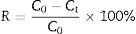Cellulosimicrobium cellulans CWS2, a novel strain capable of utilizing benzo(a)pyrene (BaP) as the sole carbon and energy source under nitrate-reducing conditions, was isolated from PAH-contaminated soil. Temperature and pH significantly affected BaP biodegradation, and the strain exhibited enhanced biodegradation ability at temperatures above 30°C and between pH 7 and 10. The highest BaP removal rate (78.8%) was observed in 13 days when the initial BaP concentration was 10mg/L, and the strain degraded BaP at constant rate even at a higher concentration (50mg/L). Metal exposure experimental results illustrated that Cd(II) was the only metal ion that significantly inhibited biodegradation of BaP. The addition of 0.5 and 1.0g/L glucose enhanced BaP biodegradation, while the addition of low-molecular-weight organic acids with stronger acidity reduced BaP removal rates during co-metabolic biodegradation. The addition of phenanthrene and pyrene, which were degraded to some extent by the strain, showed no distinct effect on BaP biodegradation. Gas chromatography–mass spectrometry (GC-MS) analysis revealed that the five rings of BaP opened, producing compounds with one to four rings which were more bioavailable. Thus, the strain exhibited strong BaP degradation capability and has great potential in the remediation of BaP-/PAH-contaminated environments.
Polycyclic aromatic hydrocarbons (PAHs) are well known organic pollutants with 2–7 fused benzene rings and are specially recognized for properties of carcinogenicity, teratogenicity and mutagenicity. Typically, compounds with more aromatic ring structures are associated with higher toxicity and pose greater threats to human beings, animals, plants and microorganisms.1 Among the 16 priority PAHs listed by the US Environmental Protection Agency (EPA), benzo(a)pyrene (BaP), with five benzene rings, was notable as the first reported carcinogen and is regarded as representative of PAHs.2 As a highly hydrophobic, non-volatile compound, BaP is degradation-resistant and more inclined to accumulate in the environment over time, which leads to considerable environmental concern.3 PAHs are common hazardous contaminants in environmental assessment reports and organic pollutant-related studies and widely exist in surface water, soil and even groundwater in many regions of China. Zhao et al.4 conducted a comprehensive investigation of PAHs in agricultural soil in the coal production area surrounding Xinzhou, China. The BaP equivalent of seven carcinogenic PAHs (major carcinogenic contributors including BaP) accounted for more than 99% of the 16 PAH concentrations. Liao et al.5 found that the BaP concentration of coking plant soils could reach 110±18.0mg/kg, while the contamination depth reached more than 10m. Wang et al.6 noted a peak BaP concentration in groundwater from a coal gangue stack area of 46.4ng/L. In addition, because BaP is strongly adsorbed onto soil, it exhibits reduced mobility and bioavailability, thus slowing biodegradation, especially in deep soil where oxygen is scarce. Remediation measures for BaP contamination are urgently needed and technologies for the removal of BaP are in high demand.
As an cost-effective method, bioremediation techniques do not require secondary treatment and can be applied at large scales.7 BaP is easily degraded under aerobic conditions in soil, and typical microorganisms reported to degrade BaP are white-rot fungi, Lasiodiplodia theobromae, algae, Rhodococcus, Alcaligenes denitrificans, Sphingomonas sp., and Stenotrophomonas maltophilia.8 However, oxygen is generally insufficient in real cases due to consumption during the biodegradation of aromatics with fewer rings, leaving a considerable amount of BaP in anaerobic environments.9 Furthermore, anaerobic or anoxic limitations cause great concern for the degradation of BaP in deep soil and groundwater. Thus, anaerobic biodegradation of BaP is viewed as an imperative technique in practical site contamination operations. Recent studies have demonstrated that BaP can be degraded without oxygen by microorganisms in river sediment by adding electron acceptors, surfactant or co-metabolic substrates.10 Thus far, only a minority of BaP-degrading bacteria under anaerobic conditions have been reported to be isolated successfully. Liang et al.11 isolated one facultative anaerobe Pseudomonas sp. JP1, which can degrade a variety of PAHs with 3–5 rings except pyrene under nitrate-reducing conditions. The BaP removal rate was only 30% after 40 days of cultivation at a concentration of 10mg/L. In the research mentioned above, the challenge of BaP anaerobic biodegradation lies in the scarcity of available degrading bacteria, and therefore, the isolation of anaerobic degradation bacteria has become a research topic of great interest.
As a cellulolytic and hemicellulolytic microorganism, Cellulosimicrobium sp. produces a variety of degradative enzymes (β-glucosidase, protease, glycoside hydrolase and chitinase), and is widely applied in biodegradation of celluloses and xylans and in alcoholic fermentation.12 Naeem et al.13 used an indigenous chromium-resistant bacterial strain A8 identified as a Cellulosimicrobium sp. in tannery effluent treatment and 98.6% of Cr(VI) was reduced to the less toxic Cr(III) form at the concentration of 900mg/L (with a 1800mg/L tolerance limit), illustrating that Cellulosimicrobium sp. has a strong tolerance to high concentrations of heavy metal ions. Due to the unique properties of fibrolytic enzymes, Cellulosimicrobium sp. could be utilized in the biodegradation of BaP under anaerobic conditions.
Microbial activities can be influenced in various ways by the presence of metal ions. In particular, heavy metal ions may have a pernicious effect on degrading bacteria used for BaP biodegradation. Chen et al.14 investigated the degradation process of BaP in water and discovered that degradation ability was inhibited when the concentration of copper (Cu(II)) ions was lower than 1mg/L or higher than 10mg/L. Biswas et al.15 studied PAH biodegradation by native PAH-degrading bacteria in a mixture of heavy metals and concluded that bioavailable cadmium (Cd) was the main source of toxicity for degrading bacteria. One metal-immobilizing organoclay was found to be able to reduce (Cd) toxicity and enhance PAH biodegradation. Additionally, temperature, time, solution pH and available carbon source all affect the microbial activity during biodegradation. In this work, a BaP-degrading strain of Cellulosimicrobium was isolated from PAH-contaminated soil in a coking plant under nitrate-reducing conditions. The degradation performance under various environmental conditions was investigated.
Materials and methodsPreparation of PAH stock solutionBaP, phenanthrene and pyrene were purchased from Sigma Chemicals, USA (purity>97%). The PAHs were dissolved in acetone (CG, JT Baker USA) and stocked at a concentration of 1000mg/L. All PAH stocks were sealed in amber vials, wrapped with aluminum foil and stored at 4°C prior to use.
