In this study, physiological aspects of Lactobacillus plantarum BL011 growing in a new, all-animal free medium in bioreactors were evaluated aiming at the production of this important lactic acid bacterium. Cultivations were performed in submerged batch bioreactors using the Plackett–Burman methodology to evaluate the influence of temperature, aeration rate and stirring speed as well as the concentrations of liquid acid protein residue of soybean, soy peptone, corn steep liquor, and raw yeast extract. The results showed that all variables, except for corn steep liquor, significantly influenced biomass production. The best condition was applied to bioreactor cultures, which produced a maximal biomass of 17.87gL−1, whereas lactic acid, the most important lactic acid bacteria metabolite, peaked at 37.59gL−1, corresponding to a productivity of 1.46gL−1h−1. This is the first report on the use of liquid acid protein residue of soybean medium for L. plantarum growth. These results support the industrial use of this system as an alternative to produce probiotics without animal-derived ingredients to obtain high biomass concentrations in batch bioreactors.
In bioprocesses, various constituents can be used to match culture media compositions for lactic acid bacteria (LAB) production; however, a key issue is the cost involved. The use of agro-residues to obtain biotechnology-derived products has received great attention in recent years,1,2 and several by-products and raw materials of the food industry have been used in bioprocesses. The large availability of these materials, along with their low or even very low costs, makes them alternative sources of substrates that could be used for bacteria cultivations.3 Among common agro-residues are whey, whey permeate,1 starchy wastewater,4 corn steep liquor,2 and lignocellulosic materials.5,6 The production of soy protein isolate and soy protein concentrate, both in large demand in the world market, generates liquid acid streams of protein-rich residue as a result of the washing steps and separation of the isolate soy protein. This liquid fraction comprises sugars and proteins of low molecular weight. This agro-residue is discharged as an industrial effluent, and it has never been tested in bioprocesses before.
From the nutritional point of view, there is a growing interest of professionals and consumers in healthier diets that, in addition to providing basic nutrients, will also have beneficial effects on health, and probiotics are among the most important components of such diets.7–9 The current definition of probiotics, given by the World Health Organization, states that probiotics are live microorganisms that, when administered in adequate amounts in diets, will confer health benefits for the host, human or animal. Bacteria of the genera Lactobacillus and Bifidobacterium are classified among the main probiotics considered safe for food and feed use, and they are produced in industrial scales.10 Therefore, there is a great interest in screening for new potentially probiotic strains of LABs, such as Lactobacillus plantarum.
Traditionally, probiotics are generally added to or are components of dairy foods. However, there has been a growing demand for probiotics by non-dairy consumers due to increasing vegetarianism as a dietary preference throughout the world, and a large part of the world population has allergies to dairy products caused either by lactose or milk proteins.11–13 These issues, associated with concerns of the occurrence of bovine spongiform encephalopathy by some consumers, especially in Europe, have increased the demand for all animal-free foods,14 and there is a strong growing demand for new products in these markets. In the case of probiotics, it would be highly desirable if technology could deliver biosystems for cell growth free of any animal components to prevent the transfer of these constituents to the final product.15
Among probiotic bacteria or potentially probiotics depending on the strain, L. plantarum is a versatile lactic acid bacterium found in a variety of foods and other environments such as the human gastrointestinal-tract.16L. plantarum is safe to be used in food products.17 This probiotic has been studied by several authors that used different agro-residue substrates for biomass production, and it has been studied for its metabolites of interest, especially lactic acid. Such substrates include malt, wheat, barley extracts,18 molasses stillage, sugar beet molasses,19 coffee husks, tamarind seed powder, ground nut oil cake, wheat brans, rice brans, bengal gram powder, black gram flour, green gram flour, barley, millet, ragi, oats, corn flour, rice flour,20 york cabbage,21 quinoa, and wheat slurry.22
In this context, the aims of this research were to investigate the growth and fermentation activity of L. plantarum BL011, a strain isolated from Serrano cheese,23 using a new, alternative culture medium that is completely free of animal-derived components and is inexpensive. The growth and nutritional conditions that could have an influence on biomass formation were investigated using the Plackett–Burman design methodology. We also followed and analyzed the production of lactic acid along the process because it is the most important metabolite of LAB. Finally, the process was scaled-up to submerged cultures in batch bioreactors.
Materials and methodsMicroorganism and chemicalsThe L. plantarum BL011 strain, which was isolated by our group from Serrano cheese and described elsewhere,23 was used in this study. This strain was identified as L. plantarum BL011, and it is maintained at the Microbiology Culture Collection of BiotecLab (UFRGS, Porto Alegre, Brazil). The strain was identified at DSMA Laboratory (Mogi das Cruzes, SP, Brazil) by comparing the 16S rRNA amplicon sequences with GenBank databases (access number AB5989861), which showed 100% homology with L. plantarum genus and species. Working stocks of cultures were maintained in 20% (volume fraction) glycerol suspension frozen at −22°C±1°C.
Unless otherwise stated, all chemicals used in this research were of analytical grade and purchased from Sigma–Aldrich (St. Louis, USA).
Inoculum preparationErlenmeyer flasks (2000mL) containing 400mL of MRS24 were inoculated with 1.5mL of glycerol stock culture, and the cultures were incubated at 37°C±1°C in a rotary shaker (MA 830, Marconi, Piracicaba, Brazil) at 180rpm and grown to an optical density (OD) of 1.0 at 600nm. The cells were harvested by centrifugation (Hitachi, Himac CR 21E, Tokyo, Japan) at 3500×g for 15min at 4°C±1°C. The cell pellet was washed and resuspended directly into the cultivation broth (150mL), and the composition of the broth was varied accordingly to the experimental design described in Tables 1 and 2. This procedure was used as the standard inoculum preparation for all experiments.
