Clostridium difficile is the leading cause of infectious diarrhoea in hospitalized patients. The aim of this study was to determine the risk factors important for the development of hospital-acquired Clostridium difficile-associated disease and clinical manifestations of Clostridium difficile-associated disease. The clinical trial group included 37 hospitalized patients who were selected according to the inclusion criteria. A control group of 74 hospitalized patients was individually matched with cases based on hospital, age (within 4 years), sex and month of admission.
Clostridium difficile-associated disease most commonly manifested as diarrhoea (56.76%) and colitis (32%), while in 8.11% of patients, it was diagnosed as pseudomembranous colitis, and in one patient, it was diagnosed as fulminant colitis. Statistically significant associations (p<0.05) were found with the presence of chronic renal failure, chronic obstructive pulmonary disease, cerebrovascular accident (stroke) and haemodialysis. In this study, it was confirmed that all the groups of antibiotics, except for tetracycline and trimethoprim–sulfamethoxazole, were statistically significant risk factors for Clostridium difficile-associated disease (p<0.05). However, it was difficult to determine the individual role of antibiotics in the development of Clostridium difficile-associated disease. Univariate logistic regression also found that applying antibiotic therapy, the duration of antibiotic therapy, administration of two or more antibiotics to treat infections, administering laxatives and the total number of days spent in the hospital significantly affected the onset of Clostridium difficile-associated disease (p<0.05), and associations were confirmed using the multivariate model for the application of antibiotic therapy (p=0.001), duration of antibiotic treatment (p=0.01), use of laxatives (p=0.01) and total number of days spent in the hospital (p=0.001). In this study of patients with hospital-acquired diarrhoea, several risk factors for the development of Clostridium difficile-associated disease were identified.
Clostridium difficile is the leading cause of infectious diarrhoea in hospitalized patients.1 The clinical manifestations of C. difficile-associated disease (CDAD) range from asymptomatic colonization, mild diarrhoea, colitis, and life-threatening pseudomembranous colitis (PMC) to toxic megacolon, sepsis and death.2 These infections lead to prolonged hospitalizations, increased morbidity and mortality, and high health care costs. Diseases caused by this bacterial species are an increasing clinical challenge because of the emergence of resistant strains to metronidazole.3 Previous studies have suggested that administration of antibiotics, old age, multiple co-morbid conditions, previous hospitalizations, long stays in hospitals (facilities), and medical and surgical procedures are risk factors for developing the disease (CDAD).1,3,4 Some studies have indicated that administration of fluoroquinolones and proton pump inhibitors contribute to the emergence of CDAD.5 Disruption of the protective role as a barrier of the normal intestinal flora is common to many of these factors.
ObjectiveThe aim of this study was to determine the risk factors important for the development of hospital-acquired CDAD and its clinical manifestations.
Materials and methodsThe study (a prospective, case–control study) was performed at the Institute of Public Health of the Nis, Serbia, Centre for Microbiology from January 2013 to June 2014.
The clinical trial group included 37 hospitalized patients (Clinical Centre of Nis, Serbia) with diagnoses and only one episode of CDAD. The selection of patients was based on the following criteria:
- •
Presence of at least three unformed or liquid stools over 24h for at least 2 days.
- •
Hospitalization for at least 48h before the appearance of unformed or liquid stools.
- •
Cultivation of toxigenic strains of C. difficile from examined stool samples.
- •
A stool sample positive for C. difficile toxins.
The control group consisted of 74 hospitalized patients with diarrhoea, whose stool specimens were negative for C. difficile and toxins A/B. The patients in the control group were hospitalized for at least 48h in the clinics of the Clinical Centre in Nis. The control patients were individually matched with cases based on hospital, age (within 4 years), sex and month of admission.
In this prospective study, we processed data from the clinical trial and control group patients. Data were collected from the microbiological laboratories (type of isolated microorganism, production of toxins) and from the clinics of the Clinical Centre of Nis (sex, age, therapy applied during the 60 days before diarrhoea and its duration), including the following data: antibiotics, cytostatics, corticosteroids, X-ray therapy, non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, proton pump inhibitors, histamine 2 receptor antagonists, anticoagulants, laxatives, calcium channel blockers, antiarrhythmics, diuretics, oral drugs for diabetes, and invasive gastrointestinal procedures applied in the previous 3 months (colonoscopy, gastroscopy, nasogastric tube, enemas), the number of visits and total time spent in the hospital before the occurrence of CDAD, stay in the intensive care unit, underlying morbidities, and surgery in the previous 3 months. The laboratory data were collected from the day when stool was sent for C. difficile testing (white blood cell [WBC] count >20,000/μL, serum glucose level >150mg/dL, creatinine level >2mg/dL, alanine aminotransferase [ALT] levels >40IU, serum albumin level <2.5g/dL).
Daily lab reports (lab lists and protocols) about the isolation of pathogens in microbiological laboratories and medical documentation (histories of diseases, temperature lists) were used as source data.
