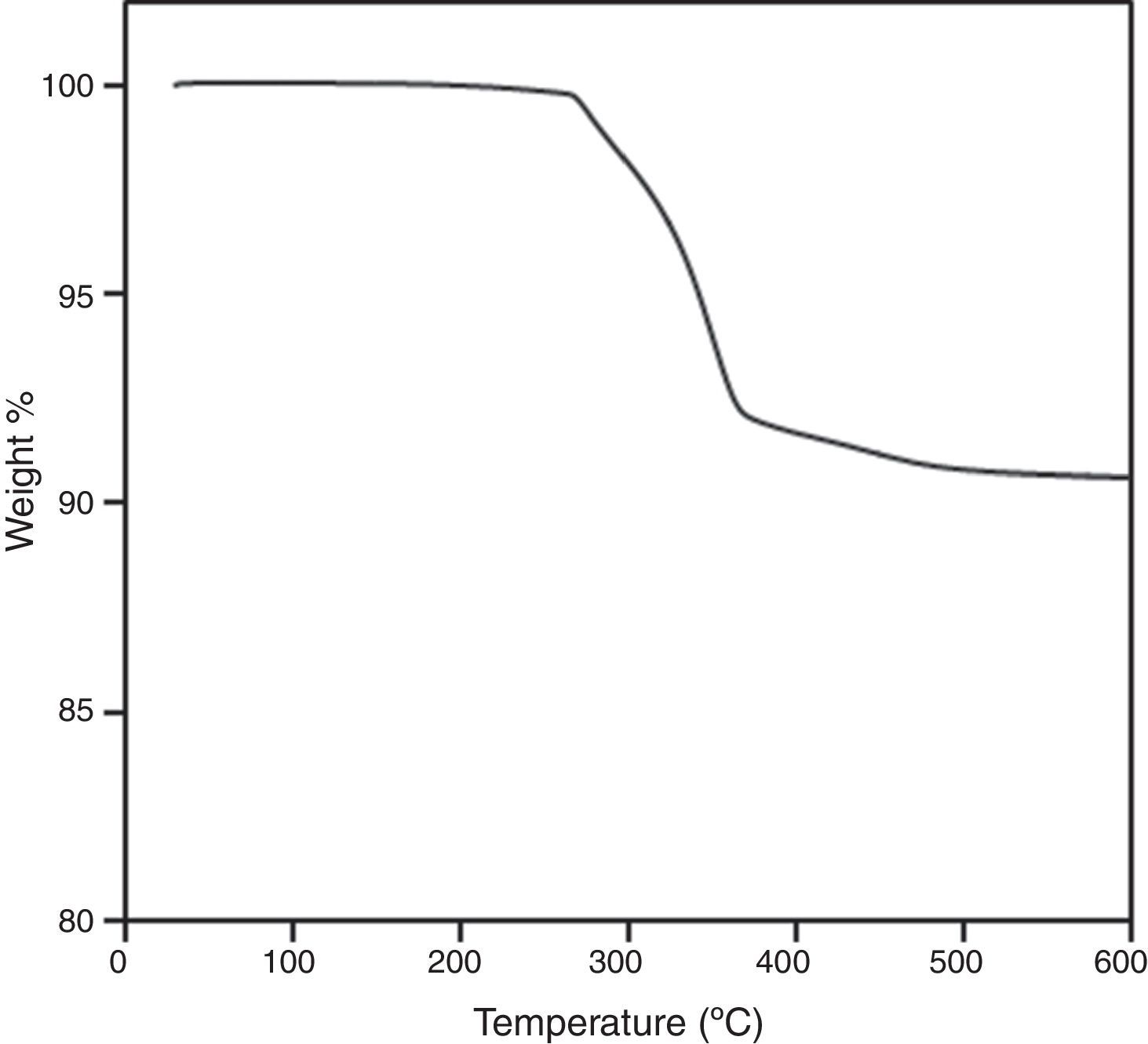
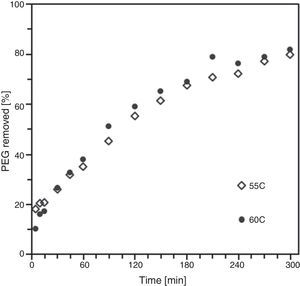
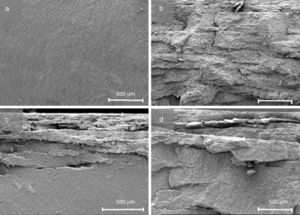
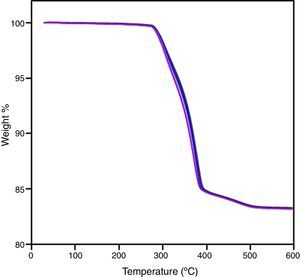
By means of the use a climate chamber, it is able to hydrate PEG before getting samples into the water leading to an easy and slow dissolution of PEG in water. Also, the use of aqueous acetone solutions at different concentrations is employed in order to easily dissolve the polymer in water. Although a high percentage of PEG extracted was achieved just in 5h (80wt.%) at 55°C and 60°C (Fig. 2), defects were present in the samples (Fig. 3). None of the debinding parameters studied have avoided defects in samples, even when aqueous acetone solutions were employed, new cracks broken out in the surface of the samples (Fig. 3). Therefore, defects during water solvent debinding of samples based on this binder composition (B1) are not related with debinding parameters such as temperature or solvent debinding rate.
Influence of binder system compositionSolvent debinding of binder compositions (B2-B5) has been tested in a recent study [23]. A composition of low and a high Mw of PEG were combined with low and high Mw of backbone polymer (CAB). The high difference between molecular weights allowed identifying the high Mw of PEG as the source of the cracks because of the swollen gel created during solvent debinding.
The dissolution mechanism of PEG in water has been extensively studied. Some authors describe the process as: water diffuses into the PEG to produce a swollen gel, and this gel gradually disintegrates into the true solution, causing the sample to expand, finally sample shrink drastically when water drained out [25]. However, there is a minimum temperature required to form the gel by physical crosslinking, this temperature is much higher than the solvent debinding temperatures. Lately, another dissolution mechanism has been proposed. Firstly, water penetrates into the samples and interacts with PEG to form hydrated complexes until the equilibrium in water content is achieved. Secondly, PEG starts to dissolve and finally PEG is transported out of the samples. The removal of the PEG is a diffusion process, swelling could occur when water molecules diffuse into the PEG to produce a hydrated layer whose volume is greater than