Preparation of culture mediumLuria-Bertani (LB) medium was used for bacterial enrichment and contained yeast extract (0.5%), tryptone (1%) and NaCl (1%). Mineral salt medium (MSM) was used as the degradation solution and contained (g/L) Na2HPO4·10H2O (10.08), KH2PO4 (1.5), NH4Cl (1), NaNO3 (1), MgSO4·7H2O (0.2), FeSO4·7H2O (0.03), CaCl2 (0.02) and 1mL microelement solution. The composition of the microelement solution was as follows (mg/100mL): H3BO3 (5.7), MnSO4·5H2O (4.3), ZnSO4·7H2O (4.3), CuSO4·5H2O (4.0), (NH4)6Mo7O24·4H2O (0.37), and CoCl2·6H2O (0.25).
All media were sterilized in an autoclave at 121°C for 30min.
Isolation and identification of BaP-degrading bacteriaSoil samples were collected at a depth of 40–80cm in a coking plant in Beijing that has been contaminated with PAHs for nearly 45 years. The total concentration of 16 USEPA priority PAHs reached 313.86mg/kg (dry weight, dw), including phenanthrene (19.6%), anthracene (6.2%), fluoranthene (26.3%), pyrene (18.1%), benzo(a)anthracene (6.5%), benzo(b)fluoranthene (8.5%), benzo(k)fluoranthene (3.0%), BaP (5.6%) and indeno(1,2,3-cd)pyrene (6.0%). BaP stock was added to the soil to guarantee BaP as the dominant contaminant, with a final concentration of 100mg/kg. The soil was sealed with pure nitrogen (99.9999%) following acetone evaporation and incubated at 25°C in the dark. BaP, as the sole external carbon source, and nitrate as the electron acceptor were added every six months to induce BaP biodegradation for 10 years. The subsequent isolation techniques were adopted from Dou et al.16 Soil samples with BaP-degrading bacteria were mixed with BaP solution (25mg/L) and the bacteria were transferred into supernatant fluid in the soil-water matrix. BaP-degrading strains were then inoculated into a BaP-supplemented MSM-agar plate (25mg/L) for further purification.
The isolated strains were identified using 16S rDNA sequence analysis. DNA was extracted from isolated microbial cells and amplified using the polymerase chain reaction (PCR). PCR amplification was conducted using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCA-3′) and 1492R (5′-TACCTTGTTACGACTT-3′) with a concentration of 20μM for both.17 Each amplification reaction was completed using 25μL PCR Master Mix, 2μL 27F, 2μL 1492R, 2μL DNA lysate, and 19μL double-distilled H2O (dd H2O). Amplification was conducted for 28 cycles (94°C for 30s, 55°C for 40s, and 72°C for 90s) in a 100-series thermal cycler after an initial denaturation at 94°C for 6min. The PCR products were tested by the Beijing Biomed Co., Ltd. The 16S rDNA sequences were aligned with previously published sequences from the GenBank database of National Center for Biotechnology Information (NCBI) using the BLAST program (http://blast.ncbi.nlm.nih.gov).
The growth characteristics of the isolated strainBased on its BaP degradation ability, one strain was selected as the target strain and used for further studies. The strain was subject to biological and ecological characterization using optical microscope and scanning electron microscope observations. Due to the importance of the carbon source for bacteria growth, various carbon sources (glucose, sucrose, soluble starch and high-density corn amylodextrins) were added to the MSM to investigate the growth behavior of the isolated strain. The results were compared with its performance on LB medium.
Culture conditionsThe isolated strain previously cultivated in 100mL LB medium at 35°C for 24h with a shaking speed of 200r/min was centrifuged at 6000r/min for 15min. The cell pellets were washed with MSM solution three times to remove residual LB and resuspended in MSM medium and used as inocula for the following BaP-degradation experiments.
BaP-biodegradation experimentBiodegradation experiments were performed in detest oxygen bottles (100mL). The PAH stock solution in acetone was spiked into bottles with MSM (20mL) broth, and the bottles were placed in a fume hood for two hours to allow complete evaporation of acetone. Dissolved oxygen in the bottle was removed by adding Na2S·9H2O (0.2g) after the evaporation of acetone. The isolated strain was then inoculated into the medium. The bottles were capped with suba-seal silicone septa, wrapped with aluminum foil to prevent photolysis and incubated at 200r/min under anaerobic conditions. Samples from individual bottle were collected at the desired time to measure residual BaP, and the removal rates of BaP were calculated to elucidate the optimal conditions. All experiments were conducted in triplicate, and error bars were calculated as the standard deviations of the measurements.
The effects of temperature, pH, BaP concentration and initial bacterial mass and individual metal ions on BaP removal rates by the isolated strain were investigated. The incubation temperatures were set at 20, 25, 30 and 35°C with a initial BaP concentration of 25mg/L. Also at a BaP concentration of 25mg/L, the initial pH of the medium was adjusted to 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 by adding 0.1M NaOH or 0.1M HCl. Five graded initial BaP concentrations at 2.5, 5.0, 10.0, 25.0 and 50.0mg/L were studied individually. The initial bacterial masses were regulated by adding various amounts of inocula. The ion concentration of Fe(II), Zn(II), Cu(II), Mn(II), Hg(II), Co(II), Pb(II) and Cd(II) was 10mg/L by adding inorganic compounds of Co(NO3)2, FeSO4, MnCl2, CuCl2, PbCl2, Zn(NO3)2, HgCl2 and CdCl2 with 10mg/L BaP in the medium, samples only contain BaP were employed as control.
All experiments about concentration and metal ions were conducted in a shaker at 200r/min at 35°C with broth pH adjusted to 7. Parallel samples in detest oxygen bottles without any inoculation were employed as blank control.
The co-metabolic biodegradation behavior of BaP by the isolated strain was also investigated. The readily utilizable carbon source glucose, in five serial concentrations, and succinic acid, d-malic acid, l-malic acid, citric acid and tartaric acid at a concentration of 10mg/L were individually chosen as co-metabolic substrates. PAHs with similar structure to BaP were added to test the carbon source utilization patterns (priorities of utilization) of the isolated strain. Three-ring and four-ring PAHs, phenanthrene and pyrene were used as representatives for the co-degradation studies. The total concentration of PAHs was adjusted to 10mg/L (1.5mg/L, 3.5mg/L and 5mg/L for phenanthrene, pyrene and BaP, respectively). The results were compared with the BaP degradation performance when BaP was the sole carbon source (BaP in control).
Research on degradation mechanism of BaPThe BaP metabolites during biodegradation process were extracted with dichloromethane after biodegradation of ten-days. The extracts were dried over anhydrous sodium sulfate, concentrated and filtered prior to analysis by gas chromatography–mass spectrometry (GC–MS).18 GC–MS analyses were carried out on Agilent equipped with a HP-5MS fused silica column (30m×0.25mm×0.25μm). Helium in 1.0mL/min was used as the carrier gas. The oven temperature program was as follows: 100°C for 1min, then linearly increased at a rate of 15°C/min up to 180°C, 180–310°C at a ramp of 5°C/min, and maintained at 310°C for 2min. The spilt flow was 24mL/min, injector temperature was 250°C. The mass spectra were recorded at 1scan/s under electronic impact with electron energy of 70eV. Mass range was between m/z 30–450. A library search was carried out by using NIST11.
