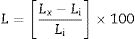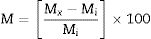The reuse of spent catalysts from residue fluid catalytic cracking (RFCC) units as pozzolanic materials in cement and concrete production offers a number of important benefits. In spite of all these benefits, the durability performance of the produced blended cement is an important issue to be considered. This study investigates the effects of RFCC spent catalyst on durability performance of hardened Portland cement paste in a highly aggressive sulfate environment. The 28-day cured paste specimens prepared from binary cement mixtures incorporating different replacement levels of 0, 10, 20, and 30% (by mass) RFCC spent catalyst at a constant water-to-cement ratio of 0.30 were exposed to 10 mass% solution of magnesium sulfate. The accelerated sulfate attack under alternative cycles of wetting and drying was studied by monitoring the changes in compressive strength, length, and mass of specimens and also by the application of XRD, SEM and EDX techniques. Based on the results and a comparison with plain Portland cement, binary cement mixtures exhibit a higher rate of deterioration in spite of their significantly improved compressive strengths resulted from pozzolanic reaction.
La reutilización de catalizadores consumidos de unidades de craqueo catalítico de fluidos residuales (RFCC) como materiales puzolánicos en la producción de cemento y hormigón ofrece una serie de beneficios importantes. A pesar de todos estos beneficios, el rendimiento de durabilidad del cemento con adiciones producido es un tema importante a considerar. Este estudio investiga los efectos del catalizador consumido RFCC en el rendimiento de durabilidad de la pasta de cemento Portland endurecido en un entorno de sulfato altamente agresivo. Los especímenes de pasta curada de 28 días preparadas a partir de mezclas de cemento binarias que incorporan diferentes niveles de 0, 10, 20 y 30% (en masa) de catalizador consumido RFCC a una proporción constante de agua a cemento de 0,30 se expusieron al 10% en masa de solución de magnesio sulfato. El ataque acelerado de sulfato en ciclos alternativos de humectación y secado se estudió mediante el monitoreo de los cambios en la resistencia a la compresión, la longitud y la masa de las muestras y también mediante la aplicación de técnicas XRD, SEM y EDX. En función de los resultados y en comparación con el cemento Portland sin adición, las mezclas binarias de cemento que incorporan catalizador consumido muestran una mayor tasa de deterioro a pesar de sus resistencias a la compresión significativamente mejoradas como resultado de la reacción puzolánica.
Sulfate attack on Portland cement (PC)-based products was first reported by United States Bureau of Reclamation in 1908 and from that time research works for improving the chemical resistance of cement-based materials and products are still being carried out [1]. The improvements achieved in these extensive and long-time research works were mostly based on the following techniques [2,3]:
- 1.
Improving the physicochemical properties of PC
- 2.
Utilizing suitable blended cements
Many researchers have studied the performance and durability of blended cements exposed to sulfate environments and claimed that supplementary cementing materials (SCMs) of either natural or artificial origins with relatively high contents of silica and alumina not only improve the long-time physical properties of PC, but also increase the chemical resistance of PC against sulfate attack [4–6]. In the experimental results, such an improvement in sulfate resistance of blended cements brought about by the effect of SCMs utilized as a partial cement substitute is quite evident [1,7–12]. In some cases, the utilized SCMs were fly ash [13], rice husk ash [14], the ash produced from combustion of agricultural wastes [15], ground granulated blast furnace slag [16,17], phosphorus slag [18], and a blend of various additives [19–23]. In such cases, in addition to the improvements happened in mechanical strength and chemical resistance of the cement, considerable environmental polluting materials could also be consumed [15].
According to previous research activities [24–29], spent catalysts from fluidized catalytic cracking (FCC) units or residue fluid catalytic cracking (RFCC) units exhibit strong pozzolanic activity. These materials have a highly porous microstructure causing a relatively high water absorption capacity which in turn considerably lowers the workability of cement paste, mortar, or concrete. The important research result to consider is that an optimal replacement of cement with FCC spent catalyst shows an opposite effect on carbonation resistance of concrete [28].
These catalysts, composed of aluminosilicates, are deactivated after some limited life time of service under operating conditions in the catalytic cracking units and therefore are disposed as industrial waste materials. Investigations have confirmed that spent catalysts of both FCC and RFCC types consisting mainly of silica and alumina and sometimes some amount of faujasite crystals are usually polluted with heavy metals and therefore must be disposed according to some specific regulations [25]. On the other hand, it is well known that encapsulation or immobilization of heavy metals-polluted materials in cement matrices is an effective way to prevent their environmental pollutions. Such a disposal method for these spent catalysts, not only results in considerable improvements in physical properties of the cement, but also effectively prevents any type of environmental pollution from such polluted materials [19,20,27–30]. The durability and long-term performance of RFCC spent catalyst- blended PC is therefore important and it is necessary to investigate the effects of aggressive environments on this cement and to evaluate its chemical resistance.
The aim of this study is to evaluate the effects of RFCC spent catalyst on durability performance of hardened PC paste against magnesium sulfate as a very aggressive salt for cement matrices. Within the scope of this study, the changes in compressive strength, length and mass of hardened cement paste specimens exposed to magnesium sulfate are measured at different time intervals and considered as measures for evaluating the extent of deterioration by salt attack.
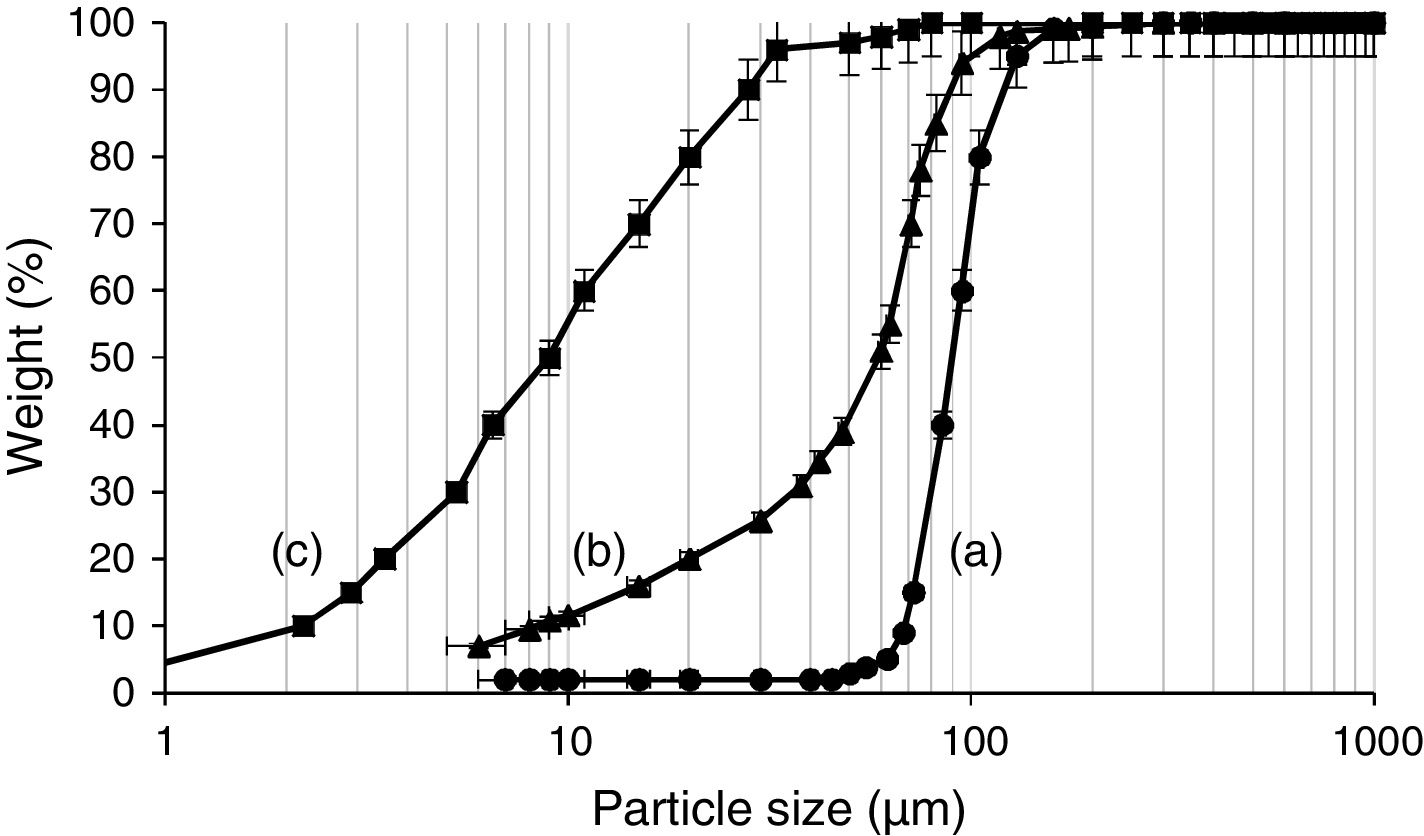
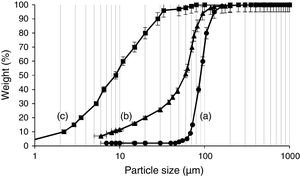
Experimental programMaterialsThe materials used in this study include RFCC spent catalyst (SC), ASTM standard type II PC of grade 42.5, silica fume and natural pozzolan. This type of PC contains less than 8% by mass calcium aluminate phase and therefore has a medium sulfate resistance [31]. Knowing that particle size distribution of SC could strongly affect both wet and dry properties of PC, the SC was therefore ground in a laboratory ball mill for 100min to obtain a relatively fine powder comparable to PC. The particle size distribution of the SC powder was determined by a laser particle size analyzer. The values of specific surface area and bulk density of ground SC were measured in accordance with ASTM C204 [32] and ASTM C188 [33] standards, respectively. Fig. 1 shows the particle size distributions of PC and SC before and after grinding. A comparison of the two particle size distribution curves (Fig. 1a and b) clearly shows that after grinding, the mass fraction of particles less than 50μm has significantly increased. As seen, in the ground SC almost 95% of the grains are in the range between 5 and 90μm, whereas such a percentage is related to the grain size of 2–35μm in PC.
