In this study a natural culture medium that mimics the synthetic yeast peptone glucose medium used for yeast fermentations was designed to screen and select yeasts capable of producing high levels of diacetyl and acetaldehyde. The presence of whey powder and sodium citrate in the medium along with manganese and magnesium sulfate enhanced both biomass and aroma development. A total of 52 yeasts strains were cultivated in two different culture media, namely, yeast peptone glucose medium and yeast acetaldehyde-diacetyl medium. The initial screening of the strains was based on the qualitative reaction of the acetaldehyde with Schiff's reagent (violet color) and diacetyl with Brady's reagent (yellow precipitate). The fermented culture media of 10 yeast strains were subsequently analyzed by gas chromatography to quantify the concentration of acetaldehyde and diacetyl synthesized. Total titratable acidity values indicated that a total titratable acidity of 5.5°SH, implying culture medium at basic pH, was more favorable for the acetaldehyde biosynthesis using strain D15 (Candida lipolytica; 96.05mgL−1 acetaldehyde) while a total titratable acidity value of 7°SH facilitated diacetyl flavor synthesis by strain D38 (Candida globosa; 3.58mgL−1 diacetyl). Importantly, the results presented here suggest that this can be potentially used in the baking industry.
Yeasts are commonly used as starter cultures for increasing product-specific aroma production during various fermentation processes (cheese, kefir, sourdough, wine, beer, etc.), as they are capable of synthesizing natural flavors like acetaldehyde (ethanal) or diacetyl (2,3-butanedione) which in turn serve to enhance the quality of the food.1 Acetaldehyde is the most important carbonyl compound produced during alcoholic fermentation with final concentrations typically varying between 10 and 200mgL−1 depending on technological factors such as culture medium composition, pH, fermentation temperature, aeration and SO2 concentration, and on the yeast strain used.2,3 Acetaldehyde is found in a variety of foods and beverages such as cheese, yogurt, beer, and wine.4,5 Generally, acetaldehyde levels reach their peak values in the early fermentation phase; it is then partly reutilized by the yeasts during the rest of the fermentation process.6 Acetaldehyde is responsible for the green apple aroma in alimentary products and, usually, the added acetaldehyde is produced by chemical synthesis. Contrarily, by adding using acetaldehyde produced by biosynthesis renders the product safe for human consumption, and the inadvertent addition of the byproducts of chemical acetaldehyde synthesis can be avoided. Diacetyl is almost exclusively synthesized by lactic acid bacteria and is the key flavor compound naturally produced by the Leuconostoc sp.7,8 Diacetyl is an important flavoring compound that determines specific characteristics of products such as fermented milks and, at very low concentrations (up to 5mgL−1), is also responsible for the characteristic “buttery” aroma of milk products.9 Furthermore, it is known that while Saccharomyces cerevisiae produces 132.4mgL−1 acetaldehyde, less than 1mgL−1 diacetyl is produced during red wine fermentation.10–12 Thus, by using a potential fermentation mix that can produce both acetaldehyde and diacetyl during the fermentation process, in place of a chemically synthesized product, it is possible to confirm to the current global trend of replacing synthetic products with naturally obtained products, while concurrently satisfying consumer demand for natural compounds in alimentary products.
Therefore, the main objective of this study was to identify yeasts capable of biosynthesizing high amounts of acetaldehyde and diacetyl and to assess their proposed use as natural products in fermentation products during industrial production. This is necessary because any product on the market that already contains this aroma cannot be further used in patisserie products.
Materials and methodsYeast strainsThe yeasts strains utilized in this study were isolated from various sources in the laboratories of BIOALIMENT (biotechnology applied in food industry – integrated center for research and education), “Dunarea de Jos”, University of Galati, Faculty of Food Science and Engineering. Every strain was assigned a unique code in the MIUG collection of the “Dunarea de Jos” University of Galati, and simultaneously, a unique code for the microorganism collection of the “Petru Poni” Institute of Macromolecular Chemistry (ICMPP). Pure strains were stored at −80°C in YPG culture medium supplemented with 20% glycerol.13 Further, the taxonomic classification of the isolated yeasts was determined and flavor production was followed by gas chromatography (GS).