Analytical method for PAHsSamples from bottles were extracted twice with 20mL cyclohexane each time (CG, JT Baker USA). In each extraction, the mixture was vigorously shaken for 10min and subjected to ultrasonic extraction in a 25°C water bath at 40Hz for 15min. The mixture was allowed to phase separate, and the organic phase was collected. The two organic solvents were combined, and residual water was removed by adding anhydrous sodium sulfate. The solution was further diluted to the appropriate BaP concentration with HPLC-grade methanol and filtered through a nylon Millipore membrane filter (0.22μm). The PAHs were identified using HPLC with a UV detector at 254nm and a C18 reverse-phase column (dimensions 4.6mm×250mm, I.D., 5-μm particle size); methanol (CG, JT Baker USA) and ultrapure water (90/10, v/v) was used with a flow rate of 1.0mL/min as the mobile phase. The injection volume was 20μL. The entire process took 20min, and the concentration of each PAH was calculated from the curve based on the peak area with various known concentrations.
The PAH degradation rate (%) was calculated as follows:
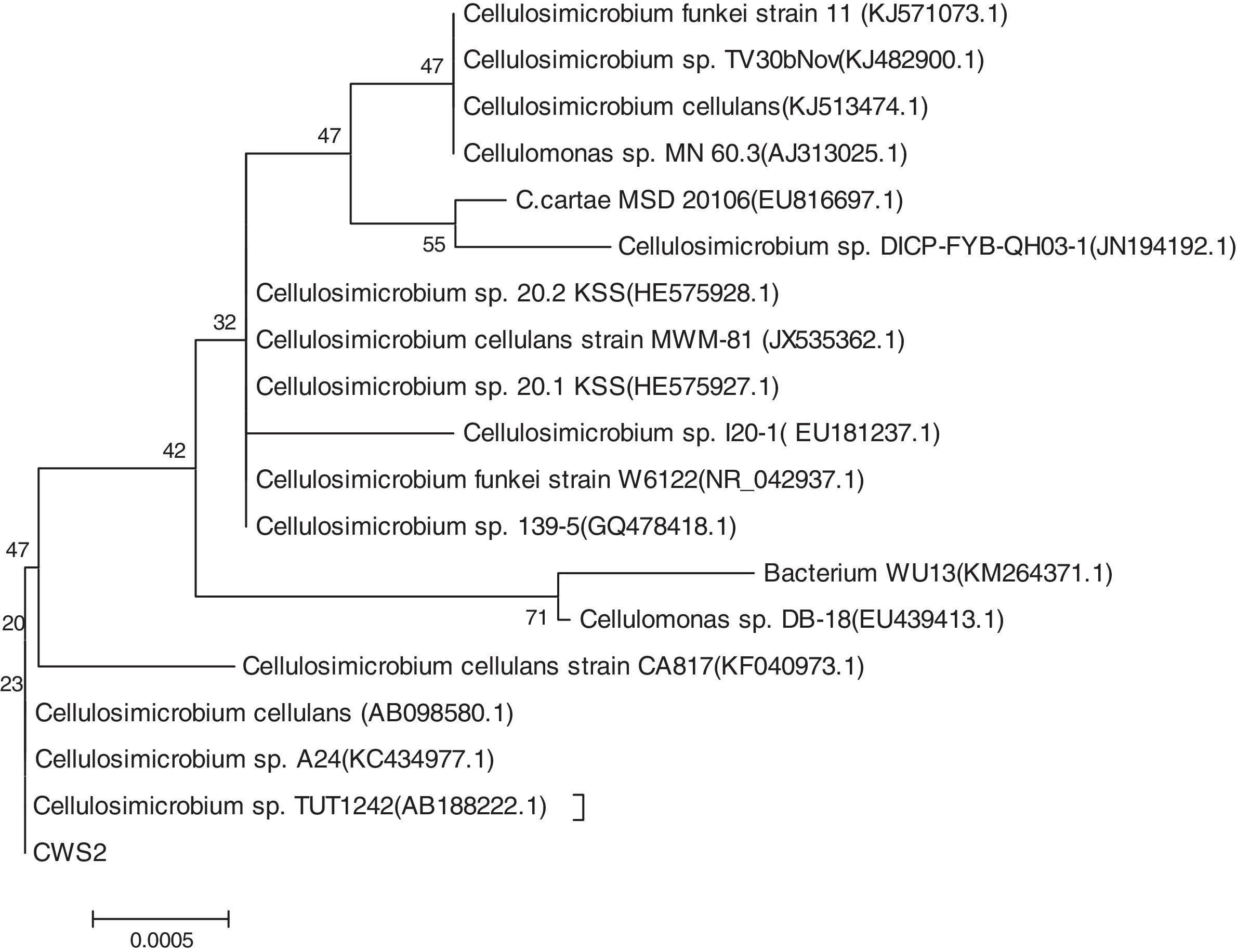
where R represents the removal ratios (%), and C0 and Ct are the initial and final PAH concentrations. All measurements were the mean values of three replicates.ResultsIsolation and characterization of BaP-degrading strainA bacterial strain using BaP as its sole carbon and energy source under nitrate-reducing conditions was isolated with plate-screening techniques after repeated domestication and enrichment. The strain was identified using 16S rDNA sequence analysis and was named CWS2. During the partial 16S rDNA sequence determination, approximately 656 base pairs of the sequence were deposited in the GenBank database. Fig. 1 shows a phylogenetic tree and the closest relatives of CWS2 listed according to the GenBank database. Based on the results, the strain CWS2 proved to have a strong relationship with Cellulosimicrobium sp. TUT124 (656bp, 99% identical), Cellulosimicrobium sp. A24 (655bp, 99% identical), and Cellulosimicrobium cellulans (655bp, 99% identical).
The CWS2 colonies on solid plates were yellow, round, non-transparent, smooth, and wet with a regular edge, and the surface of the colonies was uplifted. A single bacterium was rod-shaped with a diameter of 0.2–0.3μm and lengths of 0.6–1.0μm. The strain was determined to be Gram-positive and catalase-positive, and its oxidase activity was negative. According to these characteristics and instructions from “Bergey's Manual of Systematic Bacteriology,”19 the strain CWS2 showed high similarity to Cellulomonas. Therefore, based on the genetic results, the physiological and biochemical characteristics of the strain, the isolated strain CWS2 was recognized as a Cellulosimicrobium sp. and was named as C. cellulans CWS2. The genomic information for C. cellulans CWS2 was deposited in the GenBank under the accession number KX496338.
