This study aimed to describe a Bacillus subtilis expression system based on genetically modified B. subtilis. Abaecin, an antimicrobial peptide obtained from Apis mellifera, can enhance the effect of pore-forming peptides from other species on the inhibition of bacterial growth. For the exogenous expression, the abaecin gene was fused with a tobacco etch virus protease cleavage site, a promoter Pglv, and a mature beta-glucanase signal peptide. Also, a B. subtilis expression system was constructed. The recombinant abaecin gene was expressed and purified as a recombinant protein in the culture supernatant. The purified abaecin did not inhibit the growth of Escherichia coli strain K88. Cecropin A and hymenoptaecin exhibited potent bactericidal activities at concentrations of 1 and 1.5μM. Combinatorial assays revealed that cecropin A and hymenoptaecin had sublethal concentrations of 0.3 and 0.5μM. This potentiating functional interaction represents a promising therapeutic strategy. It provides an opportunity to address the rising threat of multidrug-resistant pathogens that are recalcitrant to conventional antibiotics.
Antimicrobial peptides (AMPs) are important components of the innate immune defense against microbial pathogens in a wide range of organisms.1 Abaecin is a major AMP found in the honeybees. It was originally isolated from the insect Apis mellifera.2 The bumblebee long-chain proline-rich peptide abaecin can reduce the minimal inhibitory concentrations.3 However, the expression of abaecin is extremely limited in honeybees. Therefore, a highly efficient expression system is needed for abaecin to allow its commercial application.
Abaecin consists of a single polypeptide chain of common amino acids and is well suited for economical production through the application of recombinant DNA technology or peptide synthesis.4 It can potentiate the activity of pore-forming AMPs from other species, for example, cecropin A and stomoxyn.5 This potentiating functional interaction represents a promising therapeutic strategy because the potency and range of combinations of AMPs are likely to extend well beyond the capabilities of individual peptides.6 It provides an opportunity to address the rising threat of multidrug-resistant pathogens that are recalcitrant to conventional antibiotics.7
The expression of foreign genes in Escherichia coli often leads to the formation of densely packed denatured peptide molecules in the form of insoluble particles called inclusion bodies.8 Inclusion body proteins are devoid of biological activity. An elaborate process involving solubilization, refolding, and purification is needed to partially recover a functionally active product.9 A yeast expression system, such as Pichia pastoris, may solve the problem of post-translational modifications.10 It needs significant investment during the later period of batch cultivation, rendering it uneconomical.11
The Bacillus subtilis expression system does not have the disadvantages of the two aforementioned expression systems and can potentially serve as an efficient expression host,12 especially for the secretion of heterologous proteins.13 Moreover, the secreted foreign proteins usually remain in biologically active forms,14 and the downstream purification is greatly simplified.15 Also, it has other advantages, such as theoretically higher yield, no aggregation of the product, and the possibility for continuous cultivation and production.16
This study aimed to describe a B. subtilis expression system based on B. subtilis cells genetically modified with a small tobacco etch virus (TEV) protease and two-cistron expression vector gene of abaecin fused to TEV.17
Materials and methodsEnzymes, chemicals, and antibioticsT4 DNA ligase, Taq DNA polymerase, and all restriction enzymes were purchased from Promega (WI, USA). The protein marker was obtained from TaKaRa Biotechnology (Shiga, Japan). Antibiotics were purchased from Sigma (MO, USA). All the other chemicals used were of the highest grade commercially available.
Bacterial strains and plasmidsThe gene encoding the antibacterial peptide abaecin, with the previously reported sequence FVPYNPPRPGQSKPFPSFPGHGPFNPK IQWPYPLPNPGH,18 was synthesized using a TEV protease cleavage locus gene, a beta-glucanase mature peptide, and signal peptide gene (gmp and gsp) as a full-length nucleotide by standard solid-phase methods at the AUGCT Biotechnology Company (Beijing, China). The recombinant plasmid named ABA containing the abaecin gene, a TEV protease cleavage locus gene, and a beta-glucanase mature peptide (gmp) with the signal peptide (gsp) were supplied by the AUGCT Biotechnology Company (Beijing, China). The E. coli DH5α, shuttle vector pGJ103, and promoter Pglv were obtained from National Feed Engineering Technology Research Center (Beijing, China). Preparation of plasmid DNA from E. coli cells and transformation of B. subtilis were carried out using standard procedures.19
Plasmid construction and expressionAfter cloning using T vector, an intact plasmid including the promoter Pglv was inserted into plasmid pGJ103 (3.2kb), resulting in the construction of pGP (3.45kb). The plasmid containing the genes of abaecin and TEV protein, with gmp and gsp, was digested with EcoRI and BamHI, while pGP was digested with EcoRI and BamHI. The two digested products were linked by T4 DNA ligase, yielding recombinant plasmid pGPA (Fig. 1). The operon, including the inducible promoter spac, and the TEV protease gene were inserted between the site BamHI and SacI of plasmid pGPA. The plasmid pGPA was transformed into E. coli DH5α, and the positive transformant was screened at a final concentration of 5μg/mL chloromycin. The positive clones were further identified with the restriction endonuclease digestion of BamHI and SacI. The sequence was identified by the SinoGenoMax Company (Beijing, China).