that of the un-hydrated material but without forming a gel [26,27]. The dimensional change caused by the swelling of the PEG that involve high stresses in the compact, and the backbone polymer must maintain the shape of the compact during solvent debinding [28]. The solubility of PEG in water is attributed to hydrogen bonding between water molecules and the oxygen of the polymer. The chemical structure of PEG is -(O-CH2-CH2)n-OH therefore the higher is the molecular weight the higher is the hydration of PEG leading to a more difficult dissolution and more occupied volume [29,30]. In order to study the influence of binder system in solvent debinding step taking into account this considerations, new binder systems have been studied (B6, B7) whose compositions are reflected in Table 1. On one hand, four different grades of molecular PEG have been combined (B6) in order to obtain a more gradual dissolution of PEG without producing catastrophic distortion in the samples. In this way, low molecular weight is easily dissolve leaving enough space in the sample that can be occupied by the swelling of the rest of hydrated PEG without distortion of the parts. On the other hand, as the high Mw is the cause of defects, the study of decreasing its content in binder is carried out by B7 that imply an increasing of the bonding strength of the compact.
The main influence in this system based on PEG, CAB and Zircon (57vol.%) is the molecular weight of PEG. Despite the use of gradual Mw of PEG and the decreasing of the high molecular weight content, the use of high Mw of PEG derives in cracks after solvent debinding, it can be observed in Fig. 4. System based on B6, backbone polymer is not able to maintain the shape and broken layers are inwards built from the surface just after 5min into the water. The flowability of the system based on B7 is affected and the injection stage was more difficult showing surface defects in the samples. Although, a high percentage of PEG removed was achieved (80%) with a similar kinetics than previous tests and cracks along the edges were minimized, blistering in the surface appeared after solvent debinding. Regarding these results, feedstock composed of 57.5vol.% of powder, requires at least 70vol.% of low molecular polymer to guarantee the flowability of the feedstock.