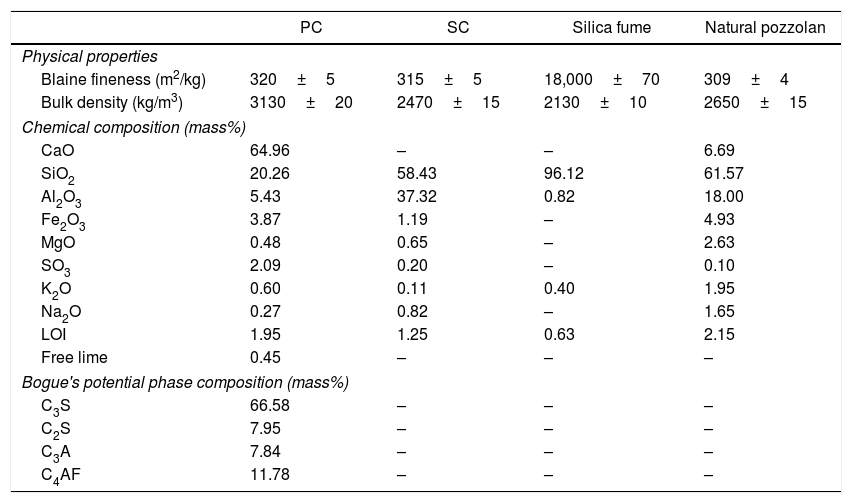
The chemical composition and physical properties of SC, PC, silica fume, and natural pozzolan are presented in Table 1. To determine the chemical composition of the SC, wet analytical methods were applied in accordance with ASTM C114 [34] and for the results to be enough accurate, two individual samples were analyzed separately and the average values were reported as the result. In these test methods, silicon dioxide (SiO2) is determined gravimetrically after the dissolution of sample in HCl. The ammonium hydroxide group, namely aluminum, iron, titanium, and phosphorus are precipitated from the filtrate, after SiO2 removal, by means of NH4OH. The precipitate is ignited and weighed as the oxides. The Fe2O3 content of the sample is determined on a separate portion of the sample by reducing the iron to the ferrous state with SnCl2 and titrating with a standard solution of K2Cr2O7. The Al2O3 content is obtained from the ammonium hydroxide group by subtracting the separately determined constituents that usually are present in significant amounts in the ammonium hydroxide precipitate. Magnesium is precipitated as magnesium ammonium phosphate from the filtrate after removal of calcium. The precipitate is ignited and weighed as Mg2P2O7. The MgO equivalent is then calculated. To determine the sulfur content, sulfate is precipitated from an acid solution of the sample with BaCl2. The precipitate is ignited and weighed as BaSO4 and the SO3 equivalent is calculated. The sample is ignited in a muffle furnace at a controlled temperature. The loss is supposed to represent CO2 and the total moisture in the sample. This test method covers the determination of Na2O and K2O by atomic absorption or flame photometry. For PC, the relative errors related to CaO and SiO2 contents were less than 3% and the relative errors of the remaining components were less than 5%. For SC and natural pozzolan, the relative errors of the Al2O3 and SiO2 contents were less than 4% and the relative errors of the remaining components were less than 6%. For silica fume, the relative errors of the SiO2 the remaining constituents were less than 2 and 5%, respectively. The obtained results revealed that the catalyst was mainly comprised of SiO2 and Al2O3. As seen, these two components account for over 95% of the total mass of the material. This SC is therefore a relatively high siliceous material and according to ASTM C618 [35], it could chemically be considered as a relatively good artificial pozzolana. Silica fume and natural pozzolan were used in pozzolanic activity measurements as reference materials for comparison purposes.
Chemical composition and physical properties of the used materials.
| PC | SC | Silica fume | Natural pozzolan | |
|---|---|---|---|---|
| Physical properties | ||||
| Blaine fineness (m2/kg) | 320±5 | 315±5 | 18,000±70 | 309±4 |
| Bulk density (kg/m3) | 3130±20 | 2470±15 | 2130±10 | 2650±15 |
| Chemical composition (mass%) | ||||
| CaO | 64.96 | – | – | 6.69 |
| SiO2 | 20.26 | 58.43 | 96.12 | 61.57 |
| Al2O3 | 5.43 | 37.32 | 0.82 | 18.00 |
| Fe2O3 | 3.87 | 1.19 | – | 4.93 |
| MgO | 0.48 | 0.65 | – | 2.63 |
| SO3 | 2.09 | 0.20 | – | 0.10 |
| K2O | 0.60 | 0.11 | 0.40 | 1.95 |
| Na2O | 0.27 | 0.82 | – | 1.65 |
| LOI | 1.95 | 1.25 | 0.63 | 2.15 |
| Free lime | 0.45 | – | – | – |
| Bogue's potential phase composition (mass%) | ||||
| C3S | 66.58 | – | – | – |
| C2S | 7.95 | – | – | – |
| C3A | 7.84 | – | – | – |
| C4AF | 11.78 | – | – | – |
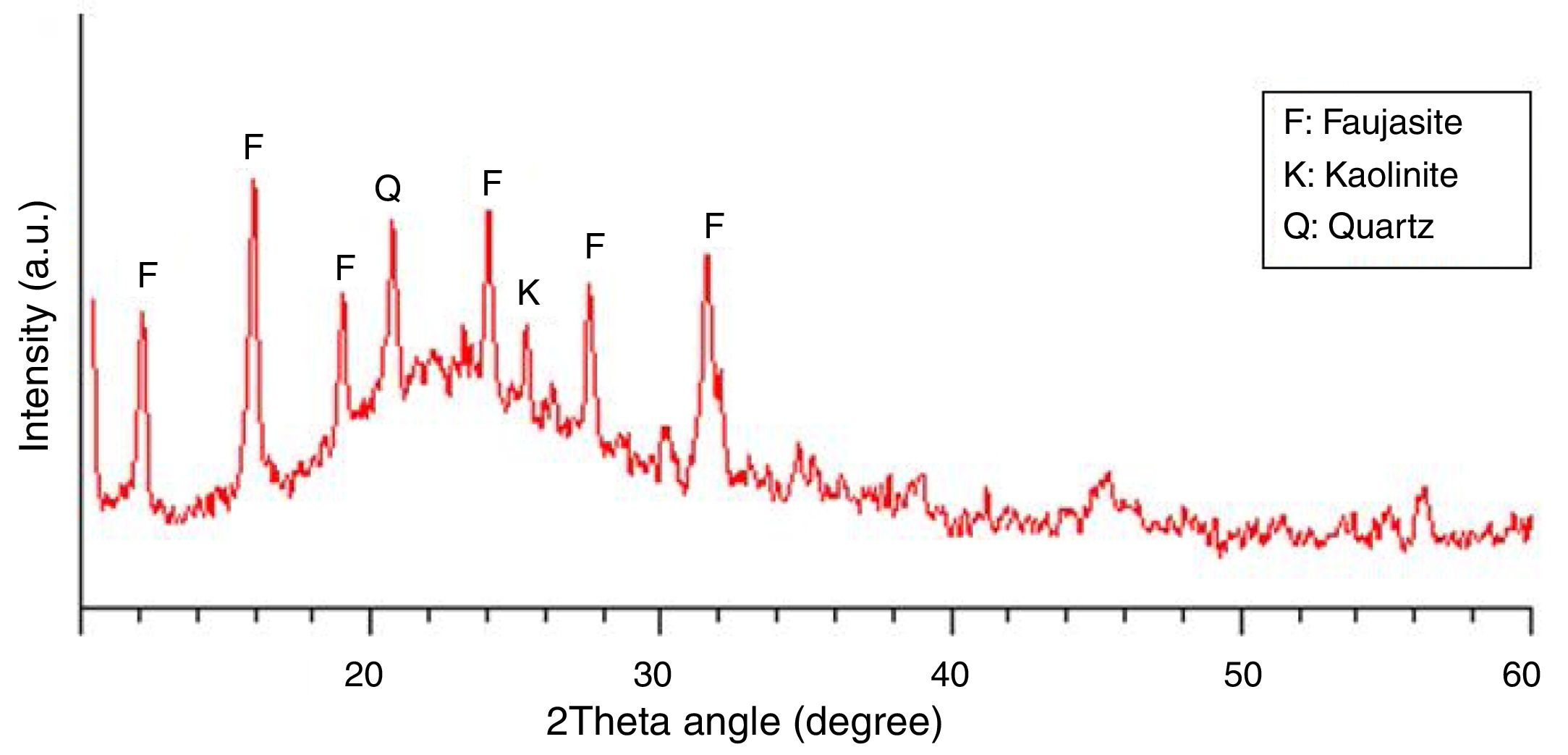
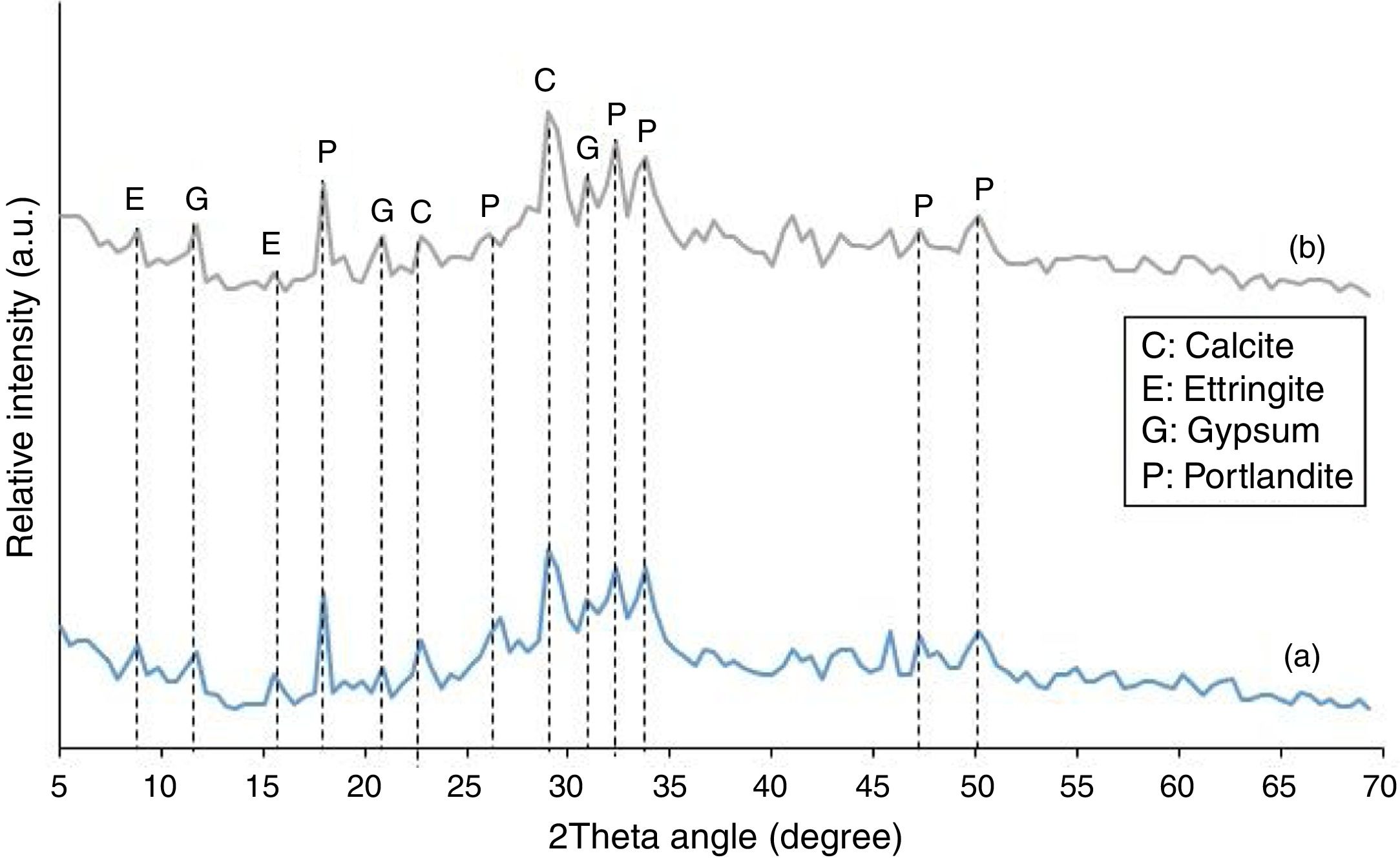
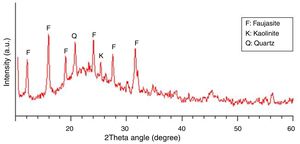
The mineralogical phase composition of the SC was determined using powder X-ray diffractometry (Cu, Kα radiation). Fig. 2 depicts the X-ray diffraction pattern of the material. As seen, the shape of the pattern and the broad diffuse halo at 2θ=23° clearly show that the SC is mainly an amorphous material. The few minor crystalline mineral phases present in the material are faujasite, quartz, and kaolinite.