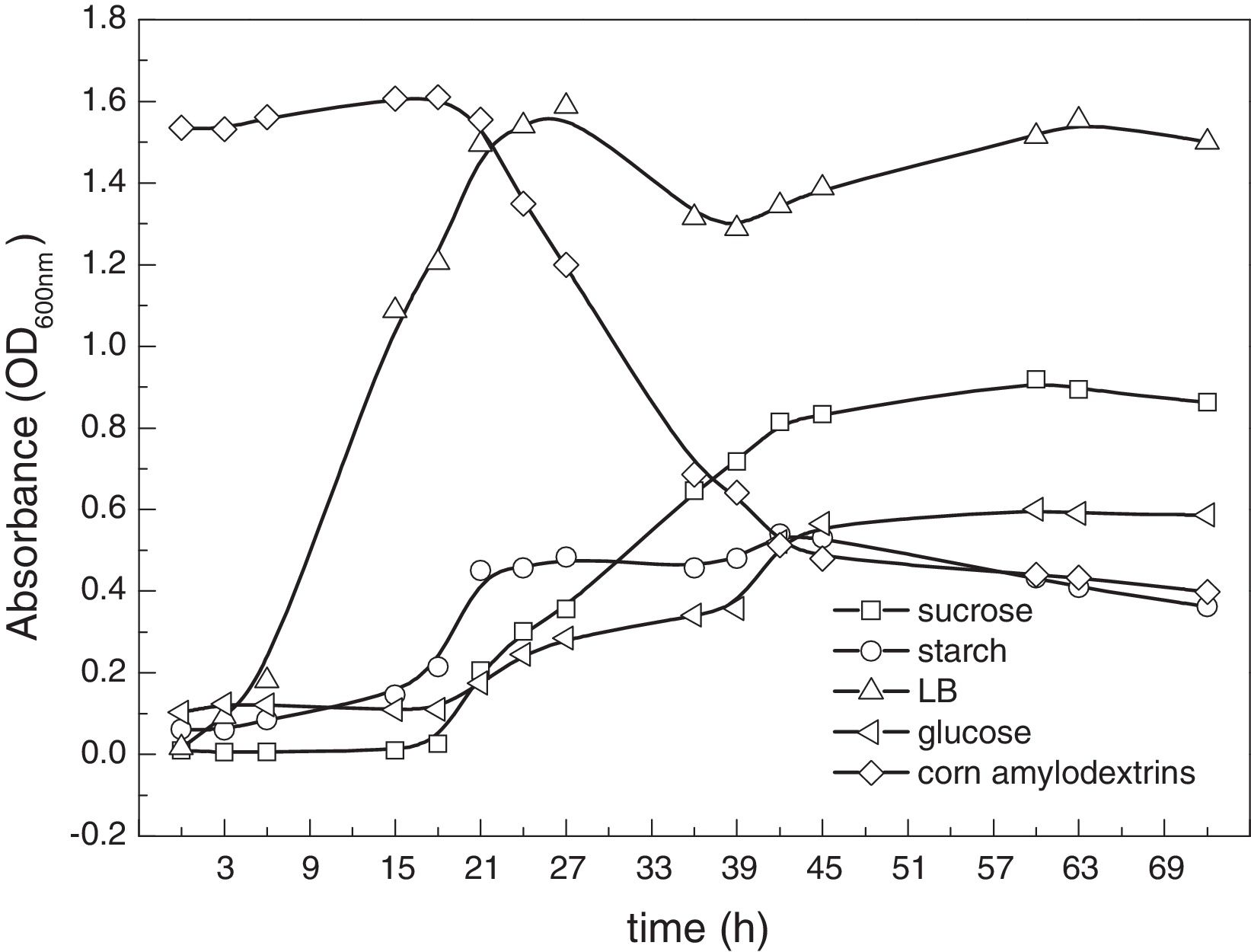
To further investigate its growth behavior, CWS2 was cultivated on other carbon sources including glucose, sucrose, soluble starch and corn amylodextrins. From the growth curves of the strain CWS2 (Fig. 2), different lag phases were exhibited and the longest was 16h. Specifically, CWS2 began growing immediately from the time of inoculation and the cell number reached a peak within 24h in LB medium. A 2nd growth period was observed after a relatively short attenuated phase (between 33h and 42h). When starch was used, the cell number increased slowly after 6h and the cell density significantly increased between 15 and 24h. Among all carbon sources, CWS2 demonstrated the longest lag phase when cultivated in glucose, soluble starch and corn amylodextrin media. Interestingly, these all showed a similar linear growth period between 24 and 42h in the exponential phase. According to the overall growth behavior, LB and sucrose media provided more favorable environments for strain CWS2, and the cell density indicated higher bacterial biomass. The cell biomass of CWS2 cultured in LB medium was fourfold higher than biomass cultured in glucose medium at the stationary phase. Thus, LB medium was selected for further bacterial proliferation.
Growth curves of Cellulosimicrobium cellulans CWS2 in different MSM mediums (sucrose, starch, glucose and corn amylodextrins). All the experimental settings followed anaerobic cultivation requirements and the temperature was controlled constantly at 35°C. Bacterial cell numbers are estimated through the optical density measured at a wavelength of 600nm (OD600). For the curve of corn amylodextrins, the higher the OD600nm the higher concentration of corn amylodextrins due to its color, and the OD600nm decrease as the bacteria grow using the corn amylodextrins as carbon source.