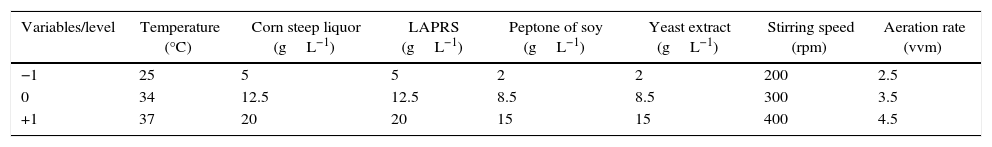
Independent variables studied in the Plackett–Burman design for the cultivation of L. plantarum BL011.
| Variables/level | Temperature (°C) | Corn steep liquor (gL−1) | LAPRS (gL−1) | Peptone of soy (gL−1) | Yeast extract (gL−1) | Stirring speed (rpm) | Aeration rate (vvm) |
|---|---|---|---|---|---|---|---|
| −1 | 25 | 5 | 5 | 2 | 2 | 200 | 2.5 |
| 0 | 34 | 12.5 | 12.5 | 8.5 | 8.5 | 300 | 3.5 |
| +1 | 37 | 20 | 20 | 15 | 15 | 400 | 4.5 |
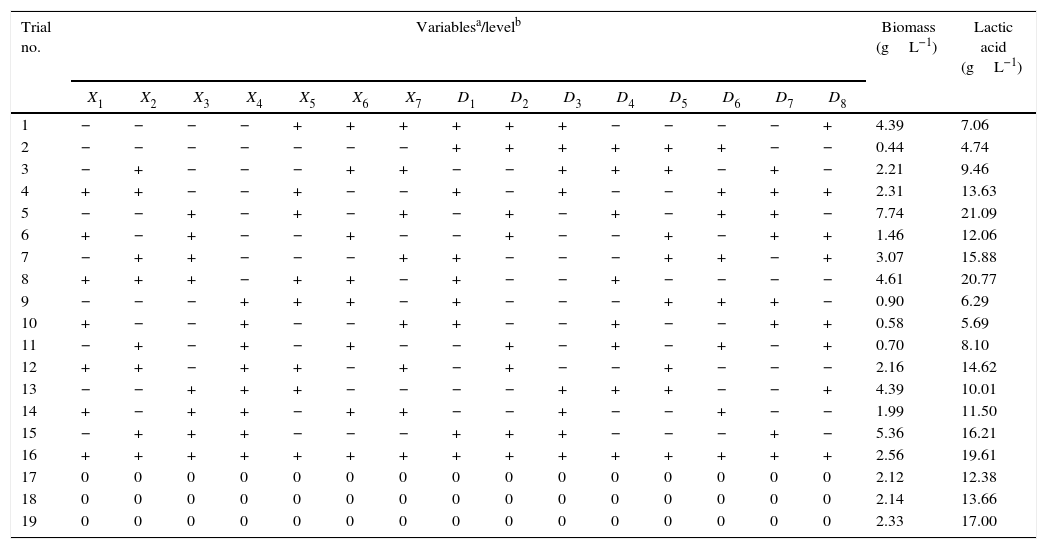
Plackett–Burman experimental design matrix for biomass and lactic acid production of L. plantarum BL011.
| Trial no. | Variablesa/levelb | Biomass (gL−1) | Lactic acid (gL−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | |||
| 1 | − | − | − | − | + | + | + | + | + | + | − | − | − | − | + | 4.39 | 7.06 |
| 2 | − | − | − | − | − | − | − | + | + | + | + | + | + | − | − | 0.44 | 4.74 |
| 3 | − | + | − | − | − | + | + | − | − | + | + | + | − | + | − | 2.21 | 9.46 |
| 4 | + | + | − | − | + | − | − | + | − | + | − | − | + | + | + | 2.31 | 13.63 |
| 5 | − | − | + | − | + | − | + | − | + | − | + | − | + | + | − | 7.74 | 21.09 |
| 6 | + | − | + | − | − | + | − | − | + | − | − | + | − | + | + | 1.46 | 12.06 |
| 7 | − | + | + | − | − | − | + | + | − | − | − | + | + | − | + | 3.07 | 15.88 |
| 8 | + | + | + | − | + | + | − | + | − | − | + | − | − | − | − | 4.61 | 20.77 |
| 9 | − | − | − | + | + | + | − | + | − | − | − | + | + | + | − | 0.90 | 6.29 |
| 10 | + | − | − | + | − | − | + | + | − | − | + | − | − | + | + | 0.58 | 5.69 |
| 11 | − | + | − | + | − | + | − | − | + | − | + | − | + | − | + | 0.70 | 8.10 |
| 12 | + | + | − | + | + | − | + | − | + | − | − | + | − | − | − | 2.16 | 14.62 |
| 13 | − | − | + | + | + | − | − | − | − | + | + | + | − | − | + | 4.39 | 10.01 |
| 14 | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | 1.99 | 11.50 |
| 15 | − | + | + | + | − | − | − | + | + | + | − | − | − | + | − | 5.36 | 16.21 |
| 16 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 2.56 | 19.61 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.12 | 12.38 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.14 | 13.66 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.33 | 17.00 |
X1 temperature, at highest level of 37°C, central level of 31°C, and lowest level of 25°C; X2 corn steep liquor concentration at highest level of 20.0gL−1, central level of 12.5gL−1, and lowest level of 5.0gL−1; X3 total sugars (LAPRS) at highest of 20.0gL−1, central concentration of 12.5gL−1, and lowest concentration of 5.0gL−1; X4 peptone of soy at highest concentration of 15.0gL−1, central concentration of 8.5gL−1, and lowest concentration of 2.0gL−1; X5 yeast extract at highest concentration of 15.0gL−1, central concentration of 8.5gL−1, and lowest concentration of 2.0gL−1; X6 stirred agitation at highest level of 400rpm, central level of 300rpm, and lowest level of 200rpm; X7 aeration rate at highest level of 4.5vvm, central level of 3.5vvm, and lowest level of 2.5vvm; D1, D2, D3, D4, D5, D6, D7 and D8 are dummy variables.
The LAPRS is the liquid fraction resulting of the washing and separation steps during the isolate soy protein production. This liquid fraction comprises sugars and proteins of low molecular weight. The LAPRS was kindly donated by the Solae Company (Esteio, Rio Grande do Sul, Brazil). This residue was collected in the industrial plant in the precipitation stage, which is the first unit operation in the wastewater treatment plant. The liquid was immediately stored in 5000mL polypropylene containers, sealed and transported under refrigeration to the laboratory where it was immediately vacuum-concentrated at 45°C±1°C for 4h and subsequently stored at −22°C±1°C until experiments. This operation was performed to concentrate the sugar content of samples. The LAPRS, as collected in the industry, had the following composition (in gL−1): total sugars, 10; protein, 2; lipids, 1; ashes (mineral salts), 2; and pH, 4.6. The analyses of protein, lipids, ashes, and total sugar were quantified according to the AOAC methods.25
Prior to use in cultivations, the LAPRS was concentrated to 50 or 25% of its original volume, resulting in final sugar concentrations of 20gL−1 (LAPRS20, for the screening variables design) and 40gL−1 (LAPRS40, used in the all other experiments), respectively.