Standard microbiological procedures were applied during bacteriological examinations of stool samples. Stool samples were inoculated on nutrient selective media and cycloserine–cefoxitin–fructose agar (CCFA) (Biomedics, Parg qe tehnicologico, Madrid, Spain) for C. difficile cultivation after alcohol-shock procedure application.
CCF agar was incubated at 37°C under anaerobic conditions for 48h. AnaeroGen sachets (AnaeroGen, OXOID, United Kingdom) were used to create anaerobic condition in jars. Anaerobic strips (Anaerobic indicator, OXOID, United Kingdom) were used to verify anaerobic conditions. A commercial API system for anaerobic bacteria (API 20A BioMerieux, France) was applied for the biochemical identification of C. difficile isolates (typical colonies were 4mm or larger in diameter, elevated, and convex, with a discrete margin, an irregular surface and a strong horse manure-like odour).
C. difficile toxins A and B were detected in stool specimens by the MINIVIDAS Clostridium difficile Toxin A/B test (BioMerieux, France). C. difficile toxin A was detected in stool specimens by the ColorPAC Toxin A test (Becton Dickinson, USA). Colonies of C. difficile were subcultivated in 5mL of brain-heart infusion broth under anaerobic conditions over four days. After incubation, liquid cultures of C. difficile were centrifuged at 3000×g for 15min. Determination of toxins was performed using previously cited tests according to the manufacturer's instruction. The same procedure was applied to the liquid cultures of reference strains C. difficile ATCC 43598 (A−/B+) and C. difficile ATCC 43255 (A+/B+), cultivated in brain-heart infusion broth under anaerobic conditions within four days.
The diagnosis of CDAD was based on the presence of the following criteria6:
- •
Diarrhoeal stools or toxic megacolon and a positive laboratory assay for C. difficile toxin A and/or toxin B in stools a toxin-producing C. difficile detected in stool via culture or other means.
Clinical manifestations of CDAD were defined as recommended by Kuijper et al.6
The SPSS (version 15) statistical package was used for the statistical analysis. The chi-square test (or Fisher's exact test as appropriate) and the t-test were used to compare demographics, co-morbid conditions, and antibiotic use between the cases and controls. Logistic regression models were used to identify patient-related risks. Variables found to be statistically significant by a univariate analysis were then entered into a multivariate logistic regression analysis to identify independent risk factors for the development of CDAD. A p-value <0.05 was defined as significant.
ResultsData were collected for the 37 patients with CDAD, and the data were compared with those for the 74 patients without CDAD. The sex ratios (M/F) for the case/control patients were 20/17 and 40/34, respectively. CDAD most commonly manifested clinically as diarrhoea without colitis (56.7%). There was only one case of fulminant colitis, which had a fatal outcome (Table 1). The MINIVIDAS C. difficile Toxin A/B test was positive for the presence of toxins in the stool samples of 37 patients with diarrhoea. The ColorPAC Toxin A test was positive for toxin A in the stool samples of one patients with diarrhoea. The isolates of 36 patients with diarrhoea were positive for toxins A and B (A+/B+). The isolates of one patient were positive for only toxin B (A−/B+) (Table 1).
The clinical manifestations of disease caused by Clostridium difficile and the production of toxins A and B by C. difficile isolates.
| The clinical manifestation | Production of certain toxins of C. difficile isolates | Numbers of patients | ||
|---|---|---|---|---|
| Excretion of toxins A and B (A+/B+) | Excretion of only toxin B (A−/B+) | n | % | |
| Numbers of patients/isolates n % | Numbers of patients/isolates n % | |||
| Diarrhoea without colitis | 21 (100) | 0 | 21 | 56.7 |
| Colitis | 11 (91.6) | 1 (8.3) | 12 | 32.4 |
| Pseudomembranous colitis | 3 (100) | 0 | 3 | 8.1 |
| Fulminant colitis | 1 (100) | 0 | 1 | 2.7 |
| Toxic megacolon | 0 | 0 | 0 | 0 |
| Total | 36 (97.3) | 1 (2.7) | 37 | 100 |
Based on the collected data, the primary diseases that were most prevalent among hospitalized patients with CDAD were hypertension, diabetes, chronic renal failure and cerebrovascular accident (stroke). In the majority of patients (54.0%), there was a presence of two or more primary diseases. The presence of chronic renal failure, chronic obstructive pulmonary disease and cerebrovascular accident (stroke) were significantly different, p<0.05. The presence of haemodialysis was statistically significant (Table 2).