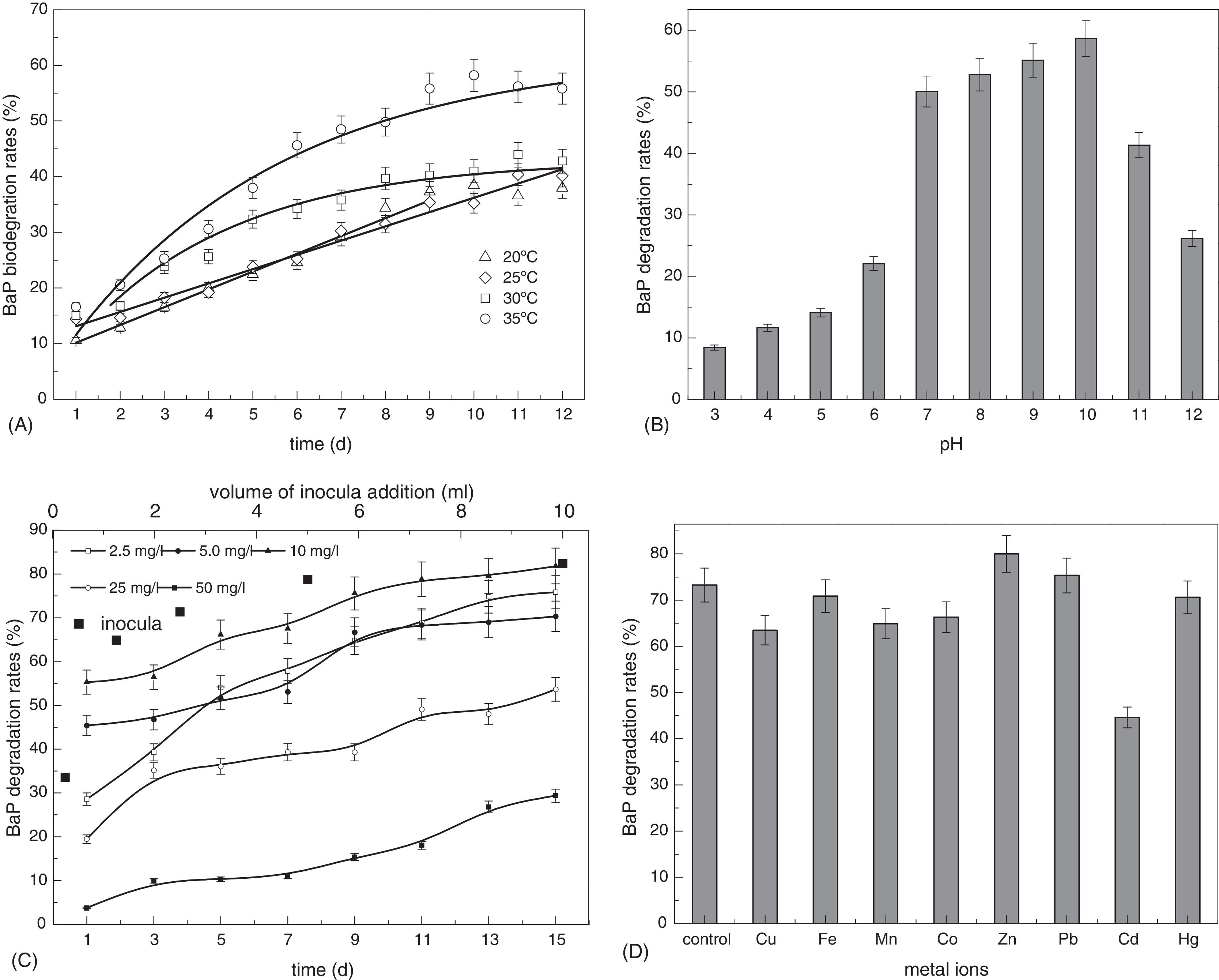
A BaP concentration of 25mg/L was adopted in the investigation of temperature influence on bacterial behavior. As shown in Fig. 3A, no significant difference was observed in BaP degradation behavior at 20 and 25°C within 12 days. BaP was degraded faster at both 30 and 35°C in the first 5 days compared to the other two temperature settings, but differences in degradation speed under the two conditions were widening on the fifth day. The degradation rate of BaP at 35°C was 18.0% higher than that at 30°C after 12 days. The degradation ability at 20 or 25°C was significantly lower than 30 and 35°C during the whole degradation process. The biodegradation kinetics at 20 and 25°C followed a zero-order rate model; the equations were y=6.96+3.19t, R2=0.9897 (y is the biodegradation rate (%) at time t and t is the degradation time) and y=10.60+2.56t, R2=0.977, respectively. The degradation capacity at 30°C and 35°C obeyed first-order kinetics, and the equations were y=43.04–43.04e(−0.28t) (R2=0.9163) and y=62.10−62.10e(−0.20t) (R2=0.9290). According to the fitting curves, BaP biodegradation rates using CWS2 below 30°C remained stable and were not related to the BaP concentration remaining in the medium. This indicated that degradation ability was only constrained by temperature when all other factors were suitable under the experimental settings. On the first day, nearly equivalent degradation rates (15%) were observed when the temperature was 25, 30 or 35°C. This indicated that the bacteria exhibited similar adaptation ability under these environments. Notably, almost the same degradation rate (between 38.0% and 42.8%) was obtained on the 12th day when the temperature was 20, 25 or 30°C. When temperature exceeded 30°C, the higher temperature was correlated with higher biodegradation ability for CWS2, and the total amount of BaP degraded also increased. Based on the experimental results, CWS2 cultured at 35°C was adapted in subsequent BaP degrading tests.
Effects of environmental factors on BaP biodegradation by Cellulosimicrobium cellulans CWS2 under anaerobic conditions. (A) Effect of temperature on BaP biodegradation. (B) Effect of solution pH on BaP biodegradation. (C) Effects of different initial concentrations and different volume of inocula addition on BaP biodegradation. (D) Effect of co-existing metal ions on BaP biodegradation. The initial BaP concentration was 25mg/L for all the batches in (A) and (B). The temperature was maintained at 35°C for all except in (A), and samples were measured at day 10 and 13 in (B) and (D), respectively. The solution pH was 7.0 in (C) and (D). The initial BaP concentration was 10.0mg/L in (D). The error bars represent the standard deviations of triplicate sample measurements.
As shown in Fig. 3B, C. cellulans CWS2 exhibited higher BaP biodegradation efficiency under neutral and mildly alkaline conditions. Higher BaP degradation rates were observed when the solution pH ranged from 7 to 10, and the BaP biodegradation ability by CWS2 proceeded as pH 10>pH 9>pH 8>pH 7, indicating that to a certain extent, CWS2 was favorably adaptable to pH changes. After 10 days of treatment, the BaP removal rate was 58.7% at pH 10, but only 50.0% at pH 7. BaP removal declined sharply to below 25.0% when pH was lower than 6 due to restrained activity of the degrading enzyme. In contrast, CWS2 appeared to adapt to highly alkaline environments and obtained a better removal rate with a pH as high as 12. According to these results and the typical pH values in natural water environments, pH 7 was selected for the degradation of CWS2 with high efficiency and low cost.
To examine the response of strain CWS2 to BaP concentrations, different amounts of BaP were added to the media and varying biomass of the isolated strain was dosed. As seen from Fig. 3C, there were significant differences in BaP degradation performance among the five graded concentrations. The order of BaP removal rates was 10.0mg/L≈5.0mg/L>2.5mg/L>25.0mg/L>50.0mg/L. CWS2 degraded 55.3% BaP in one day when the initial BaP concentration was 10mg/L. In contrast, 53.7% BaP was degraded by the strain in fifteen days when the concentration increased to 25.0mg/L. Thus, BaP concentration proved to be a major factor for degradation, as it directly affected the reaction time to a great extent. In particular, four degradation curves (initial BaP concentration of 2.5mg/L excluded) showed a similar rising trend within 13 days; these curves were nearly parallel. This is in accord with the typical bacterial degradation performance and indicated that the contaminants were removed at a stable level once the microorganisms were fully activated. However, when contaminants were limited to below 2.5mg/L, the bacteria degraded them at a high initial rate but failed to maintain this rate due to the limitations of remaining BaP. The final BaP degradation rate obtained at a BaP concentration of 2.5mg/L was 75.8%, close to the highest rate of 78.8% (initial BaP concentration of 10mg/L). The degradation curves at 50.0mg/L showed that CWS2 maintained a constant degradation speed even at very high BaP concentrations; 15mg/L of BaP was consumed in 15 days. It is predicted that the pace can be continuously maintained as exhibited in the low-BaP concentration curves. Thus, strain CWS2 possessed a strong BaP-degradation ability.