Cultivation proceduresScreening of variablesA Plackett–Burman (PB) design was used to determine which nutrients and conditions had a significant effect on L. plantarum BL011 biomass formation, the dependent variable, which is presented in Table 1.26 The concentrations and values for the independent variables were fixed based on the general values found on the literature for studies with this microorganism and on a previous research from our group.1 Lactic acid production was also followed in these experiments because it is the most relevant metabolite of this bacterium (Table 2). Seven real and eight dummy variables were screened in 19 trials with triplicates at the central point. The minimal and maximal ranges selected for the seven parameters are presented in Table 2, in which each column represents an independent variable, and each row represents a trial. Variables with confidence levels >95% were considered to have significant influence on biomass production.
In the investigation of variables with a significant effect on biomass production, cultivations were performed with LAPRS20 medium containing the following additives (in gL−1): MgSO4·7H2O, 0.2; and MnSO4·H2O, 0.04. This mineral solution was also used at the same concentrations for all cultivations in this study. The other components of the medium were soy peptone, corn steep liquor, and raw yeast extract (autolysed non-purified yeast extract; Prodesa, SP, Brazil), which is an inexpensive source of mineral and vitamins. A volume of 150mL of inoculum was transferred to a bioreactor filled with 1350mL of medium, and the compositions were varied in accordance with the specific assay of the PB design. These experiments were performed using a 2000mL Biostat B bioreactor (B. Braun Biotech International, Melsungen, Germany), and the pH was set at 5.5±0.21 and was controlled by the automatic addition of 10M NaOH or 1M H3PO4. The dissolved oxygen concentration (DOC) of cultures was measured using a polarographic O2 probe (Mettler-Toledo, Germany). The volumetric oxygen mass transfer rate (kLa) was calculated using the dynamic gassing out method.27 In two experiments, the DOC was kept at a minimum of 30% of saturation by controlling the agitation speed through a control-loop. The temperature, aeration rate, and stirring speed were set according to the PB design (Table 2). A confirmation experiment using the best conditions found in the PB design was performed in the same bioreactor described above, except for the LAPRS concentration, which was doubled. The medium for this experiment was composed of 15gL−1 raw yeast extract and 1500mL of LAPRS40, and the conditions were 25°C, pH 5.5±0.2, 200rpm agitation, and 4.5vvm aeration.
Saccharification and co-saccharification in the bioreactorTo hydrolyse the polymeric sugars present in LAPRS, saccharification reactions of this substrate were performed using commercial invertase through the following two different approaches: (1) saccharification followed by fermentation as independent and sequential reactions (separate hydrolysis followed by fermentation); and (2) co-saccharification and fermentation performed as a single reaction (simultaneous hydrolysis and fermentation in the same vessel).
LAPRS hydrolysis was performed using invertase from S. cerevisiae (Maxinvert L 10000, batch 409200451, DSM Food Specialties, The Netherlands. Enzyme activity (1U) was defined as 1μmol glucose released from sucrose per minute at 50°C±1°C and 42% sucrose (mass fraction)). For condition 1, 1500mL of LAPRS40 was added (18.21U) in a 2-L flask and incubated at 50°C and pH 4.6 for 3h under agitation. The other nutrients were then added to the hydrolysed solution and used for fermentation without further modifications. For condition 2 consisting of the co-saccharification and fermentation, the enzyme was directly added to the final medium in the bioreactor in the presence of cells under the conditions of the fermentation. All trials were performed in duplicates.
All cultivations were performed in the bioreactors as described above under the same conditions (25°C, pH 5.5±0.2, 200rpm agitation, and 4.5vvm aeration).
Analytical methodsSamples (10mL) of culture broth were centrifuged at 3500×g for 15min at 4°C±1°C. The cell-free supernatant was used for the quantification of sugars and lactic acid. Biomass was measured gravimetrically as dry weight of cells. Samples were centrifuged, washed twice with cold distilled water, and dried in pre-weighed plastic tubes at 80°C±1°C to constant weight in vacuum ovens. Total sugar concentration was determined by the Dubois method using glucose as a standard.28 Lactic and acetic acid concentrations were determined by HPLC (Shimadzu, Kyoto, Japan) equipped with a refractive index detector and Bio-Rad HPX-87H column (300mm×7.8mm) using 5mM sulphuric acid as the eluent at 65°C±1°C, flow rate of 0.8mLmin−1 and sample volume of 20μL. Samples were filtered through cellulose acetate 0.22μm membranes prior to HPLC injection.
Data analysisAll experimental designs and results analyses were performed using Statistica 10.0 (Statsoft, Tulsa, USA).
Results and discussionExperimental designThe Plackett–Burman design was used to evaluate the effects of concentrations of LAPRS, soy peptone, corn steep liquor, and raw yeast extract as medium components as well as temperature, stirring speed, and aeration rate as cultivation conditions on L. plantarum BL011 biomass formation (dependent variable) and lactic acid production (important metabolite). Table 2 presents the PB experimental design for 19 trials with two study levels for each variable as well as the corresponding biomass production after 48h of bioreactor cultivation. Table 3 presents the statistical analyses of the studied variables. Except for the concentration of corn steep liquor, all other variables were found to be significant at the 95% level for biomass production. The highest values of biomass formation (7.74gL−1) and lactic acid production (21.09gL−1) were obtained in test 5. The value of kLa measured under these conditions was 30h−1 (200rpm, 4.5vvm, and mid-exponential growth phase), indicating that this aeration condition could be adopted for scaling-up the process to independently allow testing of other variables.