Underlying diseases and conditions of hospitalized patients with CDAD and hospitalized patients in the control group.
| Underlying disease | Patients with CDAD (N=37) | Patients in the control group (N=74) | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Hypertension | 10 | 27.0 | 24 | 32.4 | 0.56 |
| Diabetes mellitus | 6 | 16.2 | 13 | 17.5 | 0.85 |
| Malignancy | 4 | 10.8 | 4 | 5.4 | 0.30 |
| Chronic obstructive pulmonary disease | 5 | 24.3 | 2 | 2.7 | 0.04 |
| Chronic renal failure | 8 | 21.6 | 2 | 2.7 | 0.002 |
| Haemodialysis | 7 | 18.9 | 2 | 2.7 | 0.006 |
| Peritoneal dialysis | 1 | 2.3 | 0 | 0 | 0.33 |
| Stroke, cerebrovascular accident | 10 | 27.0 | 8 | 10.8 | 0.029 |
| Schizophrenia | 2 | 5.4 | 4 | 5.4 | 0.65 |
| Other non-infectious diseases of the gastrointestinal tract | 6 | 16.2 | 12 | 16.2 | 0.95 |
| Choledocholithiasis | 1 | 2.3 | 2 | 2.7 | 1.0 |
| Hyperthyroidism | 1 | 2.3 | 2 | 2.7 | 1.0 |
| Thrombocytopenia | 1 | 2.3 | 0 | 0 | 0.33 |
| Built-in AV artificial valve | 1 | 2.3 | 0 | 0 | 0.33 |
| Absolute arrhythmia | 1 | 2.3 | 0 | 0 | 0.33 |
| Chronic cellulitis | 1 | 2.3 | 0 | 0 | 0.33 |
Univariate logistic regression in hospitalized patients identified the following significant risk factors for CDAD: applied antibiotic therapy (OR=30.13, 95% C.I.: 7.87–115.36, p=0.0001); duration of antibiotic therapy (OR=6.1, 95% C.I.: 1.6–23, p=0.001); administration of two or more antibiotics in the treatment of infections (OR=2.97, 95% C.I.: 1.19–3.04, p=0.015); administration of laxatives (OR=2.38, 95% C.I.: 1.39–4.06, p=0.001); and the total number of days spent in the hospital (OR=1.54, 95% C.I.: 1.31–1.82, p=0.001) (Table 3).
Univariate analysis of risk factors monitored in patients.
| Variable | Case group N=37 n (%) or mean±S.D. | Control group N=74 n (%) or mean±S.D. | OR | 95 C.I. | p |
|---|---|---|---|---|---|
| Sex, M/F | 20/17 | 40/34 | 1.00 | 0.70–1.44 | 0.83 |
| Age (years old) | 63.4±17.5 | 64.1±16 | 1.03 | 1.00–1.05 | 0.64 |
| Treatment | |||||
| Antibiotics | 34 (91.8) | 19 (25.6) | 30.13 | 7.87–115.36 | 0.0001 |
| Duration of antibiotic therapy (each day for the duration) | 21±5.5 | 12±4 | 6.1 | 1.6–23 | 0.001 |
| Two or more antibiotics | 20 (54.0) | 21 (28.3) | 2.97 | 1.19–3.04 | 0.015 |
| X-ray | 1 (2.7) | 1 (1.3) | 2.03 | 0.13–31.09 | 0.80 |
| Chemotherapy | 2 (5.4) | 3 (4.0) | 1.35 | 0.23–7.64 | 0.87 |
| Steroid treatment | 4 (10.8) | 9 (12.6) | 0.96 | 0.32–2.92 | 0.61 |
| Non-steroidal anti-inflammatory drugs | 7 (18.9) | 10 (13.5) | 1.40 | 0.58–3.38 | 0.45 |
| Angiotensin-converting enzyme (ACE) inhibitors | 8 (21.6) | 13 (17.5) | 1.23 | 0.56–2.70 | 0.60 |
| Beta blockers | 6 (16.2) | 19 (25.6) | 0.84 | 0.41–1.74 | 0.64 |
| Anticoagulants | 7 (18.9) | 13 (17.5) | 1.08 | 0.47–2.47 | 0.86 |
| Laxatives | 19 (51.3) | 16 (21.6) | 2.38 | 1.39–4.06 | 0.001 |
| Calcium channel blockers | 4 (10.8) | 4 (5.4) | 2.0 | 0.53–7.55 | 0.43 |
| Antiarrhythmics | 5 (13.5) | 9 (12.6) | 1.16 | 0.42–3.21 | 0.49 |
| Diuretics | 6 (16.2) | 8 (10.8) | 1.56 | 0.58–4.17 | 0.56 |
| Diabetes mellitus drugs | 10 (27.0) | 14 (18.9) | 1.43 | 0.70–2.90 | 0.46 |