Culture conditionsIn this study, we designed and used a natural culture medium, yeast acetaldehyde-diacetyl medium (YAD), that could mimic the synthetic YPG culture medium, to select yeast strains capable of producing high levels of diacetyl and acetaldehyde. Importantly, the sodium citrate, manganese and magnesium sulfate, which are part of the YAD culture medium, are consumed by the microorganisms during growth and biosynthesis, thereby rendering the YAD culture medium ‘natural’. For selecting yeast strains capable of producing diacetyl and acetaldehyde, we designed a culture medium with the following composition: 10gL−1 dextrose; 50gL−1 yeast extract; 5gL−1 sodium citrate; 10gL−1 whey powder; 0.05gL−1 manganese sulfate; 0.2gL−1 magnesium sulfate. The pH was adjusted between 6 and 7 using 0.1N HCl. The culture medium was sterilized at 120°C for 20min, and this culture medium was designated YAD (yeast diacetyl acetaldehyde). The dormant yeasts were activated by inoculating them in standard YPG medium and transferred to YAD culture medium during the log growth phase by inoculating 5mL of YAD culture medium with a 20% yeast suspension that was activated in YPG medium for 24h and had an absorbance of 0.5 at 600nm. The cultures were incubated at 30°C for 48h under static conditions in order to reduce the evaporation of volatile substances. After fermentation, the medium was filtered to remove cells and subjected to Gas chromatography for identification and quantification of acetaldehyde and diacetyl production.
Identification of the yeast strains using ID 32 C™ID 32 C™ is a standardized system of yeast identification that uses 32 miniaturized assimilation tests and a database. The ID 32 C™ strip consists of 32 cupules, each containing a dehydrated carbohydrate substrate. A semi-solid, minimal medium is added along with a suspension of the yeast to be tested. After 24–48h of incubation, growth in each cupule was read using the ATB Expression, the mini API instrument, or visually. Identification was performed using the Apiweb™ identification software.
Detection of diacetyl and acetaldehyde in culture mediumDetection of aldehydes with Schiff's reagentSchiff's test is a qualitative test for the presence of aldehyde functional groups wherein, a colorless Schiff reagent turns into a characteristic violet color when aldehyde groups are present in the sample added.14 The reaction was performed by adding 500μL of Schiff's reagent to the 5mL of medium fermented for 48h at 30°C. The media samples were assigned scores between 0 and 5 based on reaction intensity at 5s after reagent addition, with 0 denoting no acetaldehyde production; 1–3 denoting low production of acetaldehyde, 4 denoting good production of acetaldehyde, and 5 denoting very good production of acetaldehyde. Only samples with high aldehyde content (score 5) were further tested to determine if acetaldehyde was indeed present in high amounts.
Detection of diacetyl with Brady's reagent2,4-Dinitrophenylhydrazine (DNPH, Brady's reagent) is a chemical compound which can be used to qualitatively detect the presence of ketone or aldehyde functional groups. The test is considered positive if a yellow (aliphatic), orange or red (aromatic) precipitate is present. DNPH does not react with other carbonyl-containing functional groups such as carboxylic acids, amides or esters.15 The test was performed by adding 250μL of Brady's reagent to 2.5mL of medium fermented for 48h at 30°C. As diacetyl is an aliphatic compound, only those strains that gave a yellow precipitate were selected for further investigation. Similar to Schiff's reaction, the samples were evaluated based on intensity of the yellow precipitate and this was assigned a score between 0 and 5, where 0 denotes no diacetyl production, 1–3 low production of diacetyl, 4 good production of diacetyl and 5 very good production of diacetyl. Further, results from both the Schiff test and the DNPH test were considered together in order to select strains capable of producing both acetaldehyde and diacetyl. For identification of diacetyl and acetaldehyde in the culture medium, the same strain was simultaneously inoculated into both YAD and YPG media.