Construction of recombinant plasmid pGPA. After cloning using T vector, an intact plasmid including the promoter Pglv was inserted into plasmid pGJ103 (3.2kb), resulting in the construction of pGP (3.45kb). The plasmid containing the genes of abaecin and TEV protein, with gmp and gsp, was digested with EcoRI and BamHI, while pGP was digested with EcoRI and BamHI. The two digested products were linked by T4 DNA ligase, yielding recombinant plasmid pGPA.
The plasmid pGPA extracted from the positive transformant was transformed into the expression vector B. subtilis 1A747 with chloramphenicol resistance. The clones were picked with shaking at 37°C overnight in Luria-Bertani (LB) broth containing 50μg/mL of chloramphenicol. After this, the culture was inoculated into new LB broth at a ratio of 1:100 with shaking at 37°C until the optical density at 600nm reached 0.8−1.0. The culture was induced with 1% glucose and incubated at 37°C for an additional 24h with a rotation speed of 250rpm. Then, the supernatant was collected by centrifugation. Various pH values of the culture medium (pH 3.0−7.0) were tested for the optimal expression of recombinant proteins. It was found that pH 6.0 of the medium was the optimal condition for the expression of abaecin (data not shown).
Purification of the recombinant abaecinThe supernatant was first filtered twice using an Amicon ultrafiltration device (Millipore, MA, USA). The supernatant, containing proteins ranging from 3 to 10kDa, was dialyzed overnight in 0.1M sodium acetate and then applied to a CM-Sepharose CL-6B column (Pharmacia Biosciences, NJ, USA) pre-equilibrated with 0.1M sodium acetate (pH 5.0). The column was washed with 0.1M acetate buffer, and the proteins were eluted with a linear gradient of 0.1–1.0M sodium acetate (pH 5.0). A sample (100μL) of the filtrate was collected and applied to semi-preparative reversed-phase high-performance liquid chromatography (HPLC) on a C18 column (250×4.6 mm2, 5μm, and 300×Å), equilibrated in 0.1% trifluoroacetic acid and 18% acetonitrile. The bound protein was eluted with a linear gradient of acetonitrile (18% – 45%, v/v) in 0.1% trifluoroacetic acid. The flow rate was 1.0mL/min, and the absorbance of eluted protein was monitored at 280nm. The peak of the chimeric abaecin eluted at 31% acetonitrile from reversed-phase HPLC was determined by analyzing fractions on Tricine-sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).20 The fractions containing abaecin were collected for the next step. The sequence of recombinant abaecin was determined by the SinoGenoMax Company (Beijing, China)
Antimicrobial activitiesGrowth inhibition assays: E. coli K88ac was purchased from the China Veterinary Culture Collection Center (Beijing, China). The bacteria were cultivated and incubated in LB medium (0.5% NaCl, 0.5% yeast extract, 1% tryptone, pH 7). All strains were then stored at −80°C with 20% sterile glycerol until needed.21
Growth inhibition was determined in sterilized 96-well plates in a final volume of 200μL using the microdilution assay as described previously.22 A stock solution of the peptides was diluted tenfold in the culture medium and 100μL of LB medium. The bacteria (2×107 to 4×107 cells/mL) were added to 100μL of LB medium and peptide solution (serial twofold dilutions). The OD600 was measured every 20min for 16h in an Eon Microplate Spectrophotometer (BioTek Instruments, VT, USA). Control cultures with no AMPs were included in the assays.
ResultsPlasmid constructions and expressionThe recombinant plasmid ABA with a restriction enzyme EcoRI cleavage site at the 3′-terminus and a BamHI cleavage site at the 5′-terminus was linked with pGP (digested with EcoRI and BamHI) by T4 DNA ligase. As shown in Fig. 1, the constitutive expression vector pGPA, containing the operon of inducible promoter spac and TEV protease gene, was constructed. B. subtilis supplied a favorable base for expression. After a three-step construction process, the recombinant plasmid containing the fusion gene of abaecin and TEV was transformed into B. subtilis 1A747 for expression.