Degradation tests were carried out at a BaP concentration of 10.0mg/L at 35°C and pH 7 for 15 days. The biomass dosages added at 20mL MSM were 0.25, 1.25, 2.5, 5.0 and 10.0mL. The results in Fig. 3C indicate that biomass could be a major limiting factor in BaP biodegradation and should be properly studied when bacteria are used to remediate BaP-contaminated soil or water.
Metallic ions are typically simultaneously present in contaminated water with PAHs, and thus, their roles in the decomposition of BaP by strain CWS2 were investigated. Fig. 3D shows how various metallic ions influenced biodegradation. Most ions (not including Zn(II)) inhibited BaP biodegradation, and the inhibitory effect of Cd(II) was most obvious. The trace elements Fe(II), Zn(II), Cu(II), Mn(II) and Co(II) are important for organisms to maintain cellular functions and normal physiological activities. However, high concentrations of those ions result in serious side effects. As shown in Fig. 3D, BaP biodegradation was inhibited by Cu(II), Mn(II) and Co(II) in a limited range (removal ratios decreased below 10%). While the strain CWS2 demonstrated tolerance to Fe(II) and Zn(II). The strain exhibited obviously higher tolerance to Zn(II) due to the fact that the BaP removal rate increased under current experimental settings. In this study, no obvious inhibition effect on BaP degradation was observed with the addition of Pb(II) and Hg(II).
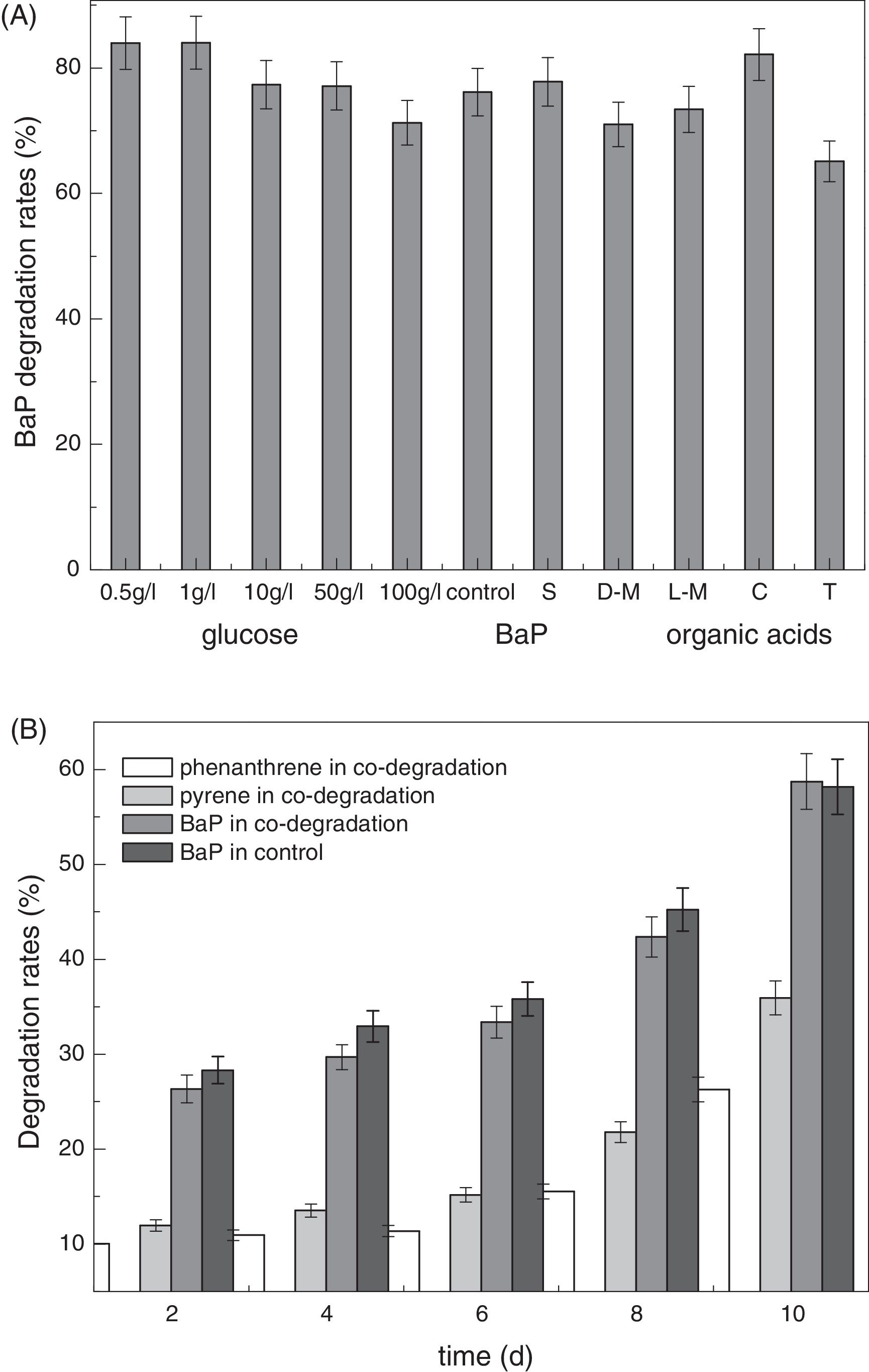
Effect of additional carbon sources on BaP biodegradationAs a common energetic material, glucose is easily biodegradable and is utilized preferentially by bacteria in the presence of various carbon sources, particularly in anaerobic environments such as fermentation conditions. Accordingly, glucose is frequently used as a co-substrate to promote microorganism growth for degradation of organic pollutants and has also been widely used as a co-substrate for anaerobic biodegradation of PAHs.10 As discussed above, strain CWS2 began to thrive after a lag phase of 16h in a glucose substrate, and the bacteria reached maximum biomass in 45h. As illustrated in Fig. 4A, the proper addition of glucose can enhance BaP degradation. Glucose at concentrations of 0.5 and 1.0g/L had a positive effect on BaP biodegradation, and the removal efficiency of BaP increased by 10% compared with the control. However, no evident increase in BaP removal efficiency was observed when the glucose concentration was above 10g/L. BaP degradation was slightly inhibited once the glucose concentration surpassed 50g/L. Thus, when glucose served as the carbon source for bacterial growth, proper addition can enhance BaP biodegradation. LMW organic acids are ideal carbon sources for most microorganisms, as they are metabolic intermediates of various carbon sources. It is observed in Fig. 4A that the addition of LMW organic acids had different effects on the degradation of BaP. Relatively, citric acid promoted BaP biodegradation, while tartaric acid slightly inhibited degradation. Other organic acids (succinic acid, d-malic acid, l-malic acid and tartaric acid) influenced BaP degradation in a limited range.
Effects of co-substrates on BaP anaerobic biodegradation by Cellulosimicrobium cellulans CWS2. The initial BaP concentration was 10mg/L. The concentrations of phenanthrene and pyrene added into the medium were 2.5 and 7.5mg/L, respectively. The S, D-M, L-M, C and T represented succinic acid, d-malic acid, l-malic acid, citric acid and tartaric acid, respectively. The error bars represent the standard deviations of triplicate sample measurements.
In Fig. 4B, phenanthrene and pyrene were degraded to different degrees by CWS2. However, the degradation rates of phenanthrene were significantly lower than that for pyrene, and both were much lower than the BaP degradation rates. In comparison, the BaP degradation capability of strain CWS2 revealed no significant difference in mixed PAHs solution or BaP-only solution. Thus, we concluded that strain CWS2 had a high selectivity for BaP among mixed PAHs. The biodegradation rates of phenanthrene and pyrene remained unchanged in the first 6 days and then increased. The degradation patterns of BaP under both conditions were similar, as the degradation rate increased from approximately 26% at the second day to approximately 59% at the tenth day.
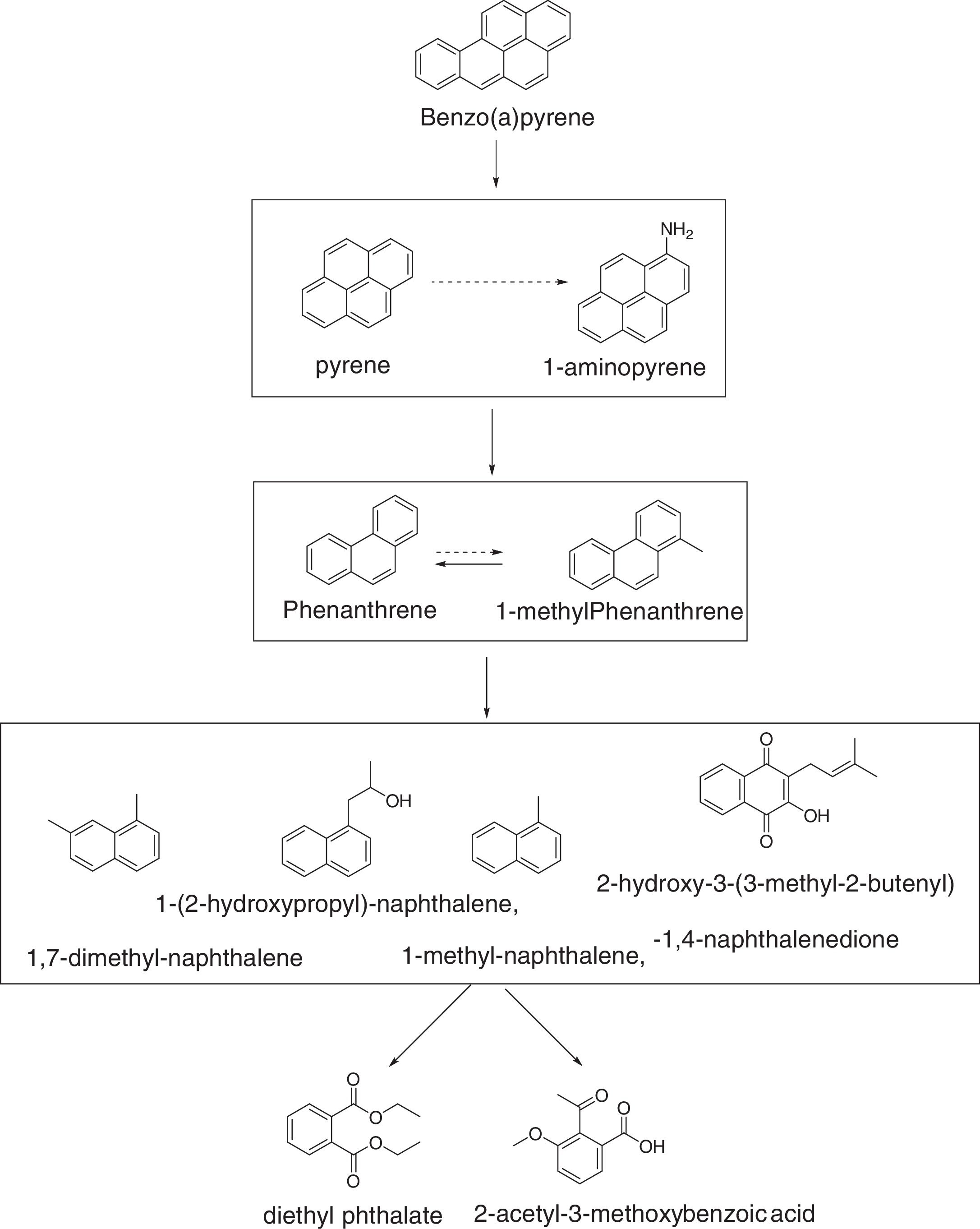
Degradation mechanism of BaPThe qualitative analysis of the metabolic intermediates of BaP biodegradation showed that some new peaks were turned up in the chromatograms of samples compared with that of blank control. Several identical products were detected based on the GC–MS library search and they were pyrene, 1-aminopyrene, phenanthrene, 1-methylphenanthrene, 1,7-dimethylnaphthalene, 1-(2-hydroxypropyl)naphthalene, 1-methylnaphthalene, 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthalenedione, diethyl phthalate, and 2-acetyl-3-methoxybenzoc acid. A possible biodegradation pathway of BaP was proposed based on the catalysis of enzymes to damage rings structures as Fig. 5 shown.
In our test, pyrene and 1-aminopyrene were the initial intermediate products detected which were not generated from the first opening of BaP, and the relationship between pyrene and 1-aminopyrene were still unclear. Phenanthrene and 1-methylphenanthrene were intermediate products with three rings. Though four products with two rings were detected, it was difficult to figure out how they were degraded. Finally, these compounds were broken down to some benzene compounds with methoxy and carboxyl.
DiscussionIsolation and characterization of BaP-degrading strainThe isolated strain was initially identified as Cellulosimicrobium sp., and some related studies on degradation of organics have been reported. Liu et al.20 isolated a strain capable of high phenol tolerance from rhizosphere soil in a sewage-treatment plant, and ideal degradation rates were reached when the phenol concentration was 500–1500mg/L. Analysis based on 16S rDNA gene sequences demonstrated that the strain belonged to C. cellulans. Davolos and Pietrangeli21 studied bacterial strains in samples from a PAHs wastewater treatment in Italy and detected Cellulosimicrobium. The Cellulosimicrobium species listed above all showed similar behaviors as the strain we identified, thus the strain Cellulosimicrobium has the potential to degrade PAHs.
Biodegradation of BaPTemperature and pH affect not only the physiological growth of microorganisms but also the activity of their enzymes. Although microorganisms can survive under wide temperature and pH ranges (even in extreme environments), appropriate temperature and pH conditions must be determined to maximize growth and related physiological activities. In this way, functional microbes can be thoroughly utilized in industrial applications. As observed in the BaP degradation by strain CWS2, the pH of the medium decreased due to microbial production of acid metabolites. The substantial accumulation of low-molecular-weight (LMW) organic acids from sources of microbial metabolism changed pH markedly and influenced metabolic pathways.22 These metabolic pathways were also affected by carbon sources, and accumulation of metabolites was also related to the amount of carbon sources. Thus, the type and concentration of substrate played an important role in the growth of microorganisms.
Metal ions are essential elements for microorganisms and are critical to enzymatic activities; these ions will thus affect microbial metabolism (catabolism and anabolism). However, they can be harmful or even lethal to microorganisms when improper metal ions bind to the enzyme, resulting in inactivation and denaturation. The biodegradation of BaP by microorganisms in a solution is typically completed by two steps: absorption to the microbial cells surface, and transport into cells followed by metabolism.23 The uptake of heavy metals by organisms operates via the same mechanism: adsorption of metal ions on the cell surface and intracellular binding of heavy metals to thiol-containing compounds. The results from Chen et al.24 indicated that the activities of manganese peroxidase and lignin peroxidase were generally inhibited by Cd. Cd(II) is cytotoxic to cells and directly and severely impacts enzymatic activities involved in bacterial metabolism. Chen et al.25 investigated the removal mechanisms of BaP with Cu(II) at the cellular interface of Stenotrophomonas maltophilia and concluded that the cell wall was the main adsorption site for Cu and functioned as a barrier for the transportation of BaP. A study of mechanisms of Cu- and Zn-binding using Pseudomonas putida CZ1 through chemical modification of biomass by Chen et al.26 indicated that carboxyl functional groups in the cell walls played an important role in the binding of metal. Thus, there is a high probability that acidic metabolites of the microbes with carboxyl groups assisted in Cu(II) adsorption and reduced the transport of BaP. In the metabolism of BaP, there must be intermediates containing carboxyl groups followed by decreased BaP degradation rates. While the heavy metals Hg(II) and Pb(II) will denature proteins and lead to a loss of vitality of the microorganisms.27
Effect of additional carbon sources on BaP biodegradationBaP is not only classified as macromolecule organic matter but also as a refractory organic compound, and its toxic properties may have a strong suppression effect on the growth of microbes.28 In addition, BaP was degraded by microorganisms mainly through two routes: consumption as the sole carbon and energy source or as an alternative carbon source. Recent studies showed that BaP could be degraded by mixed bacteria, but most indicated that BaP is degraded as a co-metabolic substrate under anaerobic conditions.9 Ambrosoli et al.10 reported that phenanthrene and pyrene degradation ability were enhanced remarkably when 1g/kg glucose was added as a co-substrate. Ye et al.29 drew an opposite conclusion: sucrose exerted a suppressive effect on anthracene biodegradation. As stated above, strain CWS2 effectively utilizes BaP as a sole carbon and energy source. However, both were observed in our experimental settings, which illustrated that the amount of glucose was the primary determinant on whether BaP biodegradation will be enhanced by glucose addition.
The biodegradability of organics is mainly dependent on the complexity of their chemical structures and corresponding physicochemical properties. Organics with simpler structures will be easily used. Organic acids in our experiments had similar structures. The complexity order of the carbon sources is citric acid<tartaric acid<malic acid<succinic acid. However, the biodegradation ratios for strain CWS2 obtained from these acids did not follow this order. As discussed above, BaP biodegradation rates by CWS2 dramatically decreased when the pH of the medium was below 10. The order of pH values for these solutions was tartaric acid<citric acid<malic acid<succinic acid, which was positively correlated with degradation rates (except citric acid). Thus, citric acid addition contributed to promote the BaP degradation.
The molecular structure of PAHs determines their stability and hydrophobicity and is the main internal factor in controlling biodegradability. Generally, LMW PAHs tend to be more easily biodegraded than high-molecular-weight PAHs.30 However, microorganisms do not always follow such trends, especially domesticated bacteria used for a specific function. In our study, the isolated strain preferentially degraded BaP with different removal rates of phenanthrene and pyrene, which might occur for the reason that phenanthrene and pyrene are intermediate products in BaP metabolism. Indeed, both PAHs were detected in limited amounts in studies of BaP biodegradation behavior by CWS2.
Degradation mechanism of BaPPrevious study have reported that naphthalene, phenanthrene, pyrene and other PAHs were completely oxidized to carbon dioxide by mixed bacteria through denitrification.31 While researches on degradation of PAHs by single strain bacteria under anaerobic conditions were seldomly reported, particularly the biodegradation of HMW PAHs. Liang et al.11 firstly investigated the degradation pathway of BaP by Pseudomonas sp. JP1 under anaerobic conditions. He pointed out that the first opening of BaP ring generated 1,12-dimethyl-benzo(a)anthracene, 7,8,9,10-tetrahydrobenzo(a)pyrene, and 5-ethylchrysene, and then further degraded to pyrene, phenanthrene and 1-methylphenanthrene. Qin et al.32 also proposed the anaerobic degradation pathway of BaP by Microbacterium sp., the products were 4,5-dihydrobenzo(a)pyrene and 7,8,9,10-tetrahydrobenzo(a)pyrene during the process of first opening of BaP ring. In our test, only pyrene, phenanthrene and 1-methylphenanthrene were the same intermediates found in the above pathway, thus the degradation pathway was different from the Pseudomonas sp. JP1.
ConclusionC. cellulans CWS2 was isolated from PAH-contaminated soil in a coking plant. The strain uses BaP as the sole carbon and energy source under nitrate-reducing conditions. Temperature and pH of the broth were the main factors affecting BaP removal, followed by the BaP concentration. The highest BaP removal rate (78.8%) was observed in 13 days when the initial BaP concentration was 10mg/L, and the strain degraded BaP at a constant rate even at a higher concentration (50mg/L). In the metal exposure experiment, only Cd(II) significantly inhibited biodegradation among other metal ions. Appropriate addition of glucose enhances BaP biodegradation, while stronger LMW organic acids reduce degradation ability. Phenanthrene and pyrene were also degraded to various extents by the strain in the presence of BaP. Lastly, a possible degradation pathway of BaP under anaerobic conditions was proposed. Thus, the strain exhibited a strong BaP degradation capability under natural conditions and has great potential in the remediation of BaP-/PAH-contaminated environments.
Conflicts of interestThe authors declare that they have no conflict of interest.
This work was supported by the National Natural Science Foundation of China (51579010) and the Fundamental Research Funds for the Central Universities (2014NT32).