Powder injection molding process of optimized feedstock based on high Mw-CAB and low Mw-PEGSolvent debinding of the system based on low Mw of PEG (B3, B5) derived in free defects samples. Despite a successful solvent debinding stage have been achieved, it is really important to know if this binder is able to obtain good results until the end of the process. In order to verify the suitability of this system for PIM, whole process has been carried out for B3 that derived in defect free part after solvent debinding and exhibited acceptable rheological behavior.
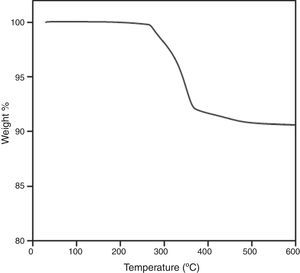
Although, a steady state is achieved at the end of mixing, homogeneity should be deeply study by other methods. The burnt-out tests to determine the amount of the binder that have been present in the feedstock is shown in Fig. 5, where it can be observed the good reproducibility of the curves of 5 different samples, therefore feedstock could have a good homogeneity.
The homogeneity of the injected samples was also studied by measuring 5 different parts of the same sample, the average density was 3.151±0.005gcm−3, it is achieved a good reproducibility.
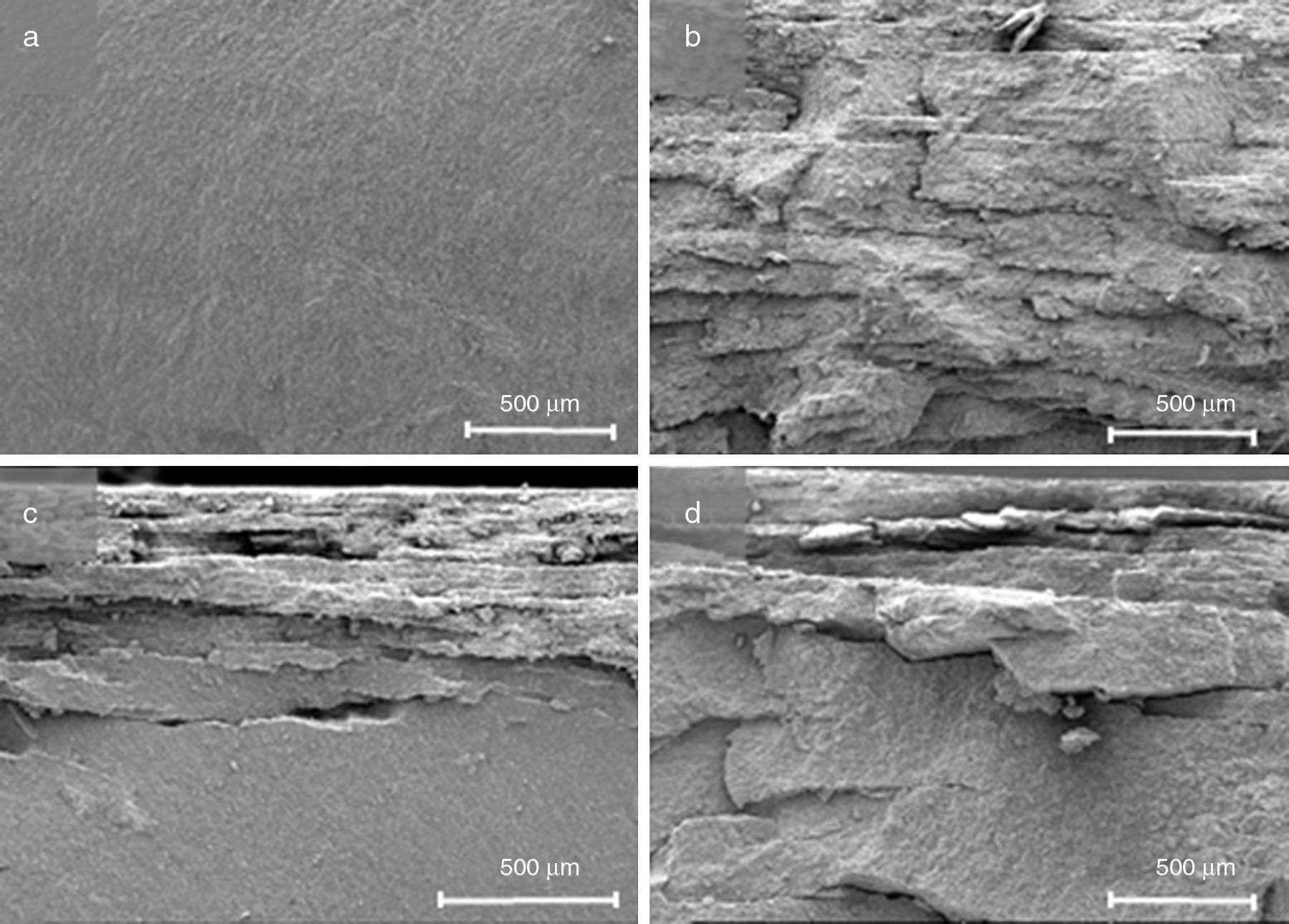
The whole PIM process has been carried out. Thermal debinding cycle was tailored after the thermo-gravimetrical analysis of a sample after solvent debinding step (Fig. 6) and sintering was performed with success. After sintering, an isotropic shrinkage was obtained in samples, as a result of using a homogeneous product. Density of sintered bars was obtained by water absorption method and an acceptable value was achieved taking into account that sintering cycle is not optimized and the high refractoriness of zircon. Despite of its moderate density value, samples shown a competitive flexural strength compare to other studies [5,31]. These results are summarized in Table 4. Fig. 7 shows the fracture surface obtained after flexural strength test. In this figure, it can be appreciated the transgranular brittle character of the fracture and the cleavage facets.