Total titratable acidity (TTA)TTA was quantified according to the method of Soxhlet-Henkel using a sodium hydroxide standard solution to neutralize the acid content of the sample (herein, suspension of the culture medium).16 The procedure used for estimating TTA is as follows. A solution containing 5g of the sample and 45mL of distilled water in titration flask was stirred on a magnetic stirrer for 2min and 2mL of phenolphthalein added. Subsequently, 0.25M NaOH solution was added to this suspension until the solution turned a light pink color. TTA was calculated using the formula TTA (°SH)=2×2.5×ν1, where ν1 is the volume of 0.25M NaOH consumed for neutralization of the acids in the sample. All titrations were performed in triplicate. This method was also used to monitor how culture medium acidity affected aroma biosynthesis during fermentation.
Quantification of diacetyl and acetaldehyde in the culture mediumDiacetyl and acetaldehyde in the culture medium were quantified using gas chromatography (GS) spectra of the analyzed samples and from equations obtained from the calibration curves for acetaldehyde and diacetyl. The concentrations used for obtaining the calibration curve for acetaldehyde were: 5, 10, 20, 40, 80, and 200μLL−1, while those for diacetyl were 2.5, 5, 10, 25, and 50μLL−1.
GS spectra were obtained using an Agilent 6890 N Gas Chromatograph with an FID Detector and a Suprawax-280 (100% PEG bonded, crosslinked) 60.0m×250μm×0.25μm capillary column with the following parameters: He gas flow set at 2mLmin−1, inlet temperature 150°C, split ratio 10:1; sample volume 1μL. The temperature program started at 40°C (maintained for 1min), and was followed by heating to 110°C at a rate of 10°Cmin−1, and then till 270°C at 40°Cmin−1.
Results and discussionTaxonomic classification of the isolated yeastsA total of 52 yeast isolates were taxonomically classified using the Apiweb™ identification software (Table 1). Thirty isolates were identified as belonging to the genus Candida spp., ten isolates belonged to genus Saccharomyces spp. (S. cerevisiae), five isolates were Cryptococcus spp., one isolate belonged to genus Geotrichum spp. (Geotrichum capitatum), and six isolates were Rhodotorula spp.
The yeasts isolates identified with ID 32 C™ and the selection of the yeast strains for acetaldehyde and diacetyl biosynthesis in liquid culture medium.
| No. | ICMPP ID code | Species | ID 32C™ (%) | YPG culture medium Schiff's reaction for acetaldehydea | YAD culture medium Schiff's reaction for acetaldehydea | YPG culture medium Brady's reaction for diacetyla | YAD culture medium Brady's reaction for diacetyla |
|---|---|---|---|---|---|---|---|
| 1 | D1 | Candida famata | 98.7 | 0 | 2 | 1 | 1 |
| 2 | D2 | Candida rugosa | 82.9 | 0 | 1 | 1 | 1 |
| 3 | D3 | Rhodotorula minuta | 99.9 | 0 | 2 | 1 | 1 |
| 4 | D4 | Rhodotorula mucilaginosa | 91.9 | 0 | 1 | 1 | 1 |