ProceduresMeasurement of pozzolanic activityIn this study, for measuring the pozzolanic activity of the SC, silica fume and natural pozzolan, thermogravimetric analysis was used. For this purpose, a homogenized mixture of from each material and calcium hydroxide with a mass ratio of 1:1 was used to prepare a paste by adding equal amount of water. The obtained paste was left in a nitrogen gas atmosphere at a constant temperature of 60°C. Then at given time intervals of 3, 7, 14, and 28 days, samples taken from the paste were dried with acetone and nitrogen gas. Finally, the dried samples were used for thermogravimetric analysis to determine the amount of reacted calcium hydroxide. For each time interval, the described experiment was repeated twice and the average value plus error percentage was reported as the result.
Preparation of specimensBinary cement mixtures were prepared by replacing PC with ground SC at different replacement levels of 0, 10, 20, and 30% by mass. Each dry binary mixture was thoroughly homogenized using a laboratory ball mill containing a few balls for 20min. Water-to-cement ratio was taken constant at 0.30 for all the mixtures and pastes were cast into cubic specimens of the size 2cm×2cm×2cm. Also, paste bars of the size 2cm×2cm×10cm were prepared to monitor the length change. The molds were stored at an atmosphere of more than 95% relative humidity at 25°C for the first 24h and then the specimens were cured in tap water at 25°C until the age of 28 days.
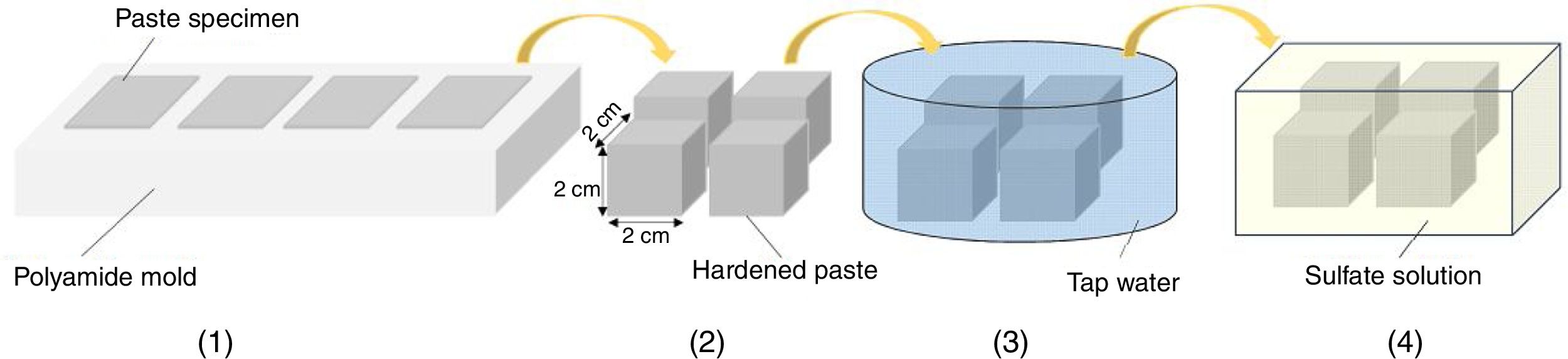
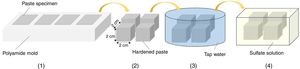
Sulfate exposure testIn order to perform a sulfate exposure test, the steps shown in Fig. 3 were followed. After paste specimens were stored in a humid chamber for 24h, they were demolded and cured in tap water for 28 days. After 28 days of curing, the specimens were exposed to sulfate solution in accordance with ASTM C1012 [36]. Among the sulfate salts, magnesium sulfate that is the most aggressive one was selected. The concentration of the salt solution was considered at a relatively high level of 10 mass% and alternative wetting-drying cycles were applied to accelerate the process of deterioration. After each two days of full immersion, the specimens were exposed to open air atmosphere and allow to dry for 24h. The ratio of the salt solution volume to the total exposure surface was kept constant at 10cm3/cm2. The solution temperature was kept constant at 25°C throughout exposure time. The sulfate solution was renewed once per month. The sulfate exposure test was continued for about 4 months. A similar trend was applied for paste bars.
Measurement methods and complementary techniquesChanges in compressive strength, length and mass of the specimens were measured and considered for determining the extent of deterioration. For each measurement, three specimens were used and the average value was reported as the result. For measurement of mass, the specimens were gently weighed as soon as removing their surface free salt solution with a towel. The mass measurements were done with 0.01g accurate digital balance. Enough number of 28-day cured paste specimens were also stored in tap water at 25°C for comparison purposes and for an accurate evaluation of the extent of deterioration. The length of specimens was measured at different time intervals using an ordinary 150-mm digital calipers with a rated accuracy of 0.02mm and length changes were determined using the following equation [36]:
where L (%) is length change at the age of x; Lx (mm) is average length of three bars at the age of x; Li (mm) is average initial length of the same three bars after 28 days of curing.The mass changes were calculated by the following equation:
in which M (%) is the mass change; Mi (g) is average mass of three specimens after 28 days of curing; and Mx (g) is average mass change of the same three specimens at the age of x.Laser particle size analyzer (Sympatec, GmbH, HDD) was used for determination of particle size distribution of SC before and after grinding. The pozzolanic activity of the SC was measured using a thermogravimetry equipment (Netzsch model 429). The mineralogical phases were determined with a JEOL JDX-8030 X-ray diffractometer using Cu-Kα radiation at 40kV and 30mA. For this purpose, PC and blended cement paste specimens after 120 days of exposure to sulfate attack were used. The microstructural and elemental analyses were carried out using a Cambridge Stereoscan 360 Scanning Electron Microscope device at an accelerating voltage of 30kV. Fourier transform infrared (FTIR) spectroscopy was performed using a Shimadzu FTIR 8400s Spectrophotometer in transmittance mode from 400 to 4000cm−1 using standard KBr technique.