| Proton pump inhibitors | 8 (21.6) | 9 (12.6) | 1.99 | 0.75–4.23 | 0.30 |
| Histamine 2 receptor antagonists | 13 (35.1) | 15 (20.2) | 2.13 | 0.92–3.25 | 0.14 |
| Medical interventions | |||||
| Colonoscopy | 6 (16.2) | 11 (29.7) | 1.11 | 0.44–2.72 | 0.92 |
| Gastroscopy | 4 (10.8) | 10 (13.5) | 0.78 | 0.27–2.38 | 0.47 |
| Nasogastric tube | 7 (18.9) | 7 (9.4) | 2.0 | 0.76–5.28 | 0.26 |
| Surgery | 10 (27.0) | 12 (16.2) | 1.67 | 0.79–3.50 | 0.27 |
| Enema | 11 (29.7) | 13 (17.5) | 1.99 | 0.84–3.41 | 0.22 |
| Hospitalization | |||||
| Stay in the intensive care unit | 21 (56.7) | 28 (37.8) | 2.16 | 1.0–2.25 | 0.059 |
| Number of hospital stays | 5±1 | 3±0.5 | 1.46 | 0.91–2.33 | 0.11 |
| Total number of days spent in the hospital | 64±15 | 29±10 | 1.54 | 1.31–1. 82 | 0.001 |
| Laboratory data | |||||
| White blood cell (WBC) count >20,000/μL | 17 (45.9) | 21 (28.3) | 2.15 | 0.98–2.68 | 0.067 |
| Glucose level >150mg/dL | 5 (13.5) | 9 (12.6) | 1.13 | 0.40–3.08 | 0.84 |
| Creatinine >2mg/dL | 4 (10.8) | 2 (2.7) | 4.36 | 0.77–20.85 | 0.18 |
| Alanine aminotransferase (ALT) >40IU | 7 (18.9) | 10 (13.5) | 1.49 | 0.58–3.38 | 0.45 |
| Albumin <2.5g/dL | 18 (48.6) | 22 (29.7) | 2.24 | 1.01–2.65 | 0.0513 |
Factors analyzed by univariate logistic regression were introduced into the multivariate model with adjustment for all the analyzed parameters. Statistically significant risk factors for the risk of CDAD included the following: applied antibiotic therapy (OR=160.60, 95% C.I.: 7.34–359.87, p=0.001); duration of antibiotic therapy (OR=1.4, 95% C.I.: 1.50–2.43, p=0.01); laxatives (OR=1.58, 95% C.I.: 1.09–3.23, p=0.01); and total number of days spent in the hospital (OR=1.14, 95% C.I.: 1.06–1.23, p=0.001) (Table 4).
Multivariate analysis of risk factors for hospitalized patients.
| Variable | OR | 95 C.I. | p |
|---|---|---|---|
| Antibiotics | 160.60 | 7.34–359.87 | 0.001 |
| Duration of antibiotic therapy (each day for the duration) | 1.4 | 1.50–2.43 | 0.01 |
| Two or more antibiotics | 2.2 | 0.8–5.9 | 0.099 |
| Laxatives | 1.58 | 1.09–3.23 | 0.01 |
| The total number of days spent in the hospital | 1.14 | 1.06–1.23 | 0.001 |
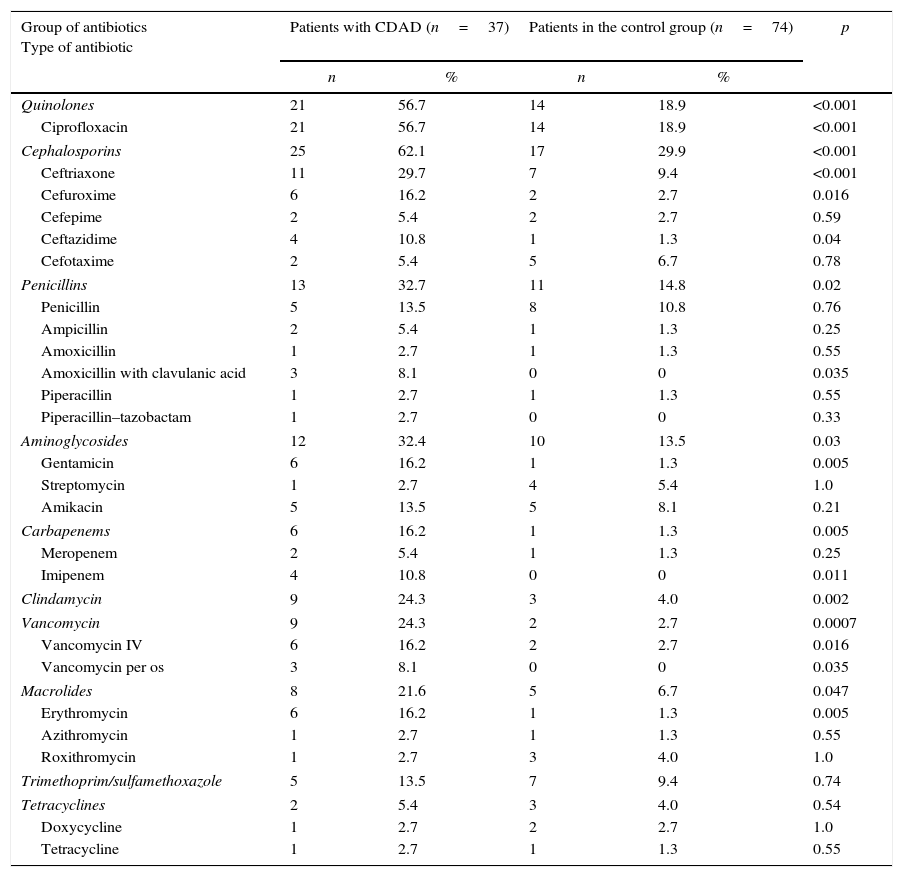
In the treatment of infections in hospitalized patients, the most commonly used antibiotics were from the groups of cephalosporins, quinolones, penicillins and aminoglycosides. In addition to the tetracycline group antibiotics and trimethoprim–sulfamethoxazole, there was a statistically significant difference for all the other groups of antibiotics compared to the control group of hospitalized patients (p<0.05) (Table 5). Statistical analysis of the main data on individual antibiotics administered to hospitalized patients found that ciprofloxacin, ceftriaxone, gentamicin, clindamycin and erythromycin were factors associated with a high risk of developing CDAD (p<0.01). Additionally, other antibiotics (0.01≤p<0.05) including imipenem, vancomycin administered orally and intravenously, cefuroxime, ceftazidime, and amoxicillin with clavulanic acid, were found to be significant risk factors (Table 5).