| 5 | D5 | Saccharomyces cerevisiae | 98.5 | 0 | 5 | 1 | 1 |
| 6 | D6 | Candida pelliculosa | 99.9 | 0 | 4 | 1 | 1 |
| 7 | D7 | Cryptococcus uniguttulatus | 99.9 | 0 | 2 | 1 | 1 |
| 8 | D8 | Cryptococcus terreus | 99.9 | 0 | 1 | 1 | 1 |
| 9 | D9 | Candida famata | 99.2 | 0 | 2 | 1 | 1 |
| 10 | D10 | Candida famata | 98.7 | 0 | 2 | 1 | 1 |
| 11 | D11 | Candida lipolytica | 99.9 | 0 | 2 | 1 | 1 |
| 12 | D12 | Candida krusei | 85.7 | 0 | 1 | 1 | 1 |
| 13 | D13 | Candida lipolytica | 99.9 | 0 | 4 | 1 | 1 |
| 14 | D14 | Candida lipolytica | 99.9 | 0 | 2 | 1 | 1 |
| 15 | D15 | Candida lipolytica | 99.9 | 0 | 5 | 1 | 5 |
| 16 | D16 | Candida lipolytica | 99.9 | 0 | 1 | 1 | 1 |
| 17 | D17 | Candida colliculosa | 99.9 | 0 | 5 | 1 | 1 |
| 18 | D19 | Candida holmii | 87.9 | 0 | 2 | 1 | 1 |
| 19 | D20 | Candida pelliculosa | 87.0 | 0 | 1 | 1 | 1 |
| 20 | D21 | Candida lipolytica | 99.9 | 0 | 5 | 1 | 1 |
| 21 | D22 | Candida pelliculosa | 99.9 | 0 | 3 | 1 | 1 |
| 22 | D23 | Candida colliculosa | 99.9 | 0 | 2 | 1 | 1 |
| 23 | D24 | Candida famata | 96.9 | 0 | 2 | 1 | 1 |
| 24 | D25 | Cryptococcus albidus | 99.5 | 0 | 4 | 1 | 1 |
| 25 | D26 | Candida lipolytica | 99.9 | 0 | 3 | 1 | 1 |
| 26 | D27 | Candida sphaerica | 97.2 | 0 | 4 | 1 | 1 |
| 27 | D28 | Geotrichum capitatum | 98.1 | 0 | 2 | 1 | 1 |
| 28 | D29 | Candida lambica | 92.7 | 0 | 4 | 1 | 1 |
| 29 | D30 | Candida colliculosa | 99.9 | 0 | 5 | 1 | 1 |
| 30 | D31 | Cryptococcus terreus | 99.9 | 0 | 2 | 1 | 1 |
| 31 | D32 | Candida lambica | 99.9 | 0 | 2 | 1 | 1 |
| 32 | D33 | Rhodotorula minuta | 99.9 | 0 | 4 | 1 | 1 |
| 33 | D34 | Candida lambica | 96.4 | 0 | 3 | 1 | 1 |
| 34 | D35 | Saccharomyces cerevisiae | 99.6 | 0 | 3 | 1 | 1 |
| 35 | D36 | Saccharomyces cerevisiae | 99.7 | 0 | 1 | 1 | 1 |
| 36 | D37 | Saccharomyces cerevisiae | 99.7 | 0 | 5 | 1 | 1 |
| 37 | D38 | Candida globosa | 99.9% | 0 | 5 | 1 | 1 |
| 38 | D39 | Saccharomyces cerevisiae | 99.6% | 0 | 3 | 1 | 1 |
| 39 | D47 | Saccharomyces cerevisiae | 99.9 | 0 | 2 | 1 | 1 |
| 40 | D49 | Candida lipolytica | 99.9 | 0 | 5 | 1 | 1 |
| 41 | D50 | Candida krusei | 85.7 | 0 | 5 | 1 | 1 |
| 42 | D51 | Candida silvicola | 99.9 | 0 | 2 | 1 | 1 |
| 43 | D52 | Rhodotorula glutinis | 83.1 | 0 | 2 | 1 | 1 |
| 44 | D53 | Candida famata | 93.2 | 0 | 4 | 1 | 1 |
| 45 | D54 | Saccharomyces cerevisiae | 99.9 | 0 | 4 | 1 | 1 |
| 46 | D55 | Saccharomyces cerevisiae | 99.4 | 0 | 2 | 1 | 1 |
| 47 | D56 | Saccharomyces cerevisiae | 99.9 | 0 | 2 | 1 | 1 |
| 48 | D57 | Cryptococcus curvatus | 99.9 | 0 | 2 | 1 | 1 |
| 49 | D58 | Candida pelliculosa | 99.9 | 0 | 2 | 1 | 1 |
| 50 | D59 | Rhodotorula mucilaginosa | 88.2 | 0 | 3 | 1 | 1 |
| 51 | D60 | Rhodotorula mucilaginosa | 86.6 | 0 | 1 | 1 | 1 |
| 52 | D61 | Saccharomyces cerevisiae | 99.9 | 0 | 3 | 1 | 1 |
The bold values are indicating the very good producers of acetaldehyde/diacetyl.
In order to identify yeast isolates capable of both acetaldehyde and diacetyl biosynthesis during fermentation, the isolates were inoculated into both YAD and YPG media and flavor biosynthesis was compared between these two media (Tables 1 and 2) using Schiff's test and Brady's test for the determination of acetaldehyde and diacetyl, respectively.
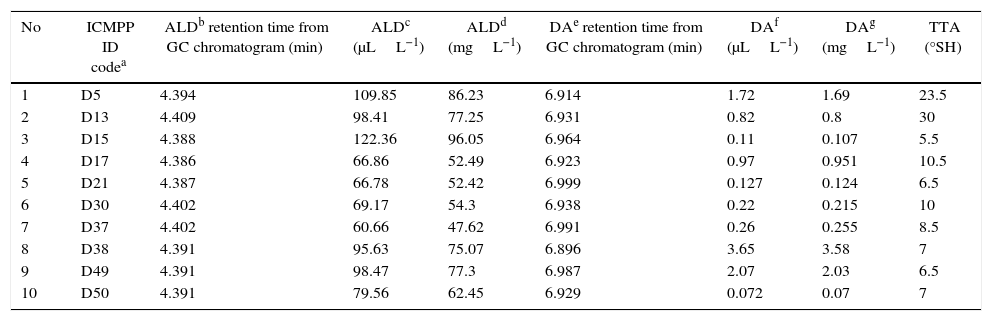
Gas chromatography and total titratable acidity (TTA) values for selected fermented mediums.
| No | ICMPP ID codea | ALDb retention time from GC chromatogram (min) | ALDc (μLL−1) | ALDd (mgL−1) | DAe retention time from GC chromatogram (min) | DAf (μLL−1) | DAg (mgL−1) | TTA (°SH) |
|---|---|---|---|---|---|---|---|---|
| 1 | D5 | 4.394 | 109.85 | 86.23 | 6.914 | 1.72 | 1.69 | 23.5 |
| 2 | D13 | 4.409 | 98.41 | 77.25 | 6.931 | 0.82 | 0.8 | 30 |
| 3 | D15 | 4.388 | 122.36 | 96.05 | 6.964 | 0.11 | 0.107 | 5.5 |
| 4 | D17 | 4.386 | 66.86 | 52.49 | 6.923 | 0.97 | 0.951 | 10.5 |
| 5 | D21 | 4.387 | 66.78 | 52.42 | 6.999 | 0.127 | 0.124 | 6.5 |
| 6 | D30 | 4.402 | 69.17 | 54.3 | 6.938 | 0.22 | 0.215 | 10 |
| 7 | D37 | 4.402 | 60.66 | 47.62 | 6.991 | 0.26 | 0.255 | 8.5 |
| 8 | D38 | 4.391 | 95.63 | 75.07 | 6.896 | 3.65 | 3.58 | 7 |
| 9 | D49 | 4.391 | 98.47 | 77.3 | 6.987 | 2.07 | 2.03 | 6.5 |
| 10 | D50 | 4.391 | 79.56 | 62.45 | 6.929 | 0.072 | 0.07 | 7 |
Acetaldehyde biosynthesis depends on the type of carbon and nitrogen sources in the medium, incubation time, agitation rate, and incubation temperature. Therefore, dextrose was added to the YAD medium as it is a very accessible carbon source for yeasts. Whey powder was added as a natural and very economical source of nitrogen, while manganese and magnesium sulfate are necessary growth factors for yeast culture. Sodium citrate in the culture medium acts as a precursor for and stimulates diacetyl biosynthesis (Table 1).17
Given the above and as can observed from the results presented in Table 1, yeasts inoculated in YPG did not produce acetaldehyde, while fermentation in YAD culture medium led to the biosynthesis of acetaldehyde due to the presence of the dextrose and whey powder in this culture medium. Nine yeasts strains produced high levels of acetaldehyde in YAD (Schiff's reaction score 5), and were selected for further analyses by gas chromatography. The nine yeast strains found to be good producers of acetaldehyde are as follows: three strains of S. cerevisiae: D5, D37 and D49, two strains of Candida lipolytica: D15 and D21, two strains of Candida colliculosa: D17 and D30, one strain of Candida globosa: D38, and one strain of Candida krusei: D50).