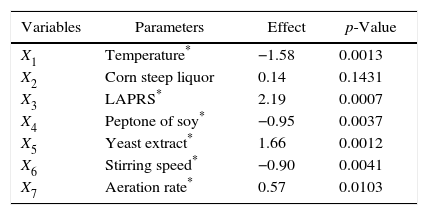
Estimated effects for biomass production calculated from the results of the Plackett–Burman design.
| Variables | Parameters | Effect | p-Value |
|---|---|---|---|
| X1 | Temperature* | −1.58 | 0.0013 |
| X2 | Corn steep liquor | 0.14 | 0.1431 |
| X3 | LAPRS* | 2.19 | 0.0007 |
| X4 | Peptone of soy* | −0.95 | 0.0037 |
| X5 | Yeast extract* | 1.66 | 0.0012 |
| X6 | Stirring speed* | −0.90 | 0.0041 |
| X7 | Aeration rate* | 0.57 | 0.0103 |
Standard error=0.58; p-values≤0.5; R2: 0.98.
Temperature and steering speed had significant negative effects. Therefore, for the next set of experiments in the bioreactor, these variables were decreased and set at 25°C±1°C and 200rpm, respectively. The aeration rate at 4.5vvm showed significant positive effects. Corn steep liquor, which was the only variable that did not have a significant effect, was excluded from the next experiments. Finally, although statistically significant, the soy peptone showed a negative effect, and because it has a high cost, it was removed from the medium composition for the next experimental step.
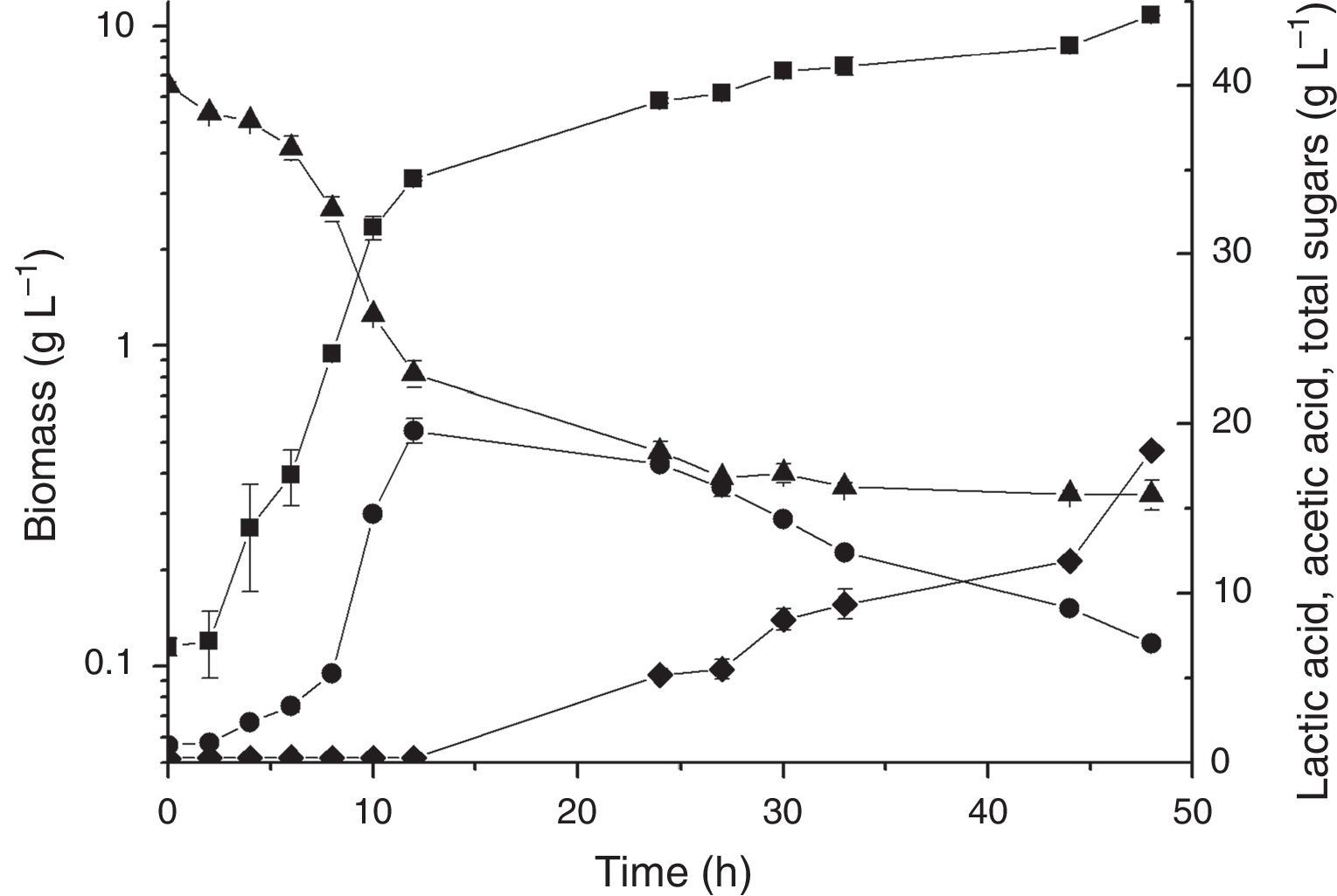
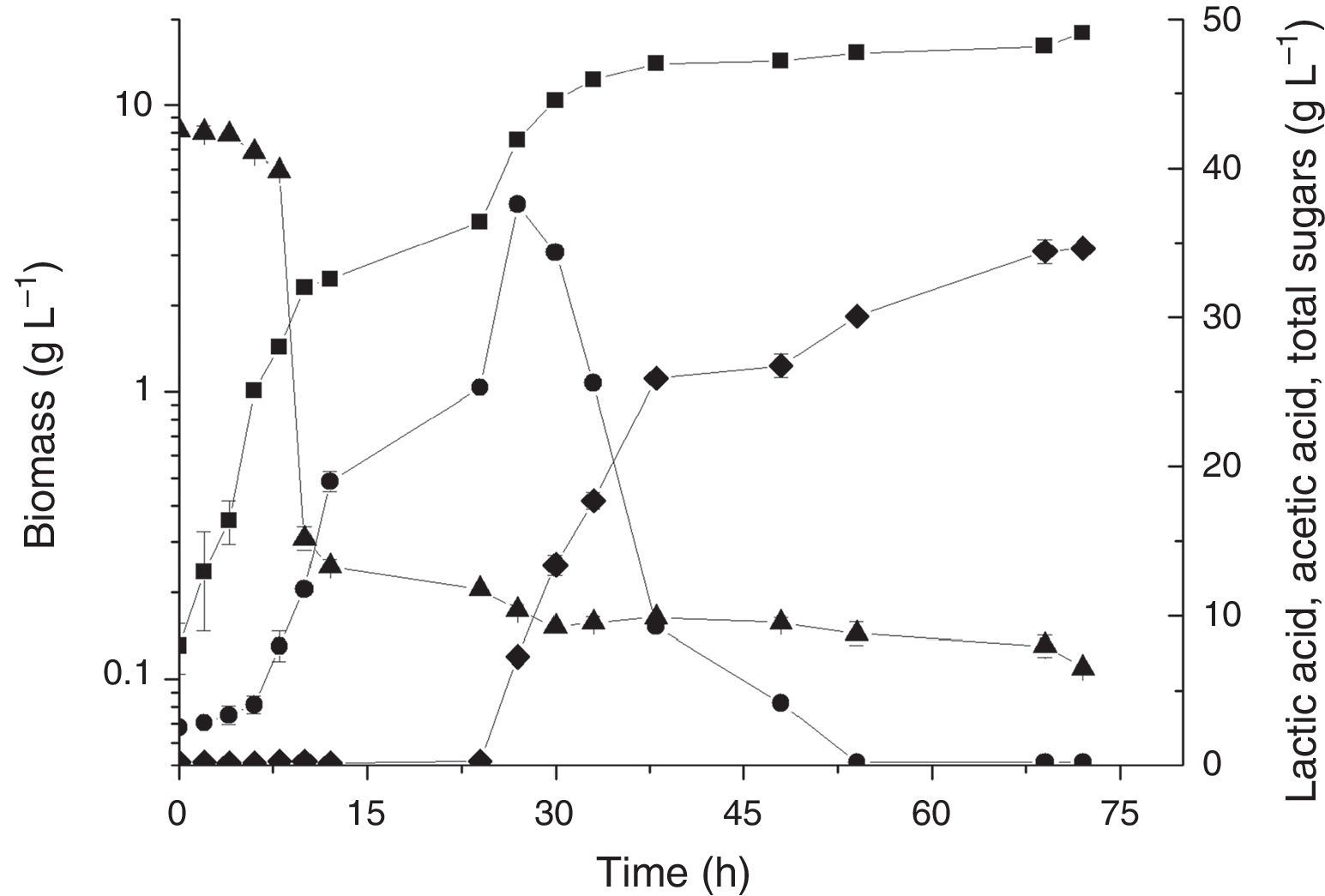
Based on the results obtained in the PB design, experiments were performed to confirm the best conditions for L. plantarum BL011 cultivation. The conditions were fixed as follows: kLa of 30h−1 (obtained using the combination of 200rpm and 4.5vvm), 25°C, pH controlled at 5.5, 15gL−1 raw yeast extract, and 1500mL of LAPRS40. The