During the initial stage of expression, the expressed fusion protein including abaecin and TEV had no antimicrobial activity. This ensured that the expressed product would not exert a deleterious effect on B. subtilis host strain, and the host strain would continuously express the fusion protein within a certain period. Also, the fusion protein was expressed at a higher level when isopropyl β-d-1-thiogalactopyranoside (IPTG) was added. The cultivation was continued for a certain period after induction with IPTG, and then the culture conditions were adjusted (data not shown) to release TEV protease into the culture medium. The targeted expressed protein abaecin was activated on the hydrolysis of TEV protease to TEV. As shown in Fig. 2, the recombinant abaecin was expressed in B. subtilis at a quite high level. The expressed protein was released directly into the culture medium and obtained from the supernatant after centrifugation.
Expression of abaecin in Bacillus subtilis 1A747. The analysis of purified abaecin on Tricine-SDS-PAGE and Western Blot revealed its molecular mass to be about 4kDa. (a) SDS-PAGE analysis of abaecin expressed. Lane 1, total cell protein (uninduced); lane 2, total cell protein (induced); lane M, low-range molecular mass marker (TaKaRa, Shiga, Japan). (b) Western blot analysis of abaecin expressed. Lane 3, total cell protein (uninduced); lane 4, total cell protein (induced). The arrow indicates the position of recombinant abaecin.
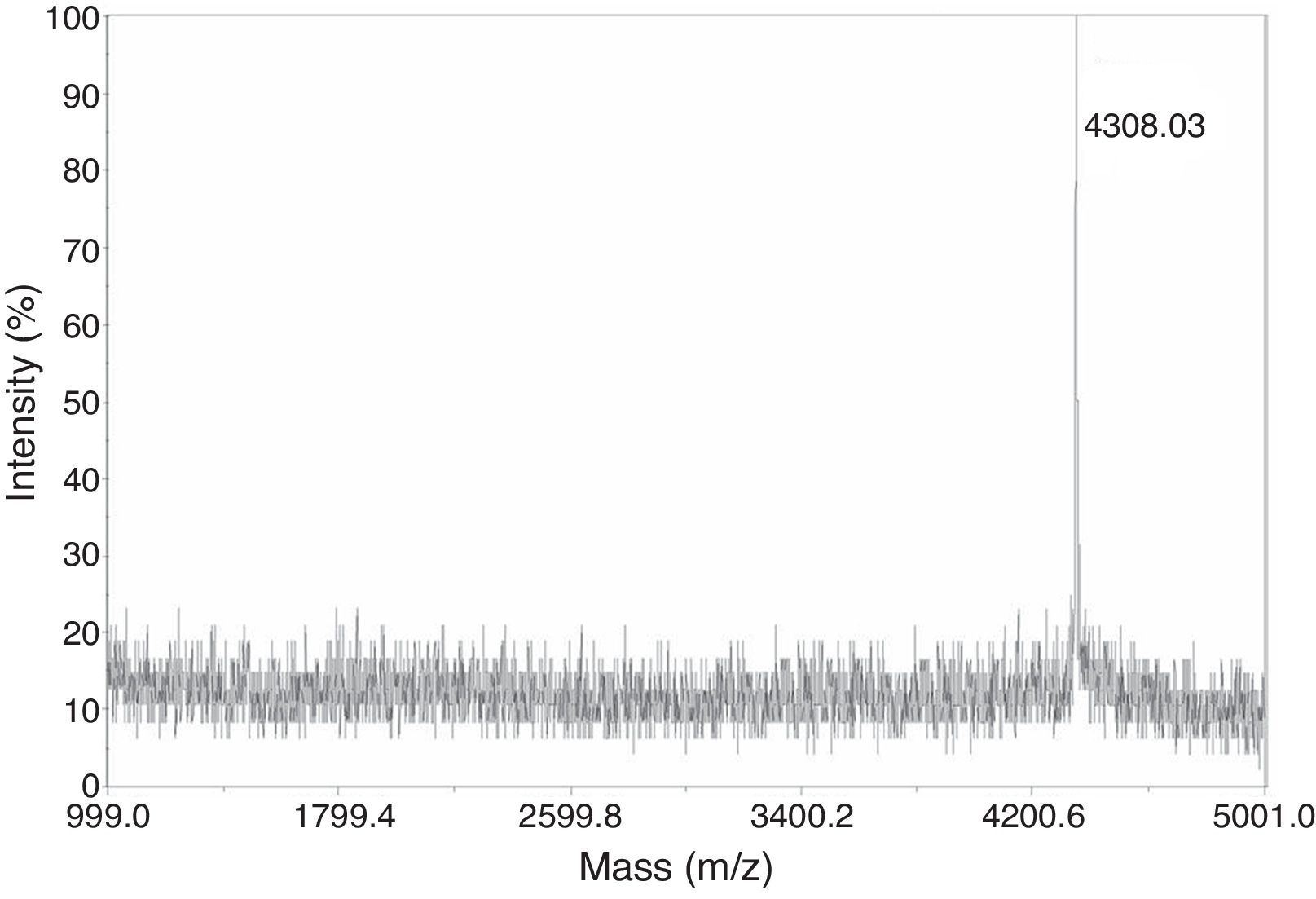
Following highly efficient purification including ultrafiltration and reversed-phase HPLC, pure chimeric antibacterial peptide abaecin was obtained from the culture medium. The analysis of purified abaecin on Tricine-SDS-PAGE revealed its molecular mass to be about 4kDa (Fig. 2). Electrospray ionization mass spectrometry of the purified abaecin demonstrated a single, nondispersed signal with a molecular mass of 3.9kDa (Fig. 3). The sequence of recombinant abaecin was identified as consistent with the theoretical sequence.
