Candida albicans is the primary causative agent of oral candidosis, and one of its key virulent attributes is considered to be its ability to produce extracellular phospholipases that facilitate cellular invasion. Oral candidosis can be treated with polyenes, and azoles, and the more recently introduced echinocandins. However, once administered, the intraoral concentration of these drugs tend to be sub-therapeutic and rather transient due to factors such as the diluent effect of saliva and cleansing effect of the oral musculature. Hence, intra-orally, the pathogenic yeasts may undergo a brief exposure to antifungal drugs. We, therefore, evaluated the phospholipase production of oral C. albicans isolates following brief exposure to sub-therapeutic concentrations of the foregoing antifungals.
Materials and methodsFifty C. albicans oral isolates obtained from smokers, diabetics, asthmatics using steroid inhalers, partial denture wearers and healthy individuals were exposed to sub-therapeutic concentrations of nystatin, amphotericin B, caspofungin, ketoconazole and fluconazole for one hour. Thereafter the drugs were removed and the phospholipase production was determined by a plate assay using an egg yolk-agar medium.
ResultsThe phospholipase production of these isolates was significantly suppressed with a percentage reduction of 10.65, 12.14, 11.45 and 6.40% following exposure to nystatin, amphotericin B, caspofungin and ketoconazole, respectively. This suppression was not significant following exposure to fluconazole.
ConclusionsDespite the sub-therapeutic, intra oral, bioavailability of polyenes, echinocandins and ketoconazole, they are likely to produce a persistent antifungal effect by suppressing phospholipase production, which is a key virulent attribute of this common pathogenic yeast.
Candida albicans is renowned as the leading fungal pathogen of oral candidosis, which manifests in a variety of clinical guises ranging from common denture associated infections in otherwise healthy individuals to systemic infections in human immunodeficiency virus disease.1 In addition C. albicans and several other Candida species cause recalcitrant infections in diabetics, asthmatics using inhalation steroids, smokers and those on cytotoxic drugs and irradiation therapy.1–4
C albicans isolates possess intriguing virulence attributes that confer them the ability to colonize epithelial cells, adhere and develop biofilms on denture materials, and invade deeper layers of the host epithelium. One of these, is its ability to secrete potent extracellular phospholipases, which degrade phospholipid constituents of the host cell membrane leading to cell disruption and subsequent epithelial invasion in certain circumstances.5 In fact a positive correlation between phospholipase activity and two major pathogenic attributes of Candida has been documented, where it was shown that C. albicans strains with the highest phospholipase activity exhibited the highest adherence to oral epithelial cells and a greatest ability to kill mice after intravenous inoculation, compared with yeasts with a low degree of phospholipase activity.5
A number of antifungal therapeutic agents are available for treatment of Candida infections, including oral candidosis. These include the two polyenes group agents, nystatin and amphotericin B, the echinocandins agent caspofungin and the azoles such as ketoconazole (an imidazole) and fluconazole (a triazole).1,6 Despite the availability of these therapeutic agents for the management of oral candidosis, failure of therapy and resultant recalcitrant infection is not uncommon.6 One reason for this may be the unique nature of the oral milieu where the diluent effect of saliva and the cleansing effect of the oral musculature tend to reduce the bioavailability of antifungal agents below that of the effective therapeutic concentrations. Hence, intra-orally, the pathogenic Candida may undergo a brief exposure to a high concentration of the antifungal agent on administration, and thereafter the drug concentration is likely to be sub-therapeutic.6
There is a single study that documents the impact of brief exposure to three antifungal agents (i.e. nystatin, amphotericin B and fluconazole) on extracellular phospholipase production of ten oral C. albicans isolates from HIV-infected individuals.7 Yet, the phospholipase production of oral C. albicans isolates obtained from susceptible patient groups such as diabetics, asthmatics using inhalation steroids, smokers and partial denture wearers following brief exposure to sub-therapeutic concentrations of antifungals with a different array of pharmacodynamics has not been studied thus far.
Though, all the aforementioned antifungal agents are not used as topically administered formulations, such preparations could be formulated for future use in the management of oral candidosis. Therefore, taken together the aforementioned information, the main aim of this investigation was to determine the extracellular phospholipase production of 50 oral C. albicans isolates obtained from diabetics (n=10), asthmatics using inhalation steroids (n=10), smokers (n=10), partial acrylic denture wearers (n=10), and healthy individuals (n=10) following their brief exposure to nystatin, amphotericin B, caspofungin, ketoconazole and fluconazole.
Materials and methodsOrganismsA total of 50 oral isolates of C. albicans recovered from oral rinse samples from patients attending the Kuwait University Dental Clinic (KUDC) for dental treatment in a previous study were included in the current study (10 isolates each were from smokers, diabetics, asthmatics using steroid inhalation, patients wearing partial acrylic dentures and healthy individuals).8 The healthy group were patients seeking dental treatment who were otherwise healthy with no medical condition or medication at the time of collecting the oral rinse samples. The diabetic patients were under oral hypoglycaemic drugs and the asthmatic patients were under steroid inhalation therapy, and were otherwise healthy at the time of attending the dental clinics. The patients who smoked more than 25 cigarettes per day were considered as smokers. Denture wearers were using partial removable acrylic dentures for a period of more than 6 months. None of the patients from whom the isolates were recovered had oral candidosis or any other systemic illness. Initially, all the yeast isolates were tested for germ tube formation. Thereafter the colony characteristics were observed using CHROMagar Candida medium (Becton Dickinson and Company, Sparks, USA) and identified by VITEK 2 yeast ID system (BioMérieux, France) as well as API 20C AUX yeast ID system (BioMérieux, Inc, Hazelwood, MO).
Antifungal agents and mediaAntifungal agents was prepared as done previously in similar studies.9 Briefly, nystatin, amphotericin B, ketoconazole and fluconazole (Sigma, St. Louis, MO, USA) were dissolved in dimethylsulphoxide (DMSO). Caspofungin (Merck and Company Inc, Waterhouse Station, NJ, USA) was dissolved in sterile distilled water. These antimycotic agents were prepared initially as 10,000μg/mL and stored at −20°C prior to each experiment as previously described.9 It was thereafter suspended/diluted in the following medium during the exposure period (1h) of yeasts. RPMI 1640 medium buffered with 0.165M MOPS (3-(N-morpholino) propanesulfonic acid) containing l-glutamine and lacking sodium bicarbonate (Sigma, USA), was dissolved in 1l of sterile distilled water and adjusted to a pH of 7.2 and filter sterilized.
Determination of minimal inhibitory concentrationMinimum inhibitory concentration (MIC) values against amphotericin B, caspofungin, ketoconazole and fluconazole were determined by E test, performed according to the manufacturer's recommendations (AB BIODISK, Solna, Sweden) as described previously.8 Freshly sub-cultured isolates (five colonies) were uniformly suspended in sterile saline, and turbidity was adjusted to 0.5 McFarland standard. The McFarland Standard 0.5 is approximately equal to 1×106–5×106cells/mL (Clinical and Laboratory Standards Institute, CLSI M44-A, 2004). This inoculum was swabbed onto the agar plates (150mm diameter) and allowed to dry for 10–15min before the E test strips were applied. RPMI 1640 agar supplemented with 2% glucose and buffered with MOPS (0.165M; pH 7.0) was used for susceptibility testing according to the method recommended by the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards; M27-A2). The plates were incubated at 35°C, and minimum inhibitory concentrations (MIC) were read after 24–48h of incubation. The point where inhibition ellipses intercepted the scale on the antifungal strip was taken as the MIC for each test isolate: complete inhibition (100%) of growth for amphotericin B and caspofungin, and marked decrease in growth intensity (80%) for fluconazole and ketoconazole. Reference strains of C. albicans, ATCC 90028, and C. parapsilosis, ATCC 22019, were used for quality control of the susceptibility testing. Interpretive susceptibility breakpoints for fluconazole were those recommended by Clinical and Laboratory Standards Institute document M27-A2. Due to the lack of defined susceptibility breakpoints for amphotericin B and ketoconazole, an isolate was considered susceptible with an MIC breakpoint of ≤1.0μg/mL for amphotericin B and 0.125μg/mL for ketoconazole. For caspofungin, isolates with MIC of ≤2.0μg/mL were considered as susceptible.
The MIC values of nystatin were determined by the broth dilution technique by performing two-fold serial dilutions of the drug in microtitre plates using an inoculum of 1–5×105 colony forming units (CFU)/mL, as done previously.9 The MIC was determined visually following 24h incubation at 37°C. The MIC was defined as the lowest concentration of the drug that inhibited growth of Candida cells, as indicated by the absence of turbidity (optically clear). The MIC was read independently by two laboratory personals. C. albicans ATCC 90028 was used as a reference strain.
Preparation of cell suspension for the phospholipase production assayA previously described method was used for this purpose.10 Briefly, yeast cells maintained on Sabouraud's dextrose agar (SDA), were inoculated onto fