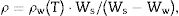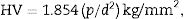Lithium disilicate glass-ceramic, modified by GeO2/SiO2 replacement to give 33.60Li2O-5.00GeO2-61.40SiO2 (mol%) composition, was investigated by the conventional melt technique. The effect of adding 0.1mol of some rare earth oxides like Y2O3, La2O3 or CeO2 on the crystallization behavior, chemical durability, Vicker's microhardness, and density of the obtained glass-ceramics were studied. The effect of In2O3 was also investigated. The glass transition temperature (Tg) was reduced slightly by the replacement of GeO2/SiO2, while it markedly increased when GeO2-containing glass was doped with different rare earth or In2O3 oxides. Different forms of disilicate phases like Li2Si2O5, Y2Si2O7 and La2Si2O7 in addition to two forms of Li2(Ge,Si)2O5 and Li2(Ge,Si)O3 solid solution were developed. LiInSi2O6 of pyroxene structure, Li6Ge8O19 and CeO2 phases were also detected. The density values of the glass-ceramics vary from 2.38 to 3.14g/cm3 and the hardness was in the range of 4150–5585MPa. The chemical durability of the modified glass-ceramics was greatly improved. The modification of glass-ceramics with GeO2 or other dopant oxides led to producing of materials with more dense and ultra-fine microstructures. Such materials are promising for superior applications as catalysis, electrolyte in fuel cells, biomedical, as well as dental restorative applications.
La vitrocerámica de disilicato de litio, modificada por reemplazo de GeO2/SiO2 para dar 33.60Li2O-5.00GeO2-61.40SiO2 (% molar), se investigó mediante la técnica de fusión convencional. Se estudiaron los efectos de la adición de 0,1 moles de algunos óxidos de tierras raras como Y2O3, La2O3 o CeO2 sobre el comportamiento de cristalización, la durabilidad química, la microdureza de Vicker y la densidad de las vitrocerámicas obtenidas. El efecto de In2O3 también fue investigado. La temperatura de transición vítrea (Tg) se redujo ligeramente mediante el reemplazo de GeO2/SiO2, mientras que aumentó marcadamente cuando el vidrio que contenía GeO2 se dopaba con diferentes tierras raras o óxidos de In2O3. Se desarrollaron diferentes formas de fases de disilicato como Li2Si2O5, Y2Si2O7 y La2Si2O7, además de dos formas de solución sólida de Li2 (Ge, Si) 2O5 y Li2 (Ge, Si) O3. También se detectaron LiInSi2O6 de estructura de piroxeno, fases Li6Ge8O19 y CeO2. Los valores de densidad de las vitrocerámicas varían de 2.38 a 3.14g/cm3 y la dureza estaba en el rango de 4150 a 5585MPa. La durabilidad química de las vitrocerámicas modificadas se mejoró enormemente. La modificación de las vitrocerámicas con GeO2 u otros óxidos dopantes condujo a la producción de materiales con microestructuras más densas y ultrafinas. Tales materiales son prometedores para aplicaciones superiores como catálisis, electrolitos en celdas de combustible, biomédicas, así como aplicaciones de restauración dental.
Glass ceramics are special series of materials in which the appropriate compositions of parent glass can be crystallized under carefully controlled conditions which lead to interesting properties of the resulting material. These materials offer a wide range of physical and mechanical properties combining the distinctive characteristics of sintered ceramics and glasses [1]. Furthermore, developing glass-ceramics demonstrate the advantage of possessing various remarkable properties in one material [2].
The lithium silicate system has attracted a great attention since development of the first glass-ceramic based on the stoichiometric composition of lithium disilicate by Stookey [3]. After this, several works were reported in the literature regarding the nucleation and crystallization mechanism in the binary Li2O-SiO2 system [4–9]. In multicomponent lithium disilicate glasses, the formation of crystalline phases is more difficult than that in the binary Li2O-SiO2 system and is mostly affected by the character and amount of oxides added to glasses as well as the nucleating agents [10]. Most of previous studies in the Li2O-SiO2 system recommended that the addition of several oxides in non-stoichiometric LD produces glass-ceramics exhibiting superior mechanical, chemical, and thermal properties. These glass-ceramics have been accepted as functional applications, such as ceramic composites, ceramic-metal sealing and all-ceramic dental restorations [11].