doubling of the total amount sugars of LAPRS from 20 to 40gL−1 was proposed because this variable produced a positive and significant effect on the PB. Fig. 1 depicts the kinetics of these cultivations. The maximal biomass obtained was 10.85±0.03gL−1, which was 28% higher than the best condition found in the PB design due to the higher sugar concentration. The highest lactic acid concentration was achieved at 12h of cultivation, reaching 19.56±0.73gL−1 with a productivity of 1.54gL−1h−1, which was similar to values of the PB design. The acetic acid concentration obtained was 18.43±0.25gL−1 in 48h of cultivation.
Time course of batch cultivations of L. plantarum BL011 in medium containing (gL−1): MgSO4·7H2O, 0.2; MnSO4·H2O, 0.04; LAPRS, 40 (total sugars); yeast extract, 15. Culture conditions: 25°C±1; 4.5vvm; 200rpm, pH 5.5±0.2; (■) dry cell weight; (●) lactic acid concentration; (♦) acetic acid concentration; (▴) total sugars concentration. The results are the mean of duplicates.
The remaining sugar left in cultivation might be a consequence of the inability of L. plantarum BL011 to completely hydrolyse all sugars present in the LAPRS because this vegetable source contains some oligosaccharides, such as raffinose and stachyose, which require enzymatic breakdown. The main enzymes involved in the hydrolysis of these sugars are alpha-galactosidase and beta-fructosidase (invertase), which showed to have negligible activities for this strain growing under the conditions of the PB in this study (results not shown).
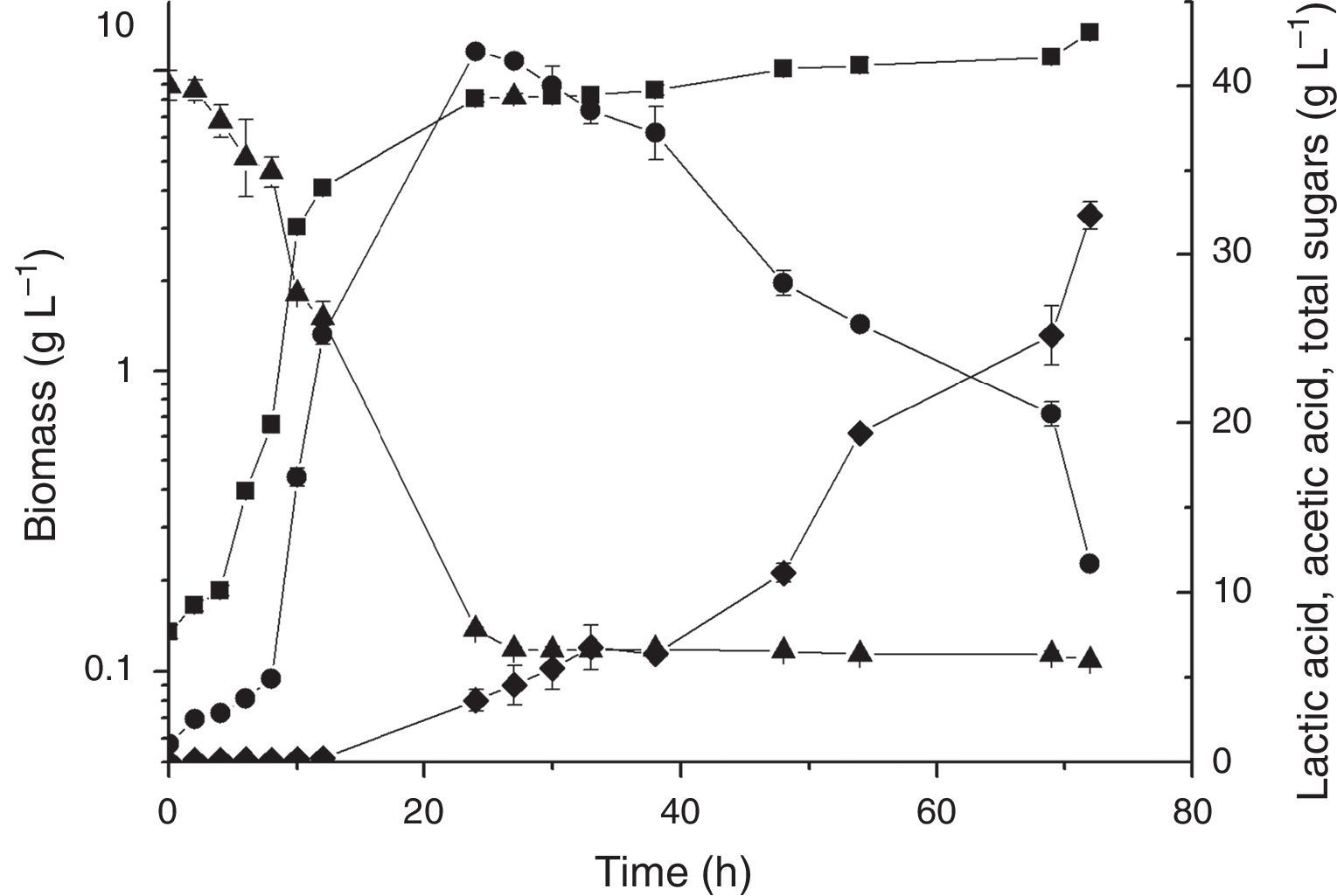
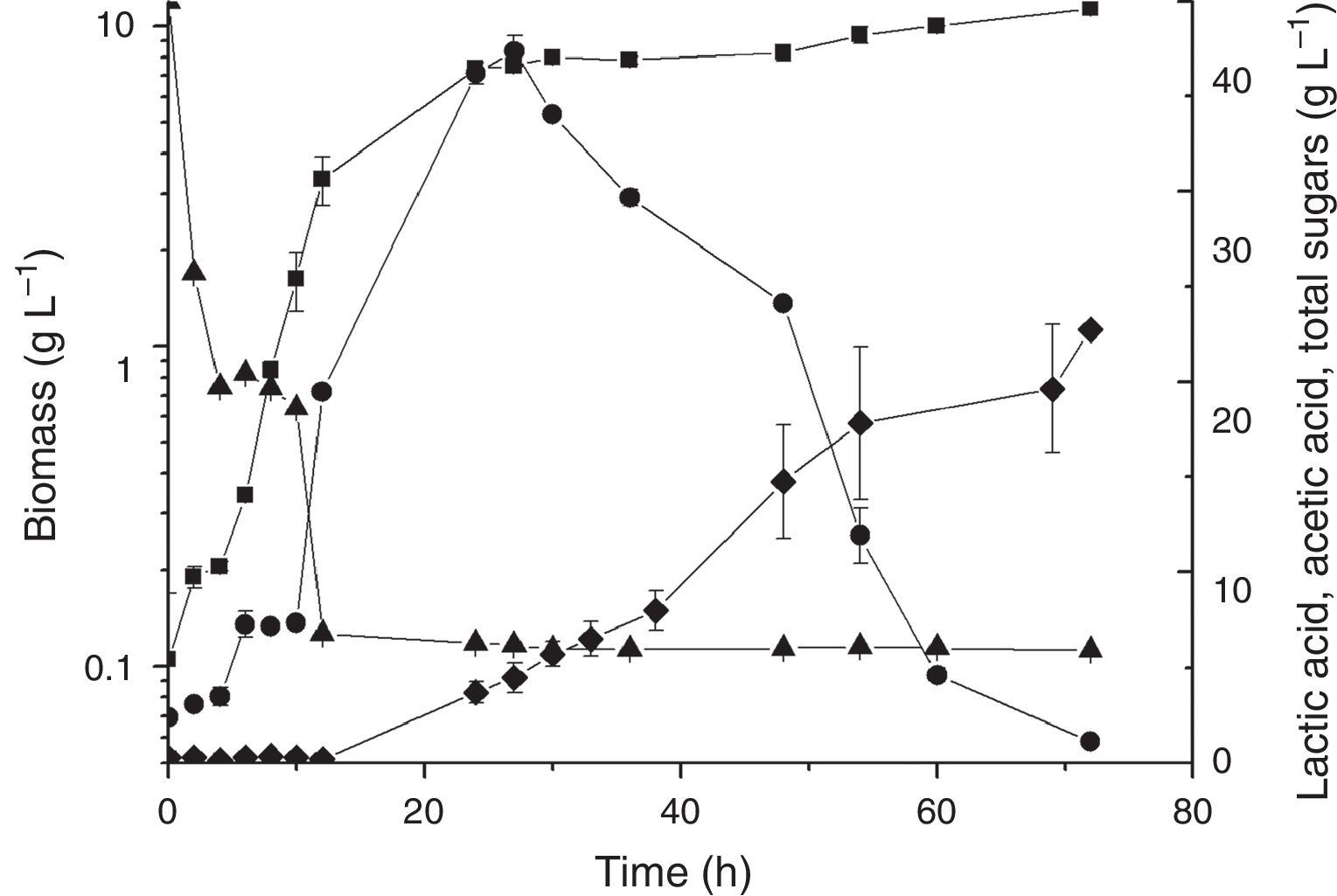
Saccharification and co-saccharification in the bioreactorBecause the absence of invertase activity for L. plantarum BL011 was confirmed, two sets of enzyme-mediated saccharification experiments were devised intending to increase the hydrolysis of sugars of LAPRS, consequently offering more sugars for bacterial metabolism. The hydrolysis of LAPRS was done using invertase because this enzyme cleaves the β-1.2 bonds in raffinose and stachyose, producing melibiose and fructose, respectively. In the first experiment, saccharification was performed independently with enzymatic hydrolysis followed by fermentation, and the results of the cultivation kinetics are presented in Fig. 2. In the second experiment, saccharification and fermentation were performed simultaneously, and the results of the cultivation kinetics presented in Fig. 3. Although the growth kinetics were similar, both showing faster consumption of sugars when compared to the PB design, the hydrolysis of LAPRS before cultivation produced better results than co-saccharification and fermentation because conditions for hydrolysis and fermentation were more ideal than the combined process with the following results: the obtained biomasses were 13.39±0.10gL−1 and 11.4±0.48gL−1, respectively; the lactic acid peaked at concentrations of 42.04±0.08gL−1 (24h) and 37.36±0.81gL−1 (24h), respectively; and the acetic acid peaked at concentrations of 32.35±0.79gL−1 (72h) and 22.71±0.19gL−1 (72h), respectively. The productivities of lactic acid measured at 24h of cultivation were 1.71gL−1h−1 and 1.46gL−1h−1 for the saccharification and co-saccharification, respectively. Comparatively, Sikder et al.29 reported a lactic acid productivity of 1.24gL−1h−1 by L. plantarum NCIM 2912 growing in sugarcane juice medium containing 140gL−1 total sugars (sucrose, 126; glucose, 8; and fructose, 6). Laopaiboon et al.30 obtained a lactic acid productivity of only 0.36gL−1h−1 in cultures of L. lactis IO-1 (JCM 7638) using hydrolysed sugarcane bagasse as a substrate (30gL−1).
Time course of batch cultivation of L. plantarum BL011 in hydrolysed LAPRS using invertase. Medium composition (gL−1): MgSO4·7H2O, 0.2; MnSO4·H2O, 0.04; LAPRS, 40 (total sugars); yeast extract, 15. Culture conditions: 25°C±1; 4.5vvm; 200rpm, pH 5.5±0.2; (■) dry cell weight; (●) lactic acid concentration; (♦) acetic acid concentration; (▴) total sugars concentration. The results are the mean of duplicates.
Time course of simultaneous saccharification and cultivation of L. plantarum BL011. Medium containing (gL−1): MgSO4·7H2O, 0.2; MnSO4·H2O, 0.04; LAPRS, 40 (total sugars); yeast extract, 15. Culture conditions: 25°C±1; 4.5vvm; 200rpm, pH 5.5±0.2 (■) dry cell weight; (●) lactic acid concentration; (♦) acetic acid concentration; (▴) total sugars concentration. The results are the mean of duplicates.
The results obtained using LAPRS40 for the growth of L. plantarum BL011 resulted in the highest biomass production compared to the literature. For instance, Brinques et al.1 reported a maximal biomass of 10.2gL−1L. plantarum using cheese whey (equivalent to 140gL−1 of lactose) as the medium carbon source. Gao et al.31 reported a final biomass production of 6.6gL−1L. rhamnosus NBRC 3863 when growing this LAB in hydrolysed fish waste. Mu et al.32 obtained a maximal cell density of 3.34gL−1Lactobacillus sp. SK007 in a medium consisting of glucose (30gL−1), yeast extract (30gL−1), and corn step liquor (47gL−1). Hwang et al.33 formulated a complex medium composed of different sugars and nitrogen sources for biomass production of L. acidophilus DGK, and these authors reported maximal biomass production of 4.54gL−1 under aerobic conditions. Finally, S. boulardii MYA-769 was produced in a medium consisting of grass juice feedstock extracted from ryegrass Lolium perenne, and it reached a biomass of 4.96gL−1.34
The results obtained using the LAPRS as the sole carbon source demonstrated that this industrial residue is a suitable substrate for the growth of L. plantarum BL011, which has not been reported in the literature. An industrial plant of soy protein isolate can produce a large volume of acid residue, such as the company that supplied the material for this study, which has a monthly discharge of approximately 5×104m3 of LAPRS (data provided by the company). When compared to other residues reported in the literature or even with synthetic media, LAPRS allows for a significant improvement of biomass formation. Moreover, the other component of the culture medium, the non-purified raw yeast extract obtained as the waste of beer production, has a low cost compared to the traditional, purified yeast extract used for cell growth, further supporting the hypothesis that the culture medium used in this work is inexpensive and suitable for Lactobacillus cultivations.
Figs. 2 and 3 show the profile of lactic acid formation, which is the most important metabolite of lactic acid bacteria, and its subsequent conversion into acetic acid by L. plantarum BL011. Lactic acid supported a small diauxic growth towards the end of cultivation, but it was mainly converted into acetic acid. L. plantarum showed flexible and adaptive behaviour for the first time in the chromosome of the WCFS1 strain, which encodes a variety of proteins related to sugar uptake and utilization,35 thus allowing L. plantarum to grow on several carbon sources. The genes encoding transporters are generally in clustered gene cassettes encoding enzymes and regulatory proteins implicated in sugar metabolism.16
The conversion of lactic acid into acetic acid under aerobic conditions results in one ATP generated via lactate dehydrogenase, pyruvate oxidase, pyruvate dehydrogenase, pyruvate formate lyase, phosphotransacetylase, and acetate kinase.36 This pathway also produces hydrogen peroxide and carbon dioxide. Hydrogen peroxide is generated by the conversion of oxygen through the manganese-dependent process. The final accumulation of acetic acid, instead of lactic acid, causes cell pH homeostasis.36
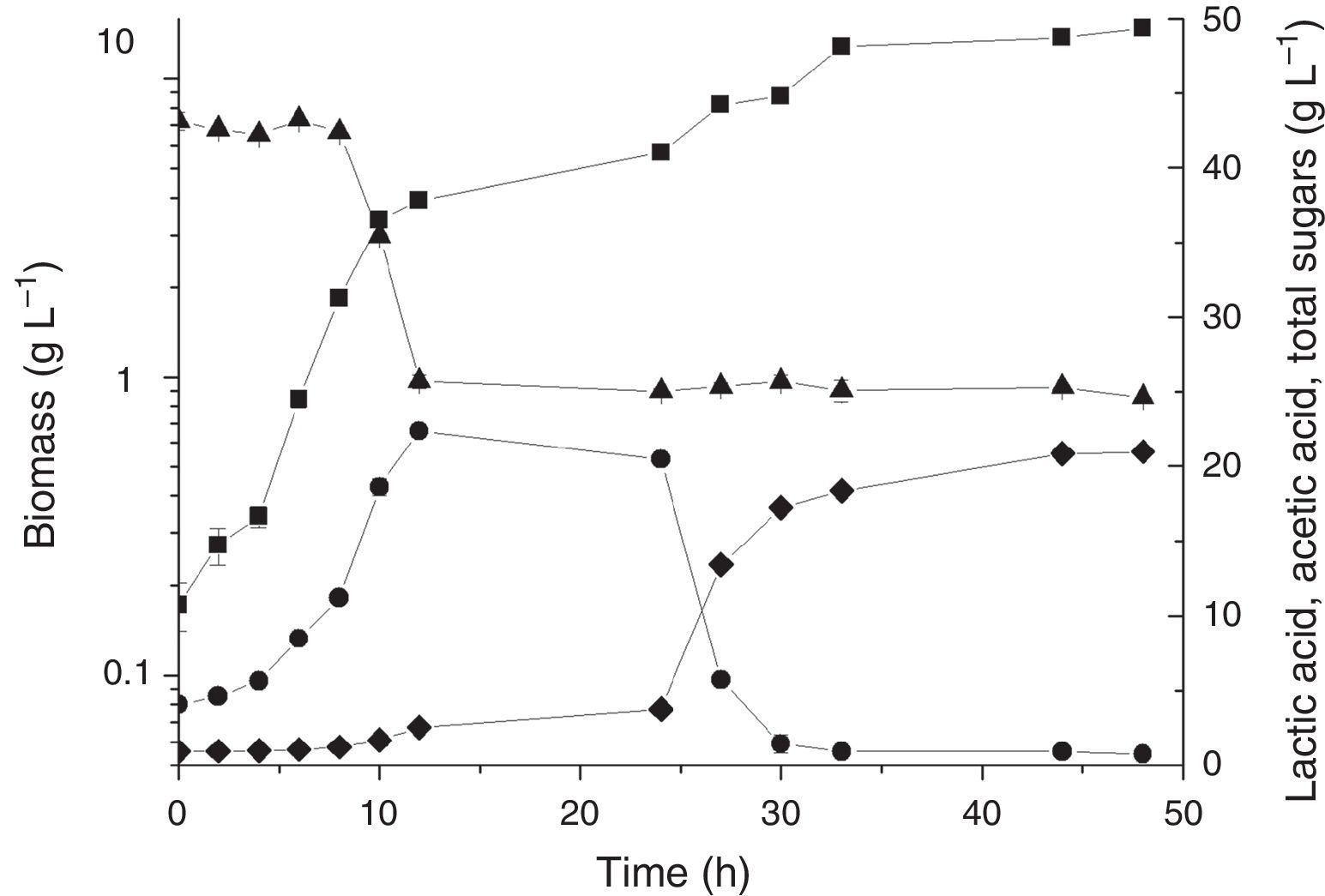
Experiments with DOC at a minimum of 30% saturationAlthough aeration and agitation speed of turbines in the bioreactor were applied to guarantee adequate oxygen supply to cultures, the DOC may eventually fall below the critical oxygen concentration, which would cause a shift from aerobiosis to anaerobiosis. Therefore, we decided to perform cultures under the best conditions, except for aeration, which was always maintained above 30% of DOC using a cascade loop in the aeration speed. The results of these experiments using the best conditions obtained for the PB design (Fig. 1) and for the saccharification (Fig. 2) are shown in Figs. 4 and 5, respectively. Fig. 4 shows a maximum biomass production of 14.86±0.19gL−1, which was approximately 37% higher than in conditions without DOC control. Lactic acid also increased up to 22.34±0.3gL−1 (compared to 19.56gL−1). In the experiments where the LAPRS was hydrolysed (saccharification), the biomass was 17.87±0.25gL−1, which was an increase of approximately 33.5% relative to the condition without DOC control, whereas lactic acid peaked at 37.59±0.4gL−1 (Fig. 5). Under the condition of DOC controlled at 30% of saturation, lactic acid was completely consumed to form biomass and acetic acid, which are the two products at the end of cultivation. The resulting biomass in the saccharification experiment is the highest amount thus far reported in the literature (Fig. 5).
Time course of batch cultivation of L. plantarum BL011 under DOC of 30% saturation or higher. Medium composition (gL−1): MgSO4·7H2O, 0.2; MnSO4·H2O, 0.04; LAPRS, 40 (total sugars); yeast extract, 15. Culture conditions: 25°C±1; 4.5vvm; 200rpm, pH 5.5±0.2 (■) dry cell weight; (●) lactic acid concentration; (♦) acetic acid concentration; (▴) total sugars concentration. The results are the mean of duplicates.
Time course of batch cultivation of L. plantarum BL011 in hydrolysed LAPRS under DOC of 30% saturation or higher. Medium composition (gL−1): MgSO4·7H2O, 0.2; MnSO4·H2O, 0.04; LAPRS, 40 (total sugars); yeast extract, 15. Culture conditions: 25°C±1; 4.5vvm; 200rpm, pH 5.5±0.2; (■) dry cell weight; (●) lactic acid concentration; (♦) acetic acid concentration; (▴) total sugars concentration. The results are the mean of duplicates.
The liquid acid protein residue of soybean (LAPRS), a vegetable carbon substrate, was successfully used to produce biomass of L. plantarum BL011 as a source of a potential probiotic culture for food applications. Lactic acid was also produced in large amounts, which should be explored if its production is of interest. The production levels of biomass and lactic acid were high when compared to other reports in the literature. The Plackett–Burman design provided an efficient method for the screening of the important cultivation variables of aeration, agitation speed, DOC, and the oxygen volumetric mass transfer, thus allowing for future scaling-up experiments. An innovative culture medium lacking lactose or any animal-derived sources for the production of microorganisms could help the development of new industrial processes where lactose-free and animal-free ingredients are required. Further research should be performed to test different bacteria or yeasts in this substrate.
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank CNPq and CAPES (Brazil) for the financial support and scholarships for this work.