Potency of the AMPs in bacterial growth inhibition assaysE. coli strain K88 showed no susceptibility in the presence of up to 200μM abaecin and entered the exponential growth phase∼4h after the initiation of cultivation. The conclusion was consistent with the findings of Rahnamaeian.23 In contrast, cecropin A and hymenoptaecin exhibited potent bactericidal activities at concentrations of 1 and 1.5μM, respectively. The sublethal concentrations of the AMPs were determined by preparing serial dilutions and repeating the growth inhibition assays. This revealed that cecropin A and hymenoptaecin had sublethal concentrations of 0.3 and 0.5μM, respectively (data not shown). Combinatorial assays were carried out by supplementing the sublethal doses of each pore-forming peptide with 20μM abaecin (Fig. 4).
Escherichia coli growth inhibition assays. (a) E. coli strain K88 in the mid-logarithmic phase was incubated with the medium (control) or with abaecin and hymenoptaecin, alone or in combination. (b) E. coli strain K88 in the mid-logarithmic phase was incubated with the medium (control) or with abaecin and cecropin A, alone or in combination. The growth rate was determined by measuring the optical density of the culture at 600nm.
Honeybee AMPs are a family of small polypeptides with great potential for antibacterial application.24 However, to date, a few reports are available detailing the heterologous expression of recombinant honeybee AMPs. Therefore, in the present study, B. subtilis was used as a host for the high-level expression and secretion of chimeric peptide abaecin. The introduction of TEV into the fusion gene offered a favorable environment for the expression of abaecin. Abaecin was produced in B. subtilis with the help of a special operon without any damage to the host strain.
Producing AMPs (such as honeybee AMPs) with high efficiency is a significant challenge for developing commercial products.25 In the present study, the B. subtilis expression system was adopted for the constitutive expression of recombinant abaecin.25 To facilitate the expression of abaecin, this system included the TEV protease gene, which could release under appropriate conditions and ensure the cleavage of the target gene.26 Also, the modified expression system did not require an inducer during expression27 and did not produce inclusion bodies.23 Therefore, the system had the advantages of high efficiency of expression at low cost, easy operation, and application to industrial production. The recombinant abaecin was expressed at a quite high level (up to about 1g/L) in bacterial cell culture.
The analysis of purified abaecin using Tricine-SDS-PAGE revealed its molecular mass to be about 4kDa, which was consistent with the theoretical molecular mass of 3.966kDa obtained from electrospray ionization mass spectrometry.
It has been shown previously that abaecin alone does not affect bacterial proliferation at concentrations of up to 200μM, but can potentiate the activity of hymenoptaecin (a pore-forming peptide also from the bumblebee) and thus reduce the minimum inhibitory concentrations of hymenoptaecin required for membrane permeabilization.28 This interaction can be specific, given that the two AMPs are naturally co-expressed in response to a bacterial challenge, but can also represent a more general mechanism.
Antibiotics are currently widely used as therapeutic agents and growth stimulants for farm animals. However, the use of antibiotics in animal feed should be completely banned due to concerns regarding the presence of residues in animal products and the development of bacterial resistance to antibiotics.29 Consequently, the development of alternatives to antibiotics needs considerable attention.30 The fact that abaecin could enhance the antimicrobial activity against specific tested organisms t suggests that one potential application of abaecin may be in the livestock industry, since bacteria such as staphylococci, E. coli, and salmonella are some of the primary organisms causing infectious diseases in livestock.31,32
In summary, a novel B. subtilis expression system for abaecin based on B. subtilis cells genetically modified with a TEV protease and the two-cistron expression vector gene of abaecin fused to TEV was developed in this study. Abaecin could enhance the effect of pore-forming peptides from other species on the inhibition of bacterial growth. These findings indicated that the B. subtilis expression system could be applied as a powerful tool for producing abaecin at a larger scale, which is expected to become a useful antimicrobial or even therapeutic agent in animals or even human beings. A further study would test this hypothesis through some practical feeding trials.
Conflict of interestThe authors declared that they have no conflict of interest.