The main conclusion from this work is the viability to process a no-synthetized zircon powders by a new method, Powder Injection Moulding, with properties far away from the conventional ones. The success of this process fully depends on the binder system design. In this study, an eco-friendly binder system with a high percentage of soluble polymer has been optimized. The first approach to the optimization of solvent debinding has been performed analyzing the influence of debinding parameters. However, in this case none of them have been able to avoid defects in the parts. After that, diferent binder compositions have been studied. It has been tailored with gradual Mw of PEG (B6) and decreasing the soluble part content (B7). This composition was compared with previous studied ones until solvent debinding (B2–B5).
Finally, binder system, based on low Mw of PEG and high Mw of CAB (B3) previous evaluated, has been studied along the whole PIM process. The importance of the binder system design lies in obtaining an extremely high quality product from the beginning, which possesses good properties in every step until the end. As the defects cannot be solved in the following stages, the initial product must have the highest quality. B3 with successful solvent debinding and with suitable rheological behavior has been studied until the end of the PIM process. Homogeneity has been verified by different methods and full PIM process has been developed without problems leading to obtain competitive properties for the zircon. Binder system based on high-Mw of CAB and low-Mw of PEG is a potential binder to obtain competitive zircon samples by PIM.
The authors wish to thank GUZMÁN GLOBAL S.L. and MIMTECH ALFA for their collaboration on the ECOPIM project (ref. IPT-2011-0931-20000) that was funded by the Spanish Ministry of the Economy and Competitiveness. Furthermore, the authors would like to acknowledge the strong support from the MULTIMAT-CHALLENGE projects (ref. S2013/MIT-2862), which was funded by the CAM-Consejería Educación Dirección General Universidades e Investigación, and from the MITICO project (ref. MAT2012/38650-C02-01), which was funded by the Spanish Ministry of the Economy and Competitiveness.
This work has participated in the Third Conference of Young Scientists of the Institute of Ceramics and Glass CSIC, held in Madrid on June 25, 2014.