Representation and statistical comparison of groups of and certain individual antibiotics administered to hospitalized patients with CDAD and hospitalized patients from the control group.
| Group of antibiotics Type of antibiotic | Patients with CDAD (n=37) | Patients in the control group (n=74) | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Quinolones | 21 | 56.7 | 14 | 18.9 | <0.001 |
| Ciprofloxacin | 21 | 56.7 | 14 | 18.9 | <0.001 |
| Cephalosporins | 25 | 62.1 | 17 | 29.9 | <0.001 |
| Ceftriaxone | 11 | 29.7 | 7 | 9.4 | <0.001 |
| Cefuroxime | 6 | 16.2 | 2 | 2.7 | 0.016 |
| Cefepime | 2 | 5.4 | 2 | 2.7 | 0.59 |
| Ceftazidime | 4 | 10.8 | 1 | 1.3 | 0.04 |
| Cefotaxime | 2 | 5.4 | 5 | 6.7 | 0.78 |
| Penicillins | 13 | 32.7 | 11 | 14.8 | 0.02 |
| Penicillin | 5 | 13.5 | 8 | 10.8 | 0.76 |
| Ampicillin | 2 | 5.4 | 1 | 1.3 | 0.25 |
| Amoxicillin | 1 | 2.7 | 1 | 1.3 | 0.55 |
| Amoxicillin with clavulanic acid | 3 | 8.1 | 0 | 0 | 0.035 |
| Piperacillin | 1 | 2.7 | 1 | 1.3 | 0.55 |
| Piperacillin–tazobactam | 1 | 2.7 | 0 | 0 | 0.33 |
| Aminoglycosides | 12 | 32.4 | 10 | 13.5 | 0.03 |
| Gentamicin | 6 | 16.2 | 1 | 1.3 | 0.005 |
| Streptomycin | 1 | 2.7 | 4 | 5.4 | 1.0 |
| Amikacin | 5 | 13.5 | 5 | 8.1 | 0.21 |
| Carbapenems | 6 | 16.2 | 1 | 1.3 | 0.005 |
| Meropenem | 2 | 5.4 | 1 | 1.3 | 0.25 |
| Imipenem | 4 | 10.8 | 0 | 0 | 0.011 |
| Clindamycin | 9 | 24.3 | 3 | 4.0 | 0.002 |
| Vancomycin | 9 | 24.3 | 2 | 2.7 | 0.0007 |
| Vancomycin IV | 6 | 16.2 | 2 | 2.7 | 0.016 |
| Vancomycin per os | 3 | 8.1 | 0 | 0 | 0.035 |
| Macrolides | 8 | 21.6 | 5 | 6.7 | 0.047 |
| Erythromycin | 6 | 16.2 | 1 | 1.3 | 0.005 |
| Azithromycin | 1 | 2.7 | 1 | 1.3 | 0.55 |
| Roxithromycin | 1 | 2.7 | 3 | 4.0 | 1.0 |
| Trimethoprim/sulfamethoxazole | 5 | 13.5 | 7 | 9.4 | 0.74 |
| Tetracyclines | 2 | 5.4 | 3 | 4.0 | 0.54 |
| Doxycycline | 1 | 2.7 | 2 | 2.7 | 1.0 |
| Tetracycline | 1 | 2.7 | 1 | 1.3 | 0.55 |
The mechanisms of settlement, maintenance and transmission of toxigenic strains of C. difficile are complex and not fully understood. Epidemiological studies have suggested that strains of C. difficile are maintained in the intestinal tracts of humans and in hospitals. Carriers of C. difficile include up to 80% of healthy children, 3% of healthy adult individuals in outpatient conditions, and up to 20% of hospitalized patients with formed stools. Carriers of toxigenic strains are less frequent (0.2–0.9% of healthy adult humans) so that the colonization of spores already present in the digestive tract is relatively rare.7 The most likely means of the development of CDAD in hospitalized patients is the input of spores, which can persist for months in hospital environments and present a constant threat for the emergence of CDAD. Spores in hospitals appear from the digestive tracts of patients with asymptomatic carrier states and patients with CDAD, and they can persist for several months on a variety of surfaces and objects (floors, furniture, medical equipment, etc.).8 Despite regular cleaning, hospital rooms showed an 8% contamination rate for samples of C. difficile spores, with contamination being higher in rooms of patients with manifest CDAD than in the rooms of asymptomatic carriers (49 and 29%).9
Both the spores and the vegetative forms of C. difficile are usually transmitted from contaminated surfaces and objects to patients by the dirty hands of medical personnel.10 Transfer of spores is facilitated by their resistance to most disinfectants and antiseptics used. Some studies have indicated that direct entry of C. difficile into the intestinal tract is also possible through contaminated objects (e.g., thermometers).11 Once C. difficile appears in health facilities, it is easily spread and maintained, so that in certain areas, the same strains are found, but there have been studies showing that invasion by new strains from the outside environment is also possible.12