Selection of the yeasts using Brady's reagentSimilar to aldehyde production, yeasts inoculated in YPG did not synthesize diacetyl, and only one strain produced diacetyl when inoculated in to YAD medium, even though sodium citrate was added to the medium. The yeast isolate with a score of 5 for diacetyl biosynthesis (C. lipolytica; D13) was then selected for further analysis by GC.
Gas chromatographic analysisThe acetaldehyde and diacetyl concentrations obtained by GC, and TTA values of the selected isolates are presented in Table 2.
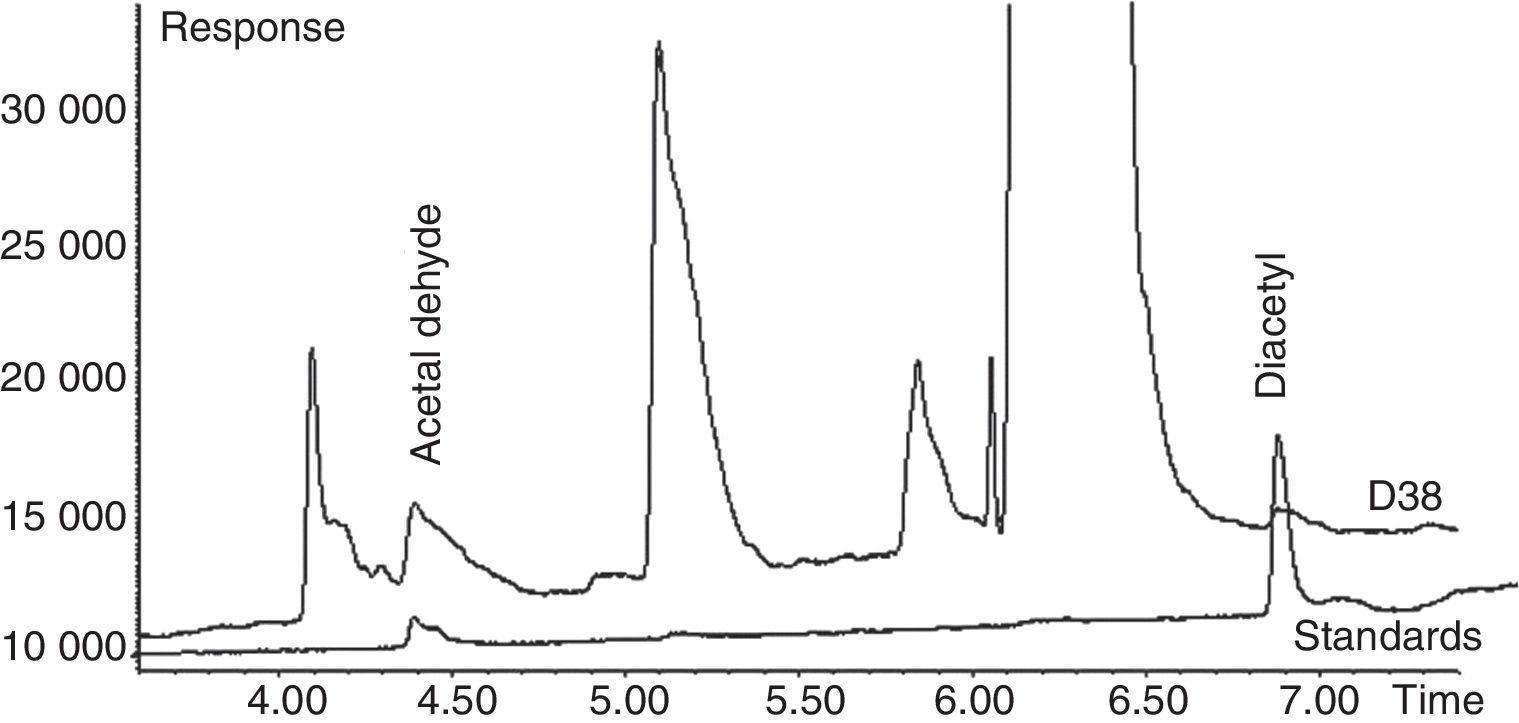
The data presented in Table 2 show that considerable amounts of acetaldehyde (75.07mgL−1) and diacetyl (3.58mgL−1) were obtained when the D38 isolate (identified as C. globosa by the API test) was used for fermentation in the YAD medium.