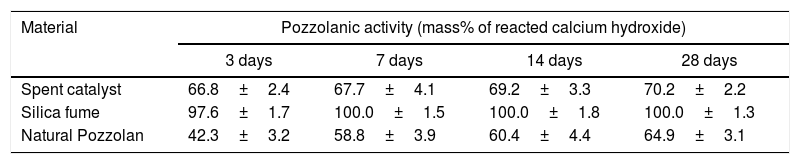
Results and discussionPozzolanic activity of spent catalystThe results of pozzolanic activity measurements for the SC and the reference materials (silica fume and natural pozzolan) are presented in Table 2. As can be seen and compared to silica fume as a material exhibiting very strong pozzolanic properties and also to a typical natural pozzolan that is currently being used by Iranian cement industry for the purpose of natural pozzolan-blended cements production, SC has a very good reactivity with hydrated lime and can be considered as a very good pozzolanic material.
Pozzolanic activity of spent catalyst and reference materials.
| Material | Pozzolanic activity (mass% of reacted calcium hydroxide) | |||
|---|---|---|---|---|
| 3 days | 7 days | 14 days | 28 days | |
| Spent catalyst | 66.8±2.4 | 67.7±4.1 | 69.2±3.3 | 70.2±2.2 |
| Silica fume | 97.6±1.7 | 100.0±1.5 | 100.0±1.8 | 100.0±1.3 |
| Natural Pozzolan | 42.3±3.2 | 58.8±3.9 | 60.4±4.4 | 64.9±3.1 |
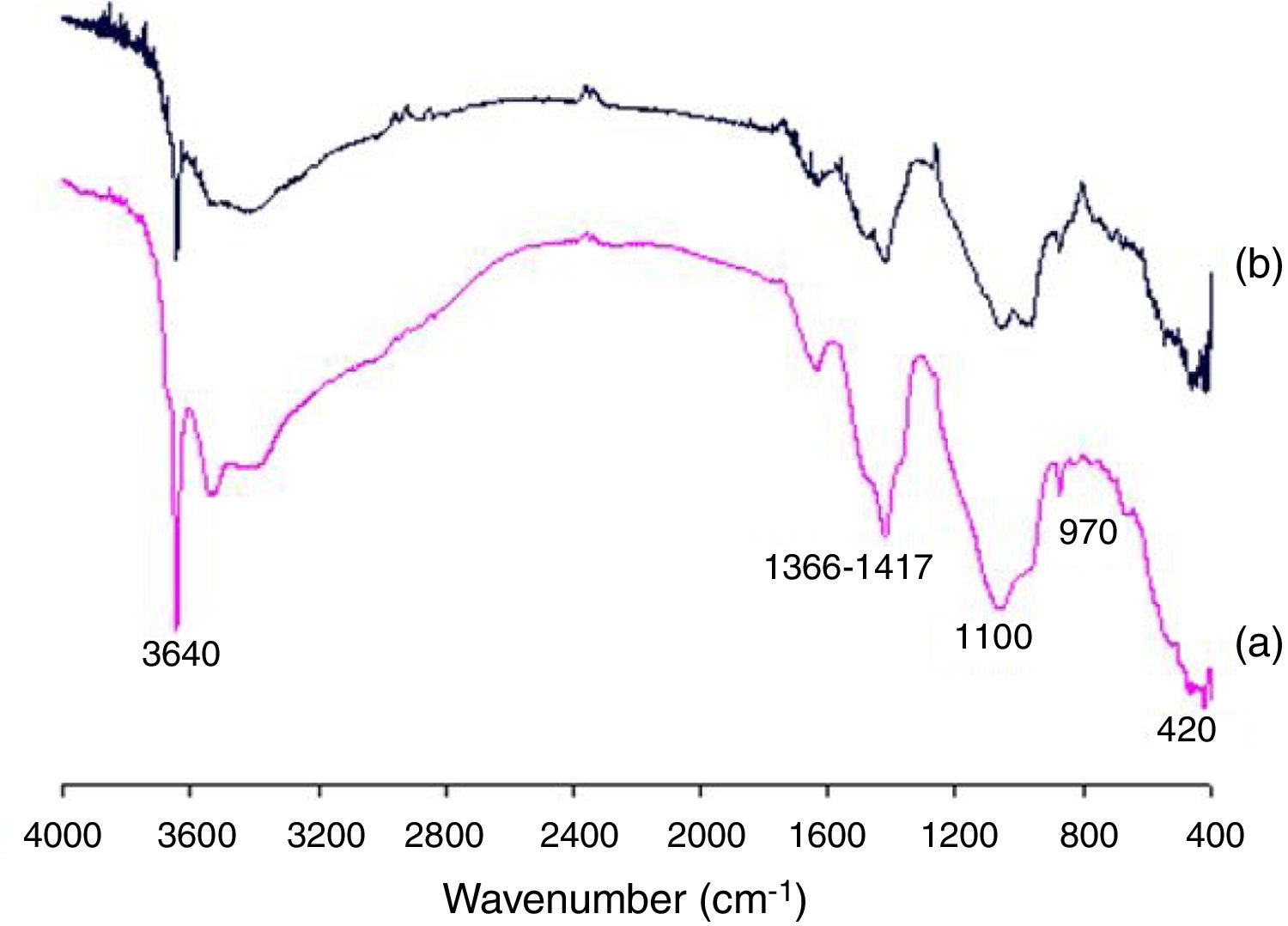
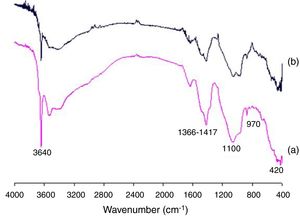
Pozzolanic activity of the SC was also confirmed by FTIR spectroscopy studies. Fig. 4 shows the FTIR spectra obtained from samples of SC/lime (1:1) after 7 and 28 days of curing. The more relevant absorption bands include 3640cm−1 for –OH of calcium hydroxide, 1366–1417cm−1 for carbonates (as lime impurities), 1100cm−1 for vibrations of valence Si-O(Al)-O, 970cm−1 for calcium silicate hydrates and 420cm−1 for calcium aluminate and calcium aluminosilicate hydrates. As can be seen, both bands at 3640 and 1100cm−1 belonging to lime and SC, respectively, noticeably decrease from 7 to 28 days of reaction while the band at 970cm−1 belonging to calcium silicate hydrates increases as a consequence of the pozzolanic reaction.
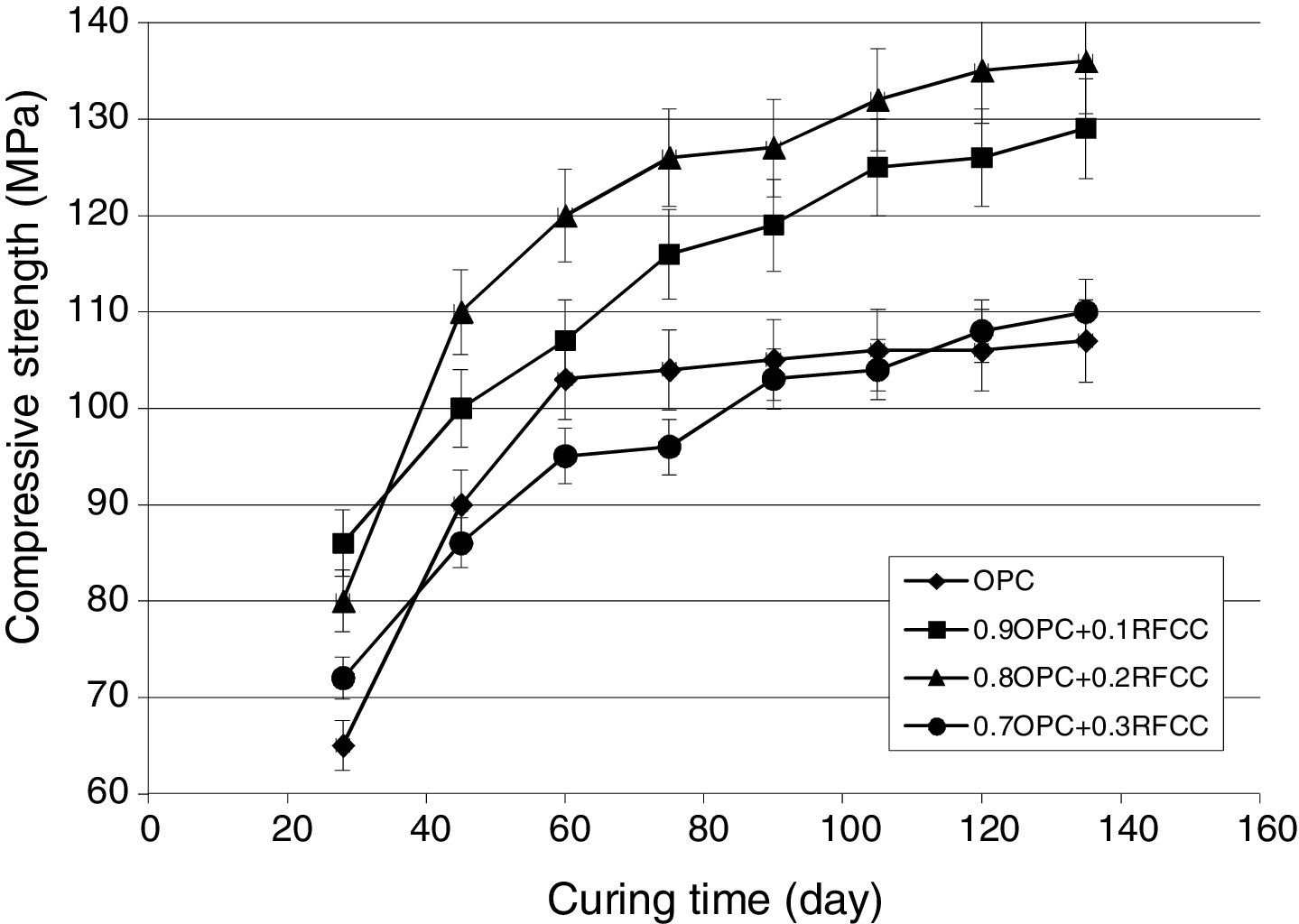
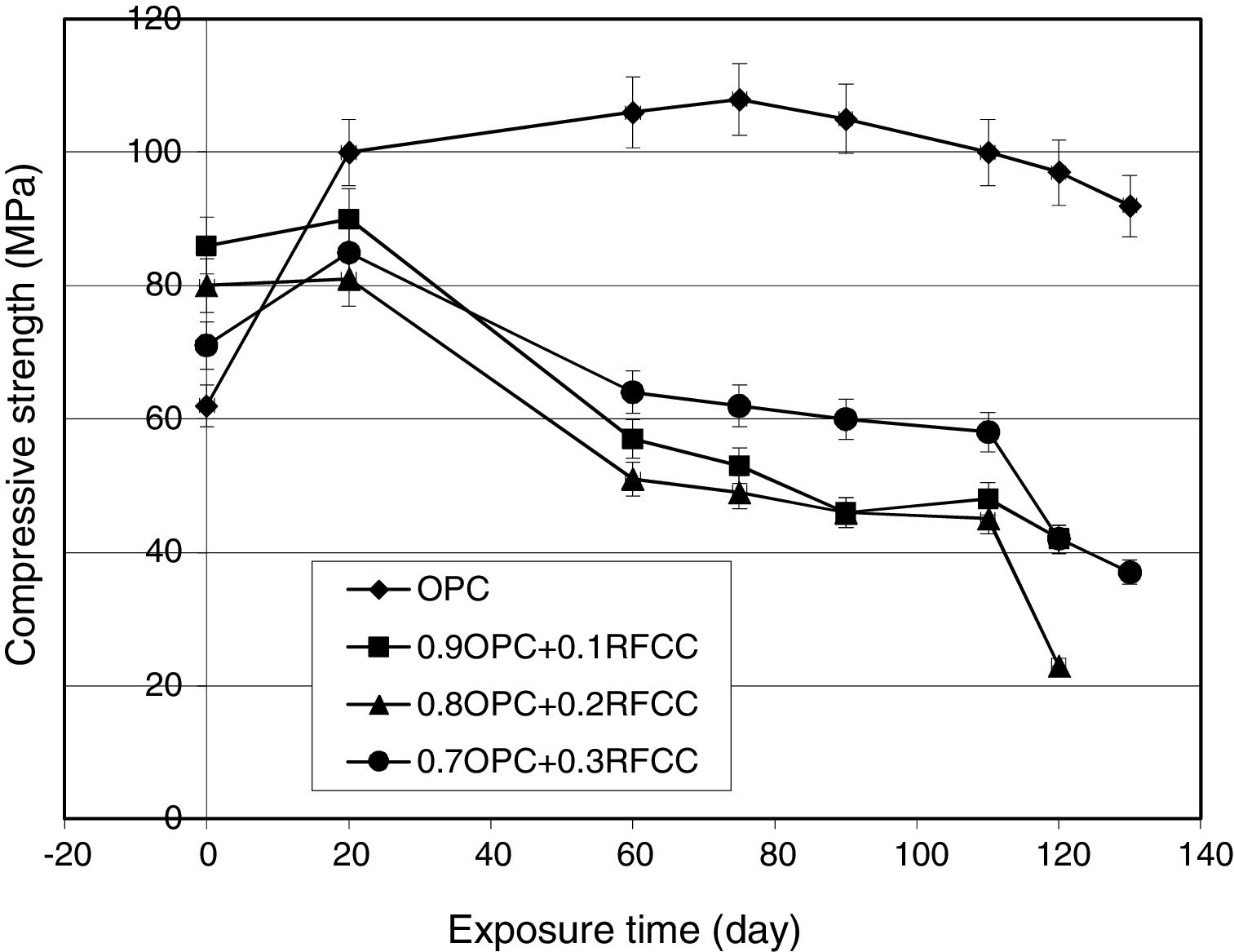
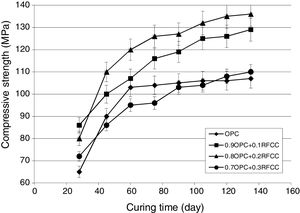
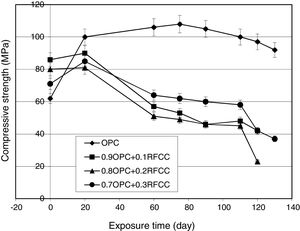
Compressive strength changesThe results of compressive strength measurements are shown in Figs. 5 and 6. Fig. 5 representing the results obtained from specimens stored in tap water not only shows the effect of SC on compressive strength of PC, but also provides the possibility of comparing the results with those obtained for specimens kept in sulfate solution (Fig. 6) and evaluating the extent of deterioration.
As seen in Fig. 5, the compressive strength continued to increase up to the age of 60 days with a higher growth rate (steeper slope) especially for mixtures incorporating 10 and 20 mass% SC. After 60 days, the compressive strength still kept increasing, but at a slower rate. Mixtures with 10 and 20 mass% replacements with the highest compressive strengths of 129 and 136MPa after 130 days of continued curing, respectively, exhibited higher values of compressive strengths at all the curing ages after the first 28 days of curing. These noticeably higher compressive strengths at replacement levels of 10 and 20 mass% are due to the pozzolanic reaction between SC and calcium hydroxide produced in the hydration of the PC phases. Such a reaction generates additional calcium silicate hydrates. These secondary hydration products are the principal components responsible for the increase in the mechanical strength of the blended cement pastes [1,8].
Specimens of all four cement mixtures exposed to magnesium sulfate attack under the condition of alternative wetting-drying cycles showed considerable compressive strength losses compared to those stored in tap water for continued curing, as seen in Fig. 6. An important point, however, is the beginning time for compressive strength reduction and the extent of deterioration. As can be seen in Fig. 6, the compressive strength of the plain PC increases up to about 104MPa after 75 days from the beginning of the exposure time and starts decreasing gradually, thereafter. All the mixtures containing SC showed a small increase in compressive strength during the first 15 days of the exposure time and after that exhibited compressive strength losses at significantly much higher rates compared to plain PC. All the mixtures containing SC, therefore, showed a significantly higher extent of deterioration compared to plain PC.
In fact, two different chemical phenomena in contrary to each other are at work for the observed changes in compressive strengths. From one side, the time progress of hydration reactions of PC phases (in plain PC specimens) along with pozzolanic reaction (in the case of SC-containing specimens) result in more matured microstructure and hence increased compressive strengths. From the other side, magnesium sulfate attack causes undesirable reactions leading to degradation of the microstructure and reduced compressive strengths. The final governing phenomenon upon continued exposure, however, is sulfate attack deteriorating all the mixtures. When PC-based materials are exposed to a source of sulfate ions, reactions between alumina containing compounds and calcium hydroxide (Portlandite) as the PC hydration product and diffusing sulfate ions produce gypsum and ettringite [37–39]. Considering Table 1, the SC contains around 37% alumina and this means that the cements incorporating SC are susceptible to the formation of gypsum and ettringite in exposure to sulfate solution. This will be confirmed in later subsections by XRD and SEM/EDX techniques. The interesting point is the significant difference in the starting time and the extent of deterioration in mixtures containing SC compared to plain PC. Replacement of PC by SC causes a considerably sooner and deeper deterioration due to sulfate attack. This is in contrary to the general expectations and the experimental results reported by the researchers for the other pozzolanic materials [1,7–12].
One of the most important parameters determining the starting time and the extent of deterioration due to sulfate attack is the permeability of the hardened cement paste, in addition to its chemical and mineralogical phase composition. If the permeability of the hardened cement paste is relatively low, the deteriorating effects of the sulfate attack, which are limited to the regions close to the exposed surfaces appear at a relatively slower rate. The reverse is also well accepted. In relatively high permeable hardened cement pastes, the increased diffusion rate of sulfate ions into internal regions results in a faster and deeper deterioration process [40–43]. The relatively high porous microstructure of the RFCC SC has been confirmed by other researchers [44,45]. Another point to be taken into account is that according to the bulk densities values in Table 1 and the particle size distributions presented in Fig. 1, it is realized that the difference between particle sizes of the SC and PC and also the difference between their bulk densities lead to a porous structure in the cement matrix. In a previous work [46], the same authors also performed capillary and gel porosity measurements on 28-day cured paste specimens of plain PC and the binary mixture containing 30 mass% SC using mercury intrusion porosimetry technique. They reported a higher total porosity of about 20.75% by volume for the binary mixture compared to the value of 17.80% by volume for plain PC. Partial replacement of PC by SC, therefore, increases the permeability of the hardened cement paste. This increased permeability along with capillary suction forces exerted by alternative wetting-drying cycles can significantly reduce the sulfate resistance of the cement paste. This means that RFCC SC reduces the sulfate resistance of PC in spite of its considerably effective pozzolanic property.
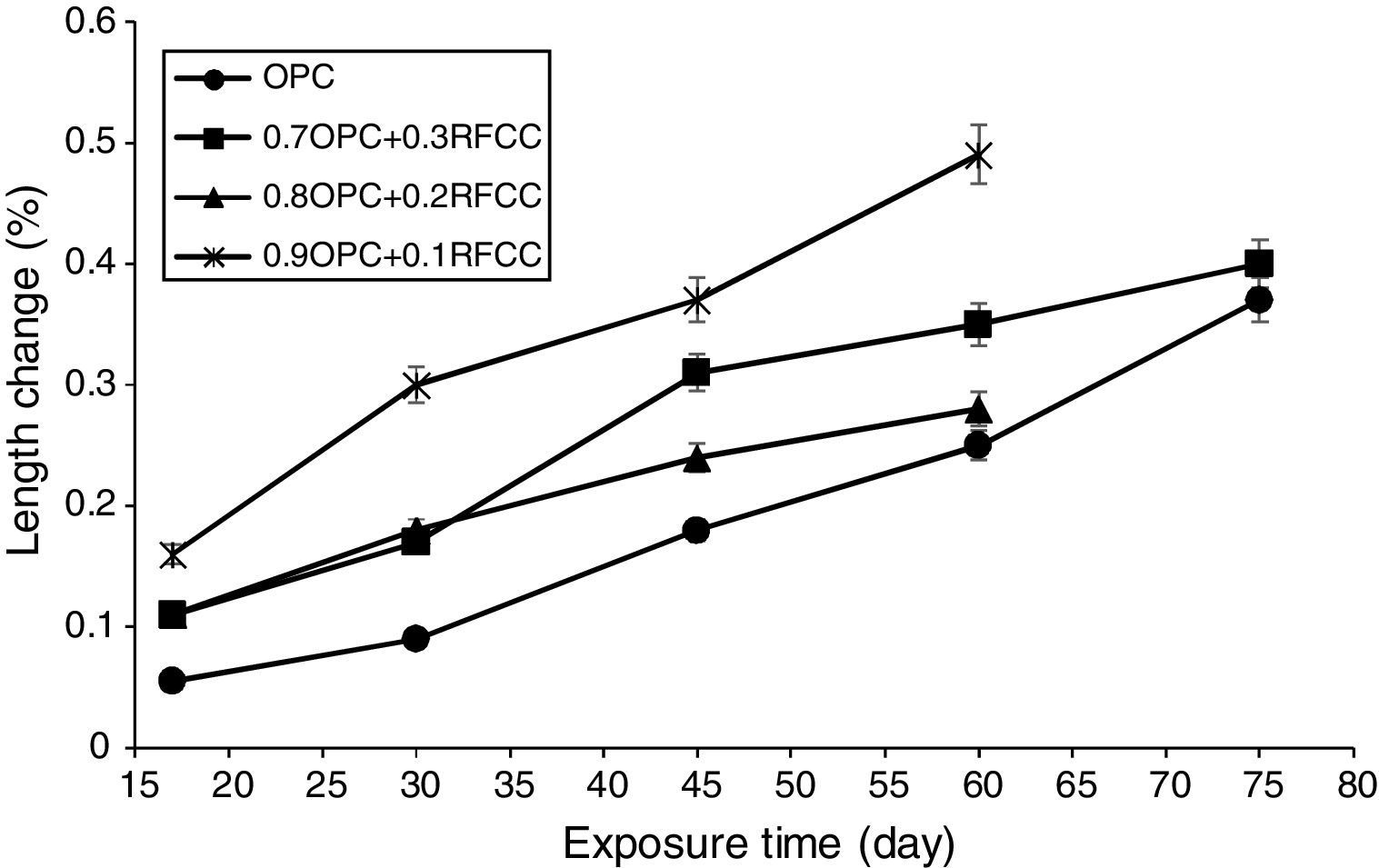
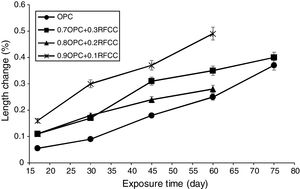
Length changesThe results of measurements of length changes are presented in Fig. 7. As can be seen, all specimens exposed to the sulfate solution experienced significant length changes.
The length changes of plain PC bar specimens and mixtures containing 30 mass% SC were measured up to 75 days of exposure, whereas the measurements of length changes for mixtures containing 10 and 20 mass% SC continued up to 60 days. The reason for this was the complete deterioration of the bar specimens due to strong sulfate attack under the condition of alternative wetting-drying cycles. In fact, mixtures containing 10 and 20 mass% SC were severely degraded and shattered into smaller pieces after 60 days of exposure. A similar trend occurred for reference bars and also mixtures containing 30 mass% SC after 75 days of exposure. A considerable increase in the length of the bar specimens is due to the formation of voluminous products such as gypsum and ettringite. From one side, the formation of gypsum and ettringite is influenced by the concentration of reactants including calcium hydroxide, calcium aluminate content of the cement mixture, and the sulfate ion present in the solution. On the other hand, it is affected by the permeability of the hardened paste under the effect of capillary suction forces exerted by alternative wetting-drying cycles [47,48].
If a pozzolanic additive, such as the SC, has a porous character, then the simultaneous effect of both pozzolanic reaction and the permeability, can be complicated. At a relatively low permeability, the formation of gypsum and ettringite is expected to be limited to surface regions, and the expansion is proportional to the amount of ettringite. At a relatively high permeability, however, due to the formation of gypsum and ettringite not only in the surface regions, but also in relatively deep regions, a more expansion is expected. The severity of the sulfate attack must also be taken into consideration. If the sulfate attack is not severe, then the progression of the pozzolanic reaction can be effective in reducing the permeability of the hardened cement paste and hence resulting in much less severe degradation. As can be seen in Fig. 7 and compared to binary mixtures, the plain PC bar specimens show the lowest expansion, which more likely is due to limited formation of gypsum and ettringite in the surface regions resulted from their relatively lower permeability.
As it is seen, the mixtures containing 20 and 30 mass% SC exhibit less expansion than the mixture containing 10 mass% SC. This can be attributed to the replacement percentage and its effect on calcium hydroxide content of the cement paste. In fact, at relatively high replacement levels, the strong pozzolanic property of SC may cause a relatively fast consumption of large amounts of calcium hydroxide in the cement paste resulting in a less permeable matrix, which less vulnerable to sulfate attack, before calcium hydroxide can participate in the destructive formation reaction of gypsum and ettringite. However, differences observed between the binary cement mixtures are difficult to explain because of the limited reliability and accuracy of the reported data from one side and lack of additional complementary evidences from the other side. In such a study, the reliability and accuracy of the reported data are limited because the non-uniform dimensional changes of the exposed paste specimens significantly affect the accuracy of the compressive strength and length changes.
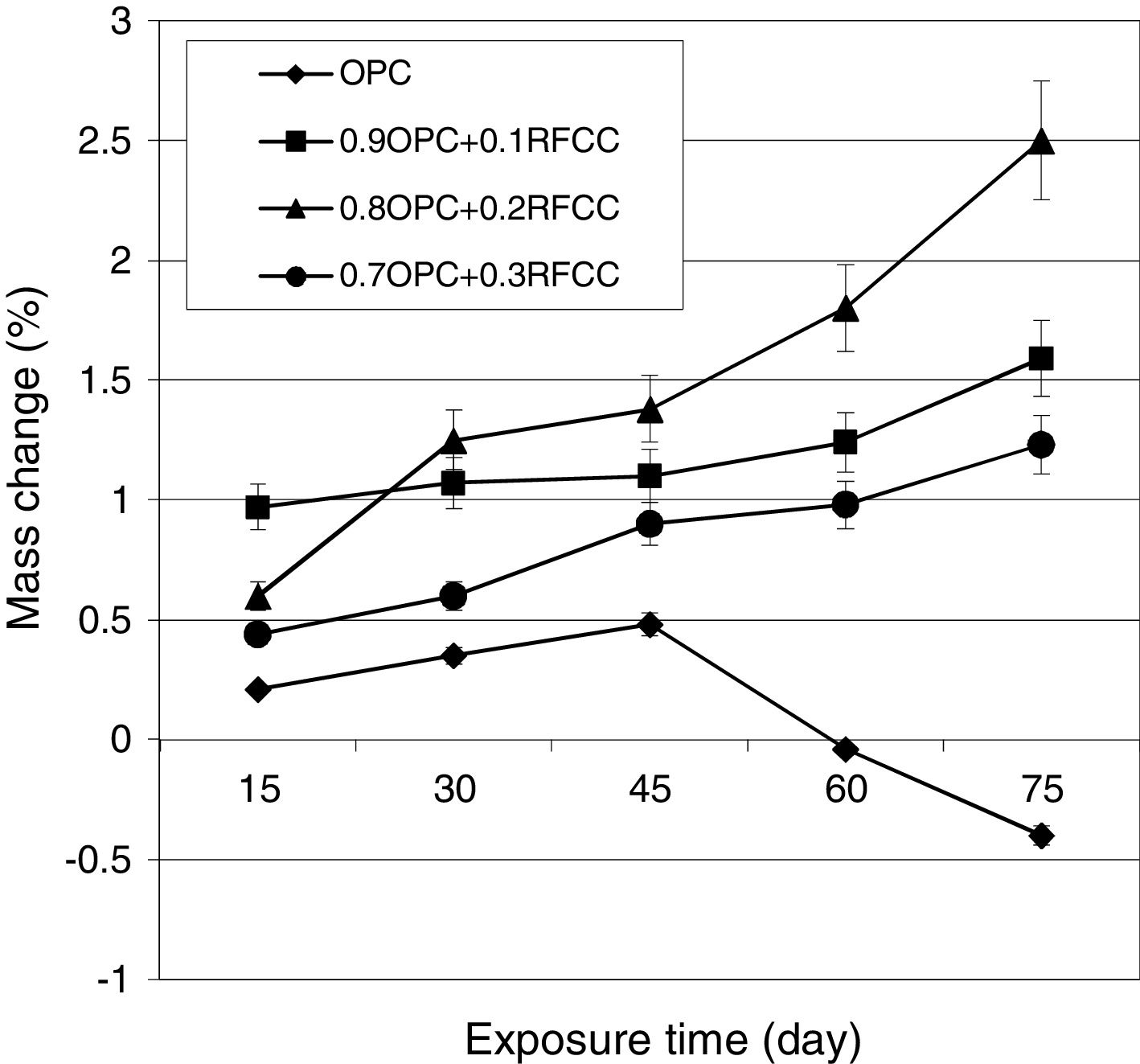
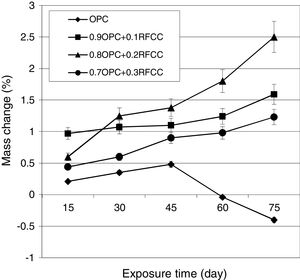
Mass changesThe results of mass change measurements are presented in Fig. 8. As seen, the plain PC specimens showed continuous mass gain during the first 45 days of exposure time and after that continued exposure resulted in significant mass losses. The specimens of the binary mixtures containing 10, 20, and 30 mass% SC, however, exhibited continuous gradual and relatively small mass gains over the whole length of 75 days of measurements with average ultimate mass gains of 1.59, 2.50, and 1.23%, respectively.
The mass losses observed for plain PC specimens were due to spalling of small pieces from the surfaces of the specimens. In fact, for plain PC specimens, the sulfate attack is mainly limited to the exposed surface regions. Intensive gypsum deposition in these areas gradually leads to intensifying disintegrating stresses, which finally result in the spalling of pieces from surface regions [49]. In natural cases of sulfate attack, this phenomenon usually does not occur during relatively short time periods about 45 days and here the main reasons for such a severe and fast attack are the type of sulfate and its relatively high concentration.
The continued mass gain of binary cement mixtures for longer exposure times compared to plain PC is due to the presence of SC, which not only reduces the concentration of calcium hydroxide inside the hardened cement paste, but also results in probably less concentrated expansion in the surface regions. It is reasonable to assume that the specimens of binary mixtures also undergo mass losses or shattering upon continued longer exposure times.
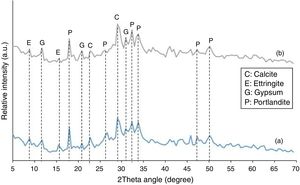
X-ray diffraction analysisThe XRD patterns of plain PC and the mixture containing 20 mass% SC after 120 days of exposure to sulfate solution are shown in Fig. 9. X-ray diffractometry analyses were performed on samples prepared from exposed surfaces. The two XRD patterns are very similar showing the presence of Portlandite, gypsum, ettringite, and calcite in both samples. No sign of anhydrous cement phases or hydration products were observed probably due to relatively very high concentrations of major crystalline phases. Reduced Portlandite content in binary cement mixture containing 20 mass% SC is due to its partial consumption in pozzolanic reactions in addition to its participation in the reactions with sulfate ions and also with atmospheric carbon dioxide. Calcite is a secondary reaction product due to the application of wetting-drying cycles. When the specimens were exposed to open air atmosphere during drying stage, part of Portlandite present in surface layers of the specimens reacted with carbon dioxide resulting in the formation of calcite. The formation of gypsum and ettringite due to the reaction of Portlandite with sulfate ion and carbonation of Portlandite due to its reaction with atmospheric carbon dioxide are common observations as reported earlier by many researchers [1,3–18,41–43]. An important difference, however, lies in the kinetics of the deterioration phenomenon. The kinetics depends on four main factors including: (1) differences in chemical and mineralogical compositions, (2) permeability of the cement paste, (3) the type of attacking sulfate and its concentration, and (4) exposure conditions including the exertion of capillary suction forces by wetting-drying cycles. Therefore, the kinetics still requires extensive research activities to well understand.
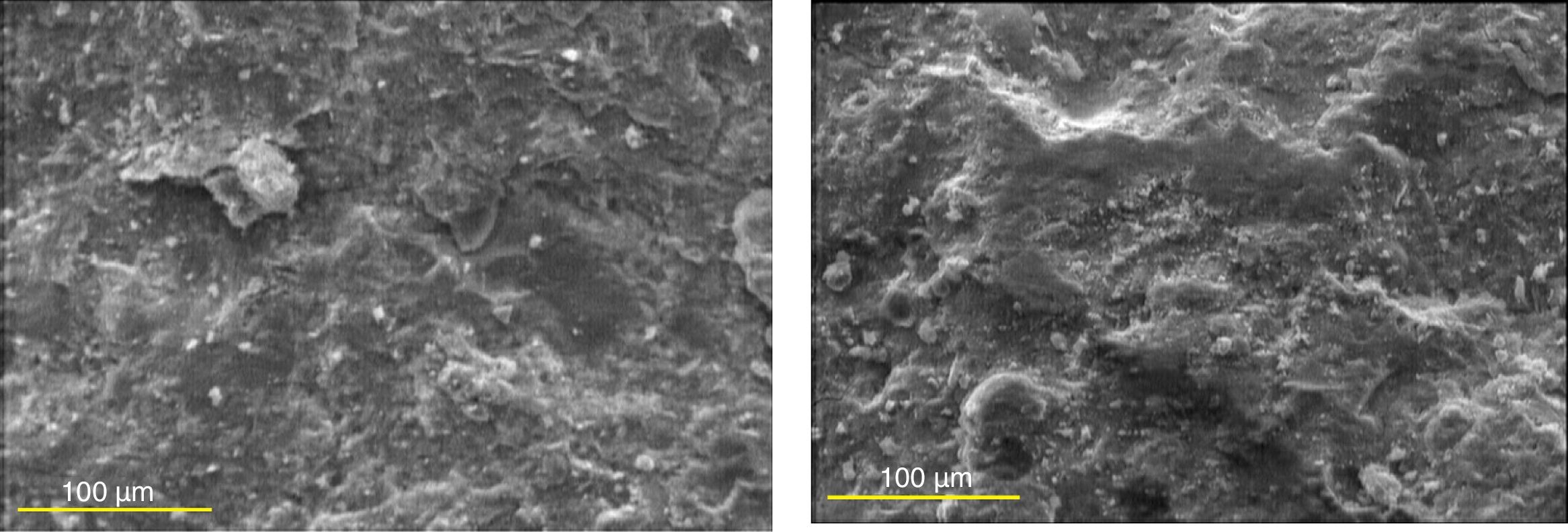
Microstructural studies by SEMFig. 10 depicts SEM micrographs of hardened pastes of plain PC and the binary mixture containing 20 mass% SC after 120 days of curing in tap water at a magnification of 300×. As can be seen, the microstructures are similar and no significant difference between them can be observed, although it is expected the microstructure of the cement paste specimen containing the SC has more uniformity and compactness due to the pozzolanic reactions and the partial consumption of Portlandite.
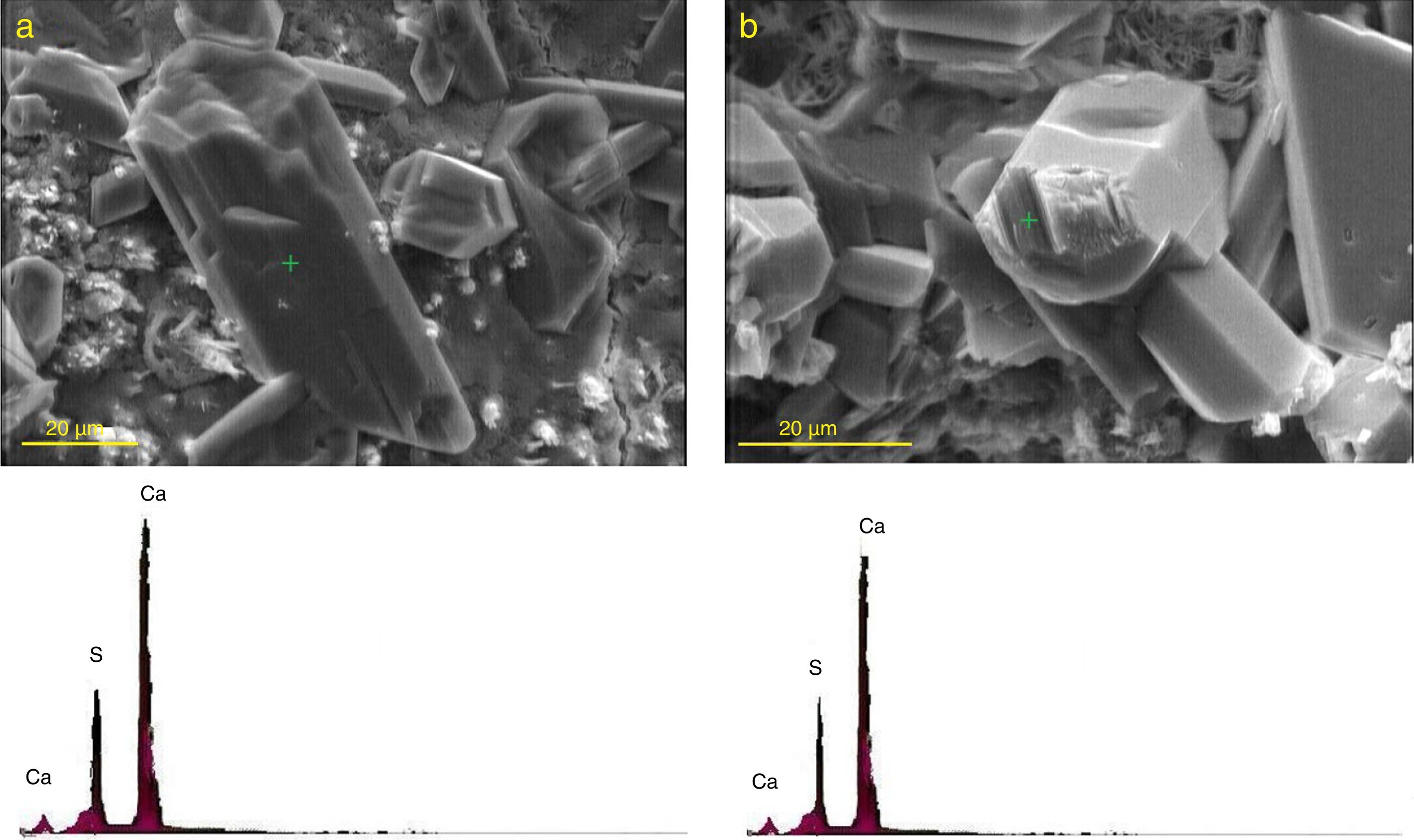
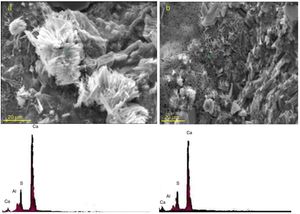
In order to track the formation and deposition of gypsum and ettringite crystals in the microstructure of exposed specimens, it was necessary to study the microstructure of the paste samples at relatively high magnifications along with the application of EDX analysis. Gypsum crystals formed in the paste specimens of plain PC and the mixture containing 20 mass% SC after 120 days of exposure to sulfate solution are shown in Fig. 11. As can be seen, elemental composition of these crystals consists of Ca and S elements, confirming the certainty of the presence of relatively large gypsum crystals in the microstructure of the paste specimens.
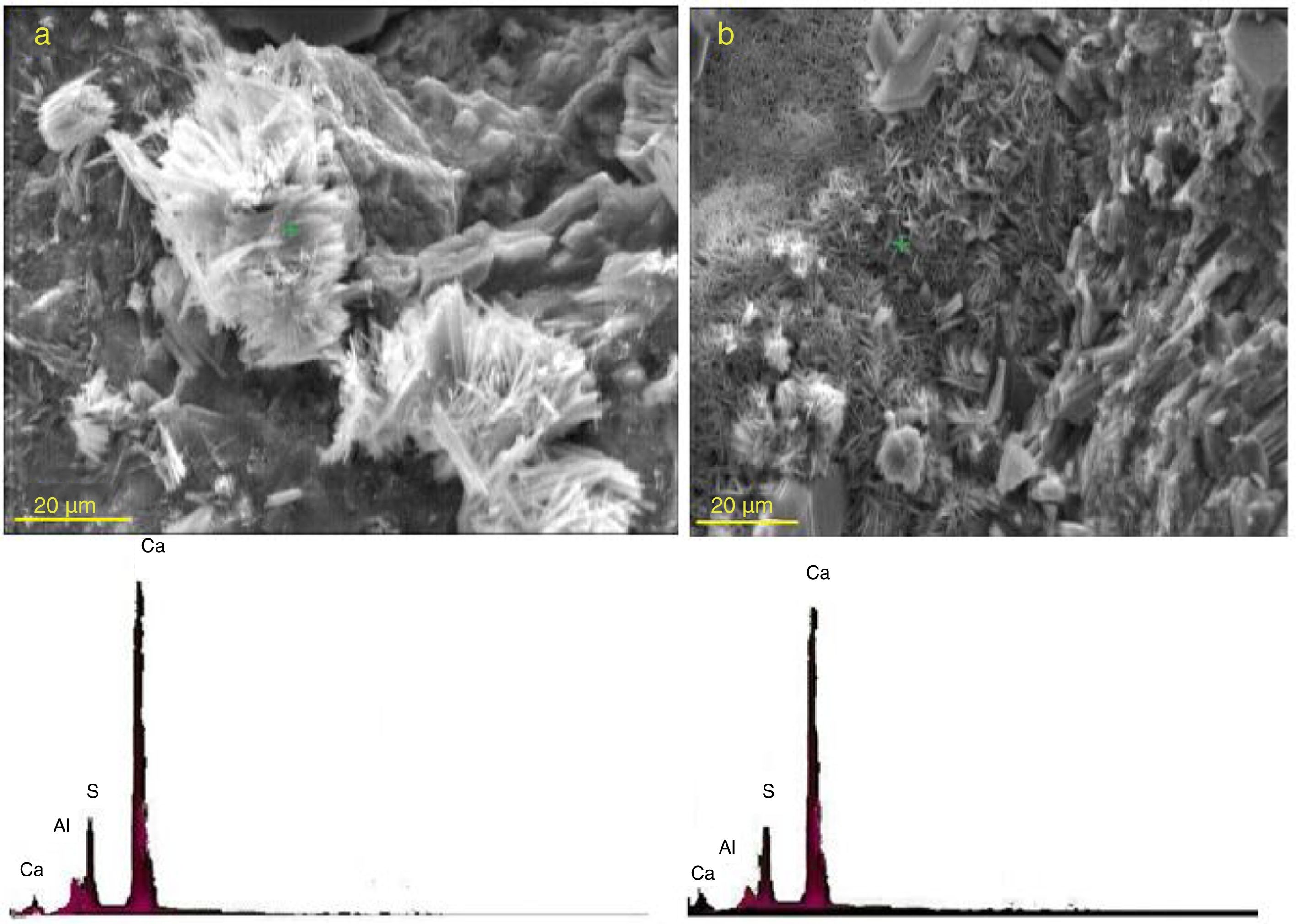
Fig. 12 shows SEM micrographs and the corresponding EDX elemental point analyses performed on needle-like crystals formed and deposited in the paste specimens of plain PC and the mixture containing 20 mass% SC after 120 days of exposure to sulfate solution. The needle-like morphology and the chemistry of Al, S, and Ca confirm the presence of ettringite crystals.
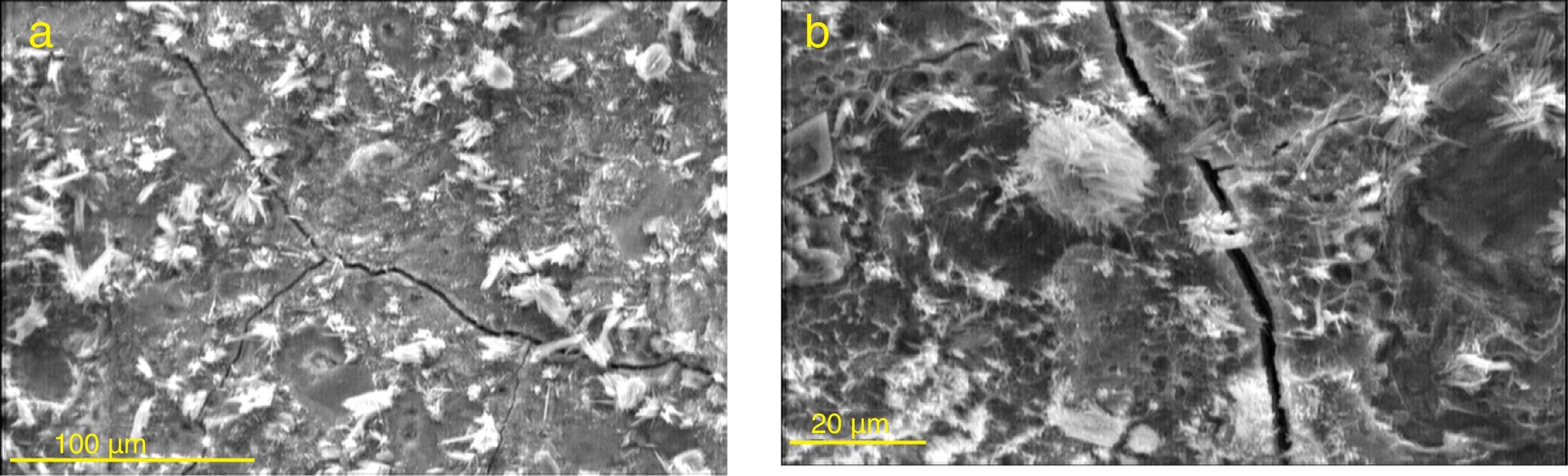
The mechanism of destruction of hardened cement paste and the resulting compressive strength loss due to the invasion of sulfate ions begin with the formation, deposition and growth of gypsum and ettringite crystals inside cement paste microstructure. The internal expansion resulted from the growth of these compounds in the hardened cement paste causes disintegrating stresses. With the continuation of the invasion process, disintegrating stresses become enough strong to overcome the microstructure tensile strength and as a result (as seen in Fig. 13) microscopic cracks are formed. The continuation of this process leads to enlarged microstructural cracks and finally dimensional expansion, mass changes (gain and loss), and loss of compressive strength.
ConclusionsExperimental results showed that paste specimens of binary mixtures incorporating different levels of 10, 20, and 30 mass% of ground RFCC spent catalyst exhibiting considerably higher compressive strengths were deteriorated faster and deeper than plain Portland cement when exposed to accelerated 10% magnesium sulfate attack. This was due to the effect of capillary suction forces exerted by alternative wetting-drying cycles. Such an odd behavior, when compared to the other pozzolanic materials, can be attributed to the effect of highly porous microstructure of RFCC spent catalyst in increasing the permeability of the hardened Portland cement paste. The results of this study clearly prove the important role of the porous microstructure of RFCC spent catalyst on permeability of the blended Portland cement paste as an important durability determining. Despite an adverse effect of the addition of RFCC spent catalyst on the sulfate resistance of PC, the results of the present study are important in three respects including: (1) proposing a promising method for the reuse of RFCC spent catalyst as a heavy metal-polluted industrial waste in the preparation of blended cements for application in sulfate-free or very low sulfate content environments, (2) the RFCC spent catalyst significantly improves the compressive strengths due to its relatively strong pozzolanic property, and (3) clarifying the fact that a higher compressive strength does not necessarily mean a better durability performance.
Conflict of interestsThe authors have no competing interests.