The primary requirement for the expression of the pathogenicity of toxigenic strains of C. difficile is a change in the normal relations of the bacteria colonizing the digestive tract, which constitute a barrier to the proliferation of pathogenic microorganisms. More than 90% of infections caused by C. difficile occur upon the administration of antibiotics. The effects of antibiotics disrupt the normal flora of the colon, allowing endogenous and exogenous microorganisms (C. difficile) to proliferate and colonize the colon.2
The presence of C. difficile in the intestinal tract leads to a number of different conditions, however, occasionally this type of bacteria does not cause any problems (such as asymptomatic colonization), but in patients with risk factors, it can lead to diarrhoea of varying severity, including life-threatening PMC, fulminant colitis and toxic megacolon. Severe forms of CDAD (PMC, fulminant colitis, toxic megacolon, etc.) usually occur in 3–5% of patients with manifest C. difficile infection.12 The most common clinical manifestation of CDAD in children is watery stool, but in 26% of children, blood can also appear in the stool. In one third of children, the disease can go into spontaneous remission, and the mortality rate is significantly lower than in adults. As in adults, CDAD in children can have a severe course, such as PMC and necrotizing enterocolitis, but these courses have occurred only in sporadic cases.13,14 CDAD usually manifests as diarrhoea in 60% of elderly patients for approximately ten days, while 32% of cases have been reported as prolonged illness (up to 18 days). Severe colitis with complications develops in 8% of patients.15
A study by Al-Eidan et al.,16 showed that the clinical manifestations of CDAD in most of hospitalized patients included diarrhoea, fever, abdominal pain, and leucocytosis. In eight patients, the diagnosis of PMC was confirmed by endoscopic examination, and nine patients with CDAD died during hospitalization. The results of research by Kyne et al.17 showed that toxigenic C. difficile isolates were cultured from samples of 87 patients, but CDAD did not develop in 14 patients (asymptomatic carriers). In 24% of 73 patients with CDAD, mild diarrhoea was present, 35.6% had a severe form of diarrhoea, and 39.7% had extended symptoms and more severe forms of CDAD. Six patients with prolonged diarrhoea had complications such as fulminant colitis, PMC and toxic megacolon. Colectomy was applied in the treatment of four patients. Three patients with colectomy died during the postoperative period in intensive care units, and one patient died within 3 weeks after surgery. The research by Changela et al.18 found that in 5 patients, the direct cause of death was C. difficile (pseudomembranous colitis, severe colitis with dehydration and fever, and multisystem organ damage with sepsis, and one patient had perforation of the intestines). The clinical manifestations of CDAD in immunocompromised patients are various, but the most commonly occurring clinical manifestations is diarrhoea of varying severity and duration. Fulminant CDAD has been described in patients with lung transplantation who received high doses of immunosuppressive corticosteroid drugs, as well as antibiotics, due to frequent lung infections. Due to immunosuppression, the digestive tracts of these patients do not form pseudomembranes as a manifestation of CDAD.19 In this study, undertaken in Nis, Serbia, CDAD most commonly manifested as diarrhoea, while 8.1% of patients were diagnosed with PMC and 2.7% of patients with fulminant colitis, which ended in death (Table 1).
However, the validity of the results of this study should be observed in the light of the limited sensitivity of the MINIVIDAS assay for diagnosis of CDAD. The sensitivity of MiniVidas assay is 63.9–93.8%, which is less that of modern PCR tests (98.3–100%).20 For this reason, the use of MINIVIDAS assay as a stand-alone test is not recommended. However, in this research, we used a two-step testing system to test C. difficile isolates. All these diagnostic methods provided validity to the results of research and the diagnosis of CDAD.
The reasons for the different clinical manifestations remain unknown, but the answer should probably be sought in the possible presence of other virulence factors in some strains of C. difficile, such as adhesion or secretion of hydrolytic enzymes or factors that depend on human hosts. Many researchers believe that host factors are more important than the virulence of the bacteria because asymptomatic children and adult carriers can be colonized with of the same toxigenic strain as patients with severe diarrhoea. Additionally, many children have high levels of toxins in their faeces, without the presence of diarrhoea or colitis. These claims have been supported by research confirming that the diseases caused by A−/B+ C. difficile could not be distinguished from the diseases caused by A+/B+ strains of C. difficile. Previous studies have confirmed that serum antibodies and local colon antibodies occurred in response to the presence of C. difficile in 60% of the population. Shim et al.21 indicated that asymptomatic colonization of C. difficile was associated with reduced risk of developing CDAD. However, this protective mechanism is not yet understood.
At the time of intestinal colonization by C. difficile, the levels of serum IgG antibodies to toxin A were significantly higher in asymptomatic carriers than among persons who developed CDAD (p<0.001).22 Additionally, higher levels of serum IgG antibodies to toxin B and antigens in asymptomatic carriers were determined, but this difference was not statistically significant. Levels of serum IgM antibodies for C. difficile were also significantly higher in asymptomatic carriers. The risk for developing CDAD is higher in patients with C. difficile colonization of the intestinal tract and low levels of serum IgG antibodies to toxin A. The risk was significantly higher if the patient also suffered from a severe underlying disease.22 Several studies have indicated that severe CDAD forms are associated with age (>70 years old), maximum leucocyte count >20,000cells/mL, minimum albumin level <2.5g/dL, maximum creatine level >2mg/dL and small bowel obstruction or ileus. Hypoalbuminaemia and elevated serum urea levels were independently associated with mortality.23,24
According to data from previous studies, 70–90% of diseases caused by C. difficile develop after the administration of antibiotics.1,3–5 The application of antibiotics in many cases results in the killing of the normal flora of the digestive tract, as confirmed by research conducted by Bignardi25 Research by Bartlett,26 performed in the late eighties of the last century, showed that CDAD usually occurred after the administration of clindamycin, penicillin (extended-spectrum effect) and cephalosporins. Analysing the results of research conducted during the nineties, Barbut10 noted that all types of antibiotics, except for aminoglycosides administered intravenously, led to the appearance of at least one case of CDAD. He also indicated that the most frequently administered antibiotics were lincosamine (clindamycin), extended-spectrum penicillins and cephalosporins. Over the last ten years, the representations of certain groups of antibiotics administered before the occurrence of CDAD have changed. Kuijper et al.6 in their research reported that, before the occurrence of CDAD, patients usually received antibiotics from the cephalosporin group (44.8% of patients), penicillin (39.8% patients), fluoroquinolones (13.3%), aminoglycosides (10.4%), macrolides (8.1%), carbapenems (7%), and clindamycin (8.9%). Similar results were published by Vesta et al.4 Before the occurrence of CDAD, antibiotics were usually administered in combinations of two or more, which increased the risk of developing the disease. This study also confirmed that all groups of antibiotics, except for tetracycline and trimethoprim–sulfamethoxazole (p>0.05), were statistically significant risk factors for CDAD in hospitalized patients (p<0.05) (Table 5).
Analysing the data on certain antibiotics administered in hospitalized patients before the occurrence of diarrhoea, Changela et al.18 determined the significant risk factors (p<0.001) for the development of CDAD, including imipenem, vancomycin (administered intravenously), piperacillin–tazobactam, levofloxacin, ceftriaxone, ceftazidime, cefepime, and clindamycin. Other antibiotics have also been determined (p<0.01) to be significant risk factors (vancomycin [administered orally], piperacillin, ciprofloxacin, cefoxitin, cefuroxime, gentamycin, erythromycin and ampicillin). The results of this study indicated that one risk factor might be the application of antibiotics to which C. difficile is sensitive in vitro (e.g., vancomycin). This paradox remains unresolved, but it is assumed that the proliferation of C. difficile from persisting spores is faster, leading to its dominance during the renewal of the colon flora.27 A more detailed analysis of the research results18 indicated that 27% of patients received one antibiotic, while 61% received multiple antibiotics simultaneously. Only 12% of patients did not receive antibiotics before the occurrence of CDAD. The study showed that antibiotics are the most important risk factors for CDAD, primarily antibiotics from the group of cephalosporins and penicillins, along with quinolones, which were not available in the eighties and nineties of the last century. The older quinolones (including ciprofloxacin) had little effect on anaerobic bacteria, while moxifloxacin and gatifloxacin have significantly increased activity against anaerobes.28,29 Considering that, in most CDAD cases, multiple antibiotics were administered simultaneously, it is not surprising that this research determined those antibiotics administered intravenously as risk factors; however, it is known that in pharmacological terms, after such administration, their concentrations in the intestines are low. However, it is difficult to determine the individual role of antibiotics in the development of CDAD. The results of research conducted in Nis determined that the administration of certain antibiotics in hospitalized patients had a statistically significant influence on the occurrence of CDAD (Table 5). Univariate logistic regression also showed that applied antibiotic treatment, duration of the therapy, and administration of two or more antibiotics for the treatment of infections had statistically significant influences on the development of CDAD (Table 3), which was also confirmed by the multivariate model for the application of antibiotic treatment and its duration (Table 4).