GC spectrum peak area of acetaldehyde (retention time approx. 4.3min) and diacetyl (retention time approx. 7.0min) (Figs. 1 and 2) demonstrated that yeast strain D15 (identified as C. lipolytica by API test) was the best producer of acetaldehyde (96.05mgL−1) in YAD medium. However, the amount of diacetyl produced by this yeast strain was very low (0.107mgL−1) even though the Brady reaction data presented in Table 1 imply otherwise. This can be explained by the fact that the Brady reagent reacts with both ketones and aldehydes, and the presence of a greater amount of precipitate was due to the presence of high amounts of the acetaldehyde, rather than diacetyl. Therefore, the volatiles produced by this strain were further analyzed by GC in order to identify the type and concentration of the compounds produced during fermentation. Isolate D38 (C. globosa) was the best producer of diacetyl (3.58mgL−1) in the same culture medium (YAD), confirming the results obtained with Brady's and Schiff's tests. Importantly, this D38 strain is also capable of biosynthesizing a substantial amount of acetaldehyde (75.07mgL−1) which is, albeit, slightly lesser than that of the D15 strain.
Given that the literature on the biosynthesis of aroma from yeasts is scant, at best on the basis of our results, we could discern only few basic inferences. The data presented in Table 2 suggest that, with respect to the yeast strain D15, an alkaline pH is more favorable for the acetaldehyde biosynthesis as the culture medium had a TTA of 5.5°SH. Such alkalinity is, however, not optimal for the synthesis of diacetyl, as the TTA of the medium fermented with D38 was 7°SH, implying that a neutral pH favors diacetyl biosynthesis.
ConclusionsWe propose a new and original method to produce acetaldehyde and diacetyl for use in the bakery industry using yeast fermentation. A natural culture medium that mimics the synthetic yeast culture (YPG) was developed and used successfully to screen potential isolates and produce substantial amounts of both acetaldehyde and diacetyl. The yeasts species were identified using ID 32 C™ tests; and gas chromatography was used to confirm acetaldehyde and diacetyl production and accurately quantify the amount produced during fermentation. Although C. lipolytica was capable of synthesizing high amounts of acetaldehyde, C. globosa could biosynthesize substantial amounts of both diacetyl and acetaldehyde under neutral pH conditions. In the light of these results, we propose this system to be tested for biotechnological transfer to industry-level fermenters and to be used during the production of fermented products.
Conflicts of interestThe authors declare no conflicts of interest.
This publication benefited from the financial support of the project “Program of Excellency in the multidisciplinary doctoral and post-doctoral research of chronic diseases”, contract no. POSDRU/159/1.5/S/133377, beneficiary “Gr. T. Popa” University of Medicine and Pharmacy of Iasi, project co-financed from the European Social Fund through the Sectoral Operational Program Human Resources Development (SOP HRD) 2007–2013, and by Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number PN-II-ID-PCCE-2011-2-0028.