It is well known that the mechanical properties of materials can be advanced by rare elements doping due to their effects on the resultant microstructure specially their purifying effect on the interface between grains and the effective solution in addition to the advantages of strengthening and toughening [12]. CeO2, a typical rare earth oxide with a cubic fluorite structure, has been widely used as stabilizer and reinforcing agent in structural ceramics [13]. Especially, it is more significant that CeO2 has profound influences on the electric properties of piezoelectric ceramics [14]. In particular, rare-earth oxides such as CeO2 and La2O3 have been proved to be favorable for the sintering property of glass-ceramics [15]. The Y2O3 and La2O3 containing silicate glasses were reported to display improvement in the alkaline durability [16] with high glass transformation temperatures (Tg), high refractive indices, very low electrical conductivity and moderate thermal expansion coefficients [17].
Silicate materials containing In2O3 are well-known materials due to their superior chemical catalytic abilities caused by the interaction between In and Si [18], as well as the high solubility of In2O3 in silicate melts which leads to improving the material durability and transparency. These materials have an important function and become widely used in many advanced applications as photoelectric thin films, a coating material for transparent electric conductors, an infrared reflective material and chemical gas sensors [19]. The current work aims to investigate the crystallization characteristics and properties of lithium disilicate glass-ceramics containing-GeO2. The influence of introducing one of different rare earth dopants Y2O3, La2O3 or CeO2 as well In2O3 on the phase formation and resultant microstructure, in addition to the chemical and mechanical properties of the prepared crystalline materials are also considered.
ExperimentalGlass batch preparation and meltingThe parent glass samples were prepared by the conventional melt technique. Preparation of 40g powder of glass batches was performed by mixing high purity (purity >99%) chemical grade of purified quartz SiO2, Li2CO3 and GeO2, with Y2O3, La2O3, In2O3 or CeO2 powders which were chosen as the raw materials according to the designed molar compositions of the studied glass as shown in Table 1. The homogeneously weighted batches were allowed to melt in Pt crucible in a Vecstar electric furnace at 1300–1350°C for 2h in air with occasional stirring to get rid of air bubbles and increase the homogeneity of the glass melt. The homogeneous bubble-free melts were cast into a pre-heated stainless steel mold to form rods, squares, and buttons with 5mm thickness and they were well annealed at about 500°C and cooled down in a muffle furnace to minimize the residual thermal stress of the glasses.
The compositions of the studied glasses.
| Sample | Oxide constitutions (mol%) | Oxide added (mol) | DTA data | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Li2O | SiO2 | GeO2 | Y2O3 | La2O3 | In2O3 | CeO2 | Tg °C | Tc1°C | Tc2°C | |
| LS | 33.60 | 66.40 | – | – | – | – | – | 478 | 615 | – |
| LG | 33.60 | 61.40 | 5.00 | – | – | – | – | 473 | 600 | – |
| LGy | 33.60 | 61.40 | 5.00 | 0.1 | – | – | – | 529 | 670 | 783 |
| LGl | 33.60 | 61.40 | 5.00 | – | 0.1 | – | – | 508 | 645 | 775 |
| LGn | 33.60 | 61.40 | 5.00 | – | – | 0.1 | – | 501 | 620 | 753 |
| LGc | 33.60 | 61.40 | 5.00 | – | – | 0.1 | 485 | 613 | – | |
The DTA measurements were performed with a differential thermal analyzer (SDTQ 600 – TA Instruments, USA) to report the glass transition (Tg), crystallization temperatures (Tc), and the optimum conditions for heat treatment processing. About 10mg powder of each glass sample was placed in an alumina crucible and subjected to a heating rate of 10°C/min from ambient temperature to 1000°C in a flowing high purity nitrogen environment. Errors in measurement are ±5°C. Therefore, according to DTA results, the annealed glasses were then heat-treated, by two-step schedules, at different temperature for nucleation and crystallization to obtain glass-ceramic materials of holocrystalline mass with a minimum residual glassy phase without deformation.
X-ray powder diffraction (XRD).Crystalline phases in the prepared glass–ceramics were identified by an X-ray diffractometer (PW1080, PANalytics, Netherlands) using Ni filtered Cu-Kα radiation (λ=1.54056Å) produced at 35kV and 25mA, with scanning speed (2θ) of 2° per minute. The diffraction pattern was recorded within Bragg angle in a 2θ=5–70° range and the phases were identified by JCPDS numbers (ICDD-PDF2 database). The International Centre for Diffraction Data (ICDD) files were used to determine the crystallized phases by comparing the peak positions and intensities.
Scanning electron microscope (SEM)Microstructures of the cross-section for glass-ceramic samples were estimated using SEM observed to analyze the morphology of crystals in the final glass-ceramics. This was done by a scanning electron microscope (SEM/EDS, Quanta FEG 250, Netherlands) with accelerating voltage 30kV. Fractured surfaces of samples were immersed in the solution (1% HF+1% HNO3) for 40s and then coated with a thin layer of gold for high resolution observation using sputtered coater.
Measurement of density (ρ)The density values of the glass-ceramic samples were evaluated by the standard principle of Archimedes method using distilled water as immersion liquid with an accuracy of ±0.001g/cm3. Five different rod pieces, for each sample free from bubbles, were weighted. The relative weights of sample rods in air and in distilled water were measured using an electrical digital balance. The densities were calculated using the equation below:
where ρ is the sample density (g/cm3), ρw (T) is the density of water at the measured temperature (g/cm3), Ws is the sample weight in air (g) and Ww is the sample weight in water (g).Vicker microhardnessVicker microhardness values were measured using a micro-hardness tester (Shimadzu, Type-HMV, Japan; with a pyramid shaped diamond indenter). A load of 100g was applied on well-polished glass–ceramic samples for 15s under normal atmospheric conditions. At least 10 indentations were made on each sample using the average indentation diagonal length to calculate hardness value in kg/mm2. The resulting indentation diagonals were measured and the hardness was calculated using the following equation:
where HV is the Vicker microhardness, p is the applied load (g), and d is the average diagonal length (mm). The micro-hardness values were converted from kg/mm2 to MPa by multiplying with a constant value 9.8.The chemical durability testThe powdered test was applied to assess the chemical durability [20] of the obtained glass-ceramics. The samples were crushed in an agate mortar and then sieved using B.S. sieves to obtain particles with diameters ranging between 0.63 and 0.32mm. The grains were washed with ethyl alcohol and then with pure dry ether three times then finally dried. The dried samples were accurately weighed (1.0g) in sintered glass crucibles (G4) and then placed in 400ml polyethylene beakers. The samples were tested in 0.1M HCl solution; 200ml of the acid solution was introduced into the polyethylene beakers. This quantity was sufficient to cover the sintered glass crucibles. The polyethylene beakers with their contents were covered by polyethylene cover to reduce evaporation. The beakers were placed in water bath regulated at 95°C. After 1h, the beakers were removed from the bath and the sintered glass crucibles were fitted on a suction pump and the whole solution was pumped through it. The sintered crucibles were dried in an oven at 110°C for 1h and then transferred to a desiccator to cool to room temperature. The chemical durability was expressed as the loss in weight of the glass-ceramic samples after immersion. Therefore, the sintered glass crucibles were reweighted and the total weight loss was calculated.
Results and discussionCrystallization characteristicsThe rare earth ions have an influence not only on the phases developed, but also on the density, glass transition temperature, softening point, as well as the mechanical and chemical stability of the glass ceramics [21]. They form the heterogeneous crystalline nuclei to initiate the phase separation in Si–O–Si glass matrix. The variations of cationic field strength (Z/r2) of dopant ions from Si4+ play key role in crystallite formation, and hence, phase separation in the modified glass [22]. The DTA curves for the studied glasses that occurred at a heating rate of 10°C/min are shown in Fig. 1. With reference to the DTA data, the glass transition temperature (Tg) of the parent glass LS is 478°C. This was noticed to decrease when the parent glass is modified by GeO2 (LG) to 473°C, while it obviously increased when the modified glass (LG) is doped with Y2O3, La2O3, In2O3 or CeO2 (Fig. 1 and Table 1). The addition of 5mol% GeO2 at the expense of SiO2 (LG glass) in the Li2Si2O5 (LS glass) led to shifting both the endothermic and exothermic peak temperatures toward lower temperatures (Fig. 1). Thus glasses containing GeO2 are melted at temperatures lower than those free of it. So, the introduction of GeO2 in the glass structure at the expense of SiO2 led to an increase of the number of non-bridging oxygens (NBO), and this was due to the fact that Ge–O bond is weaker than the Si–O bond [23].
Based on the differential thermal analysis curves (Fig. 1), the results show that, modifying GeO2-containing lithium disilicate (LG) with 0.1mol of one of different dopants like Y2O3, La2O3, In2O3 or CeO2 led to noticeable increase in both glass transition (Tg) and glass crystallization (Tc) temperatures. Therefore, according to Ray [24], the higher values of glass transition (Tg) would be expected to be achieved with the addition of different dopants in LG glass due to increasing the number and density of covalent cross-linking with the strength of cross-links between the cations and oxygen atoms in the structure of the glass samples. Thus the introduced cations can be allocated in the holes of the silicate glass structure to act as network forming ions in fourth fold coordination [25].
Fig. 2 shows the patterns of XRD of the glass-ceramic samples and Table 2 summarizes the crystalline phases developed at different heat-treatment temperatures. The X-ray diffraction analysis (Fig. 2, Pattern I) shows that, lithium disilicate-Li2Si2O5 (PDF, card No.17-0447) is the only developed phase in the parent crystalline LS glass that is heat treated at 480°C/5h–615°C/10h. Crystallization of Li2SiO3 and Li2Si2O5 phases and their ratios might depend on the thermal history including heat-treatment temperatures with duration of the process, the SiO2/Li2O ratio in the parent glass composition, and the type of nucleating agents [1]. Lithium metasilicate is exclusively formed by the low temperature treatment of glasses. With extra heating and long extent of Li2O groups, the complex layered of lithium disilicate grows in the SiO2-rich of glassy matrix. The substitution of GeO2 at the expense of SiO2 (5mol%) in the Li2Si2O5 molecule, i.e. LG sample, heat treated at 475°C/5h–600°C/10h, does not produce any new phase than that crystallized in LS glass-ceramic, i.e., no GeO2-containing phases could be detected (Fig. 2, Patterns II). This leads to an important conclusion that germanium cations (Ge4+) present in the melt can find place in the Si4+ site [26] in the lithium di-silicate structure and form Li2(Ge Si)2O5 solid solution phase. The obtained result is in agreement with our previously reported work [27,28]. Therefore, according to the petrochemical calculation [29], lithium germanium silicate phase of the probable formula-Li2 (Ge0.08 Si0.92)2O5 may be formed by the accommodation of Ge4+ in the lithium disilicate structure which has a wide range of solid solution formations [30].
Crystalline phases developed and properties of the prepared glass-ceramics.
| Sample | Heat-treatment (°C/h) | Crystalline phases developed | Density (g/cm3) | Hardness (MPa) | Weight loss % (g) |
|---|---|---|---|---|---|
| LS | 480/5–615/10 | Li2Si2O5 (LS2) | 2.38 | 4150 | 2.05 |
| LG | 475/5–600/10 | LS2 ss [Li2 (Ge Si)2O5] | 2.45 | 4455 | 1.85 |
| LGy | 530/5–785/10 | Li2 (Ge Si)O3, Y2Si2O7 | 2.88 | 4670 | 1.19 |
| LGl | 510/5–775/10 | La2Si2O7, Li2 (Ge Si)O3 | 3.14 | 5585 | 1.31 |
| LGn | 500/5–750/10 | Li2 (Ge Si)O3, LiInSi2O6 | 3.08 | 4560 | 0.98 |
| LGc | 485/5–615/10 | Li2Si2O5, Li6Ge8O19, CeO2 | 2.51 | 5050 | 1.06 |
A detailed study for the effect of doping GeO2-containing glasses, i.e. LG, with 0.1mol of Y2O3, La2O3, In2O3 or CeO2 on the crystal phase formations in the glass-ceramic materials was followed. The results indicate that dopant oxides have good solubility in the lithium silicate glass and promote the crystallization of heterogeneous nucleation. The XRD [Fig. 2, Pattern III] revealed that, the doping of GeO2-containing glass, i.e. LG, with 0.1mol of Y2O3 (LGy), treated at 530°C/5h–785°C/10h, led to appearance of d-spacing lines characteristic for Li2SiO3 (PDF, card No.29-0828) as a major phase in addition to yttrium disilicate-Y2Si2O7 (PDF, card No.38-0440) keivite phase where no lithium disilicate phase could be identified. This may be demonstrated by the affinity of yttrium oxide to react with lithium Ge-disilicate solid solution to form yttrium disilicate and lithium Ge-metasilicate solid solution phases as follows:
As a result, lithium metasilicate phase could be formed instead of the disilicate phase and accommodated germanium cation, which can replace Si isostructurally in Si4+ site in its structure to form lithium Ge-metasilicate solid solution of the probable formula-Li2(Ge0.15Si0.85)O3. Yttrium disilicate-Y2Si2O7 occurs in nature as yttrialite and is the resource of rare earth silicate compounds [31].
The heating for glass doping with 0.1mol La2O3, i.e. LGl, led to the crystallization of lanthanum disilicate-La2Si2O7 (PDF, card No.82-0729) as main phase together with secondary phase of lithium Ge-metasilicate solid solution as indicated by the XRD analysis (Fig. 2, Pattern IV) and according to the following equation:
The crystallization products of glass-ceramic, treated at 510°C/5h–775°C/10h, may be illustrated according to the DTA curve of glass LGl (Fig. 1, LGl curve). There are two exothermic peaks detected which related to the crystallization temperatures (TC) of the respective glass. The first dip was powered to the first crystalline phase of lithium Ge-metasilicate solid solution at 645°C. Along with increasing temperature, the dip of the second peak get stronger and the lines of XRD corresponding to La2Si2O7 phase was formed in the developed glass-ceramic LGl (Fig. 2, Pattern IV). In the case of In2O3-containing glass (i.e. LGn), crystallized at 500°C/5h–750°C/10h, the X-ray diffraction analysis (Fig. 2, Pattern V) revealed that the addition of In2O3 into GeO2-containing glass, led to the formation of lithium indium silicate – LiInSi2O6 phase, of pyroxene-type (PDF, card No.33-0799) together with the crystallization of lithium Ge-metsailicate-Li2(Ge Si)O3 solid solution phase. The formation of lithium indium silicate phase may be related to the reaction between lithium Ge-disilicate ss and indium oxide as follows:
3Li2(GeSi)2O5+In2O3→2Li2(GeSi)O3+2LiInSi2O6 (5)
Lithium indium silicate-LiInSi2O6 phase is one of many mineral phases related to the pyroxene family. Pyroxene glass-ceramics have materials of great interest because of the exceptional controllability of their properties. The crystals structure of these materials is able to accept a wide range of isomorphous replacement having superior physical and chemical features, and may produce the origin for fabrication of many glass-ceramic materials [32]. Thus, pyroxenes offer a fairly wide and flexible group of materials for physical investigations.
The XRD (Fig. 2, Pattern VI) revealed that, introducing 0.1mol of CeO2 (LGc) into GeO2-containing glass, thermally treated at 485°C/5h and then at 615°C/10h, led to the development of lithium disilicate-Li2Si2O5 as the main phase together with crystallization of lithium germinate-Li6Ge8O19 (PDF, card No.18-0730) and cerium oxide-CeO2 (PDF, card No.43-1002) phases. Previous works [33,34] reported that, cerium oxide could be introduced in silicate glasses as nucleating agents for accelerating the crystallization process of developed products. It seems therefore that, the CeO2 crystals were precipitated in the treated samples, at the first stage, and these precipitations probably remained from glass melting and served as nucleating centers in GeO2-containing glass and promoted the crystallization of lithium germinate-Li6Ge8O19 phase. In the second stage, phase separation occurs and the droplets of CeO2 are crystallized (Fig. 2, Pattern VI and Table 2). Cerium doped glass-ceramics have exposed importance both academically and technologically due to their superiority in combining the optical, mechanical and electrical properties [35]. As a result of the exceptional properties, doped ceria systems have wide range of applications [36] in many areas such as catalysis [37], biomedical [38], nanomedicine, and nanobiology [39], as well in solid oxide fuel cells (SOFCs) as a new type of electrolyte [40].
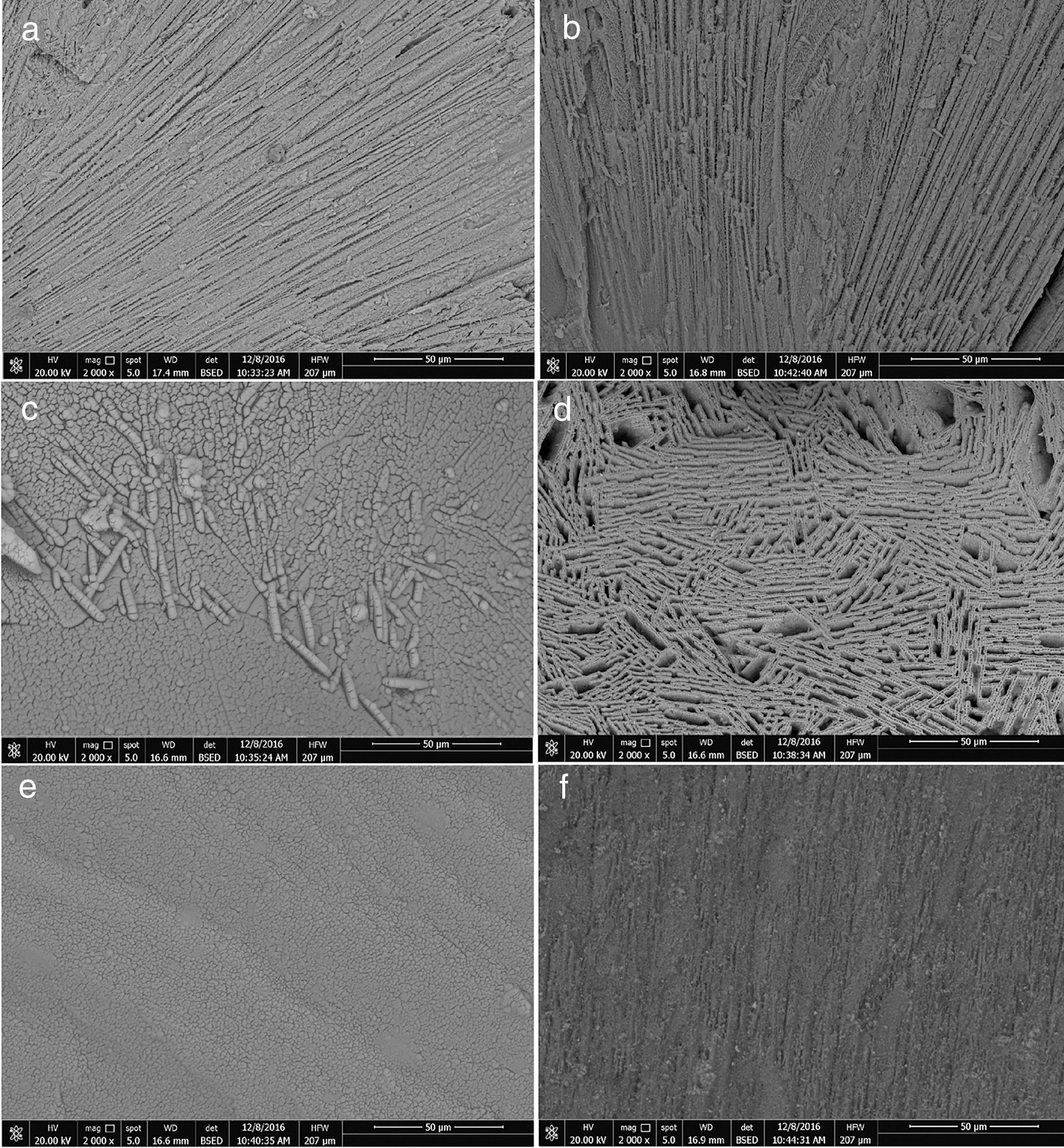
Scanning electron micrograph (SEM) of the fractured surface of the glass-ceramic samples was inspected in Fig. 3. It showed the morphology of LS parent glass with modifying glass LG and classified the effect of adding different oxides on the microstructure of the glass-ceramics. As shown in the SEM-micrographs, the crystallized parent glass LS showed volume crystallization of oriented rod-like elongated crystals (Fig. 3a). Replacement of SiO2 by 5mol% GeO2, i.e. LG, led to growth fine crystals in-between lithium disilicate elongated crystals (Fig. 3b). The microstructures of the crystalline samples were significantly different depending on the respective composition and crystalline phases accumulate as well the applied heat treatment process [1]. So, the addition of 0.1mol of different dopant oxides in GeO2-containing glass plays a key role in the modification of the developed microstructure. In the Y2O3-containing glass-ceramic, i.e. LGy, a botryoidal-like growth with tiny aggregated microstructures were formed in the crystalline sample (Fig. 3c). In the presence of La2O3, the morphology of crystalline sample LGl appears to grow as network-like growths (Fig. 3d). The addition of In2O3 in the GeO2-containing glass, i.e. LGn, led to formation of a volume of a very fine grained microstructure which differs notably from those of all other samples (Fig. 3e). On the other hand, the SEM micrograph of the crystalline specimen containing-CeO2 (LGc) showed that a volume of crystallization of ultra-fine fibrous-like growth forms was developed (Fig. 3f). Lu Pengxian et al. [41] reported that, the doping of CeO2 in the structure of perovskite ceramics led to a compact microstructure and a decrease in the crystal size. Therefore, CeO2 can suppress the grained growth and support the densification of the glass-ceramics.
Mechanical and chemical characterizationThe examined properties for the studied glass-ceramics are presented in Table 2. The density of glass-ceramic is a superior device able to explore the modification in its structure. It is affected by a competition between the viscosity of the melt and its degree of crystallization [42]. Fig. 4 shows the bulk density of the examined crystalline samples depending on the composition and type of crystalline phases formed. The density values of the investigated glass-ceramics are in the range of 2.38–3.14g/cm3. The result revealed that the GeO2/SiO2 replacement in lithium disilicate glass-ceramic increased the density value of the prepared crystalline sample from 2.38 to 2.45g/cm3. This may result from the effect of GeO2 on improving the crystallization process of the glass [19]. Thus the GeO2-containing glass samples were crystallized easier than those free of it. The results revealed that, the incorporating any of the different dopant oxides, Y2O3, La2O3, In2O3 or CeO2 into the GeO2-containing sample led to increasing the bulk density values of the investigated glass-ceramics (Fig. 4 and Table 2). This may be interpreted as; the incorporation of different dopants in the crystalline sample led to the formation of more dense microstructures (Fig. 3) and to the compactness glass-ceramic structures thus decreased the volume of the material and increased its density [43].
The mechanical properties of glass-ceramics depended on the composition of crystalline phases formed and the growing microstructure [44]. Vickers microhardness of the prepared crystalline materials were examined and represented in Fig. 5 as well as in Table 2. Hardness is one important property of silicate glass–ceramics, which is related to the degree and amount of crystalline phases formed, their grain microstructure in addition to the oxides remained in the residual glass phase [45]. The Vickers micro-hardness measured for the studied lithium di-silicate glass-ceramics was found to be in the range of 4150–5580MPa (4.15–5.58GPa) which was in the ranged with those reported by different authors [46–48]. The obtained results show that the parent crystalline sample i.e. LS, has the lowest microhardness value of around 4150MPa. Whereas, substitution of SiO2 by GeO2 in the parent glass, i.e. LG, led to increasing the hardness value to 4455MPa in similar crystalline phases obtained, the morphology of the fine crystals grows to form relatively finer crystalline grain [49]. Likewise, the integration of different rare earth oxides as Y2O3, La2O3 or CeO2 as well In2O3 in the glasses led to a significant increase in the microhardness values for the corresponding glass-ceramic samples. This may be explained by the fact that the mechanical properties of glass-ceramics can be improved by the rare elements doping due to their effects on the resultant morphological features [13]. Therefore, all doping oxides support the densification of finer microstructure (Fig. 3) in the investigated glass-ceramics.
The chemical durability of glass-ceramics generally depends on the content as well as the type of the crystalline phases formed, grain microstructure and residual glassy matrix [50]. The prepared glass–ceramics containing low alkali concentrations should possess much higher chemical durability especially at higher temperatures and higher humidity than glass–ceramics containing high alkali concentrations. The chemical stability test was carried out by measuring the weight loss percent on the prepared glass-ceramic specimens. The obtained data are given in Table 2 and graphically represented in Fig. 6. Treatment of the investigated samples by 0.1M HCl solution as an attacking agent, at a temperature of 95°C for 1 hour, revealed that the parent glass-ceramic, i.e. LS, showed the highest leaching values of the investigated glass-ceramics (Fig. 6 and Table 2). This may be attributed to the formation of less durable lithium disilicate-Li2Si2O5 as the only crystallized phase. Lithium meta- and disilicates are decomposed by the acidic solution [49]. The obtained data indicated that the leaching value was decreased by the addition of GeO2 at the expense of SiO2 in the parent crystalline sample, i.e. LG (Fig. 2, and Table 2). It is worth noting that the accommodation of GeO2 in lithium disilicate structure to give solid solution series improve the chemical stability of the resultant glass-ceramic [51]. The present results indicated that GeO2-containing glass doping with 0.1mol Y2O3, i.e. LGy, was more durable than that of the sample free of yttrium oxide (Fig. 2, and Table 2). This may be attributed to the crystallization of relatively more durable di-yttrium disilicate-Y2Si2O7 phase among the crystalline phases. With respect to the effect of adding La2O3 to the GeO2-containing glasses, it was found that the development of relatively durable lanthanum disilicate-La2Si2O7 phase led to better chemical stability of the glass-ceramic sample LGl, than that of crystalline LG sample (free of La2O3). The addition of In2O3 in the investigated LG glass greatly decreases the leachability of the corresponding crystalline sample (e.g. LGn), i.e. the chemical durability was highly improved. This may be due to the crystallization of the most durable LiInSi2O6 phase of pyroxene family [52] among the crystallization products of the material. According to Fig. 6 and Table 2, it was noted that, the leaching values of GeO2-containing glass doping with CeO2, sample LGc, were greatly lower than those of the LG sample (free of CeO2). This may be due to the formation of fine and dense microstructure glass-ceramics without pores which may provide it with better chemical stability as compared with that of porous materials [1].
ConclusionGlass-ceramic materials based on the 33.60Li2O–66.40SiO2 composition of lithium disilicate-Li2Si2O5 formula modified by partial GeO2/SiO2 replacement (5mol%) were investigated. Also, the glass–ceramics containing Y2O3, La2O3 or CeO2 rare earth oxides as well In2O3 were successfully prepared and characterized. Varieties of crystalline phases were detected in the developed glass-ceramics through the heat treatment process including lithium di- and meta-silicate solid solutions with GeO2 [Li2(Ge,Si)2O5 and Li2(Ge,Si)O3], lithium disilicate, di-yttrium disilicate, lanthanum disilicate, lithium indium silicate of pyroxene family, lithium germanate, and cerium dioxide phases. The density of glass–ceramics ranged from 2.38 to 3.14g/cm3 while the microhardness ranged between 4150 and 5585MPa. In addition, the weight loss percent toward acid attack varied from 0.98 to 2.05 values. The chemical and mechanical properties as well as density of the obtained glass-ceramics were affected by the modification processes. The existence of different rare earth oxides as well as In2O3 led to a remarkable improvement in the glass–ceramics durability and mostly enhanced the mechanical strength of the investigated materials. The obtained materials with such properties are promising for different applications as a new type of electrolyte in solid oxide fuel cells (SOFCs), catalytic converters, or biomedical, as well as dental restorative applications.