The data obtained from epidemiological studies have indicated that C. difficile infections occur more frequently in patients hospitalized for more than 8 days and in geriatric patients hospitalized for more than 36 days,30,31 which were associated with prolonged exposure to C. difficile. In this study, we also confirmed that prolonged hospitalization affected the occurrence of CDAD (Tables 3 and 4). Similar to the research by Obritsch et al.,32 this research determined a connection between the development of CDAD and the intake of laxatives, in contrast to other studies.33 Some studies have shown that nasogastric tubes, gastrostomy and faecal incontinence were risk factors for CDAD.30,31 Additionally, the use of drugs that reduce gastric acidity favour the development of CDAD. It is assumed that the reduction in gastric acidity decreases the killing of bacteria and facilitates the colonization of the digestive tract by C. difficile. It is still not clear whether a low albumin level is a risk factor for CDAD (it also occurs in the majority of patients with severe diseases but without diarrhoea) or whether it is the result of diarrhoea associated with the taking of antibiotics, which also causes protein-losing enteropathy.33
In a study by Al-Eidan et al.,16 the statistically significant risk factors for CDAD were female sex, a stay in the intensive care unit and the patient's age. In addition to their research, there have also been studies showing that women more frequently have diarrhoea during hospitalization (54%: 46%), which has also been explained by the use of antibiotics to treat urinary tract infections; however, in contrast to this explanation, there have been a greater number of studies showing no statistically significant difference in terms of CDAD patients’ sexes.18,33
The importance of the application of oncological therapy (chemotherapy, cytostatics, X-rays) as a risk factor for the development of CDAD could not be reliably determined because these patients often develop infections that require antibiotics. Previous research has shown that the administration of antibiotics and cytostatics is only a risk factor for CDAD, but there have been rare studies indicating cytostatics alone as a risk factor.34–37 The application of antibiotics increases the relative risk of developing CDAD in patients receiving cytostatic chemotherapy.
The majority of studies that have examined CDAD have shown that patient age (especially older than 60 years) is one of the most important risk factors for the development of CDAD. Age causes changes in the faecal flora, the body's resistance and immunity are weakened, and a significant number of other risk factors are also present in the elderly, such as longer hospitalization, several underlying and serious illnesses, and complications during treatment.12,33 It is interesting that there is evidence that under in vitro conditions, the faecal intestinal contents of elderly patients do not inhibit the growth of C. difficile compared to younger patients.33 For all these reasons, CDAD is common in people who are placed in nursing homes or treated in geriatric wards and hospitals that specialize in the care of the elderly and bedridden people.
According to the results of a large number of studies, the presence of a severe underlying disease is an important predisposing factor for the development of CDAD in hospitalized patients. In a study by Al-Eidan et al.,16 all 87 of their hospitalized patients with CDAD had severe underlying diseases (pneumonia or other respiratory tract infections – 46%, diabetes mellitus – 42%, ischaemic heart disease – 34.5%, hypertension – 31%, chronic obstructive diseases of the respiratory tract – 17%, kidney disease – 3.3%, and liver disease – 2.3%). The presence of two or more simultaneous diseases was found in 21 patients (24.1%). In their study, Kyne et al.38 found that CDAD was associated with extremely severe underlying disease (OR=17.6; 95% C.I.=8.8–53.5). Diseases classified according to Horn's index as severe and very severe with complications were statistically strongly associated with the development of CDAD. The probability that these patients will develop CDAD is 27%, and if Horn's index of the severity of underlying disease on admission is 1 or 2, then the probability of developing CDAD is 4%. The study also showed that the average time for colonization of the intestines by C. difficile was 6 days (3–33 days), provided that the time for colonization was the same in all groups based on Horn's index. This finding confirmed that the severity of the disease was directly associated with CDAD. These patients also have damaged immune responses and weakened general immune defences, which could play important roles in their high sensitivity to C. difficile, which colonizes the digestive tract and causes diarrhoea. The results of this research performed in Nis were consistent with previously published data (Table 2).
ConclusionIn this study, it was found that CDAD in hospitalized patients usually manifested as diarrhoea, but in a small number of cases (10.8%), it occurred in more severe forms of the disease (PMC and fulminant colitis). Additionally, several risk factors for the development of CDAD were identified (applied antibiotic therapy, duration of antibiotic therapy, laxatives and total number of days spent in the hospital). Targeting patients with these risk factors for the application of preventive measures could reduce the incidence of and morbidity due to disease caused by C. difficile.
Conflicts of interestThe authors declare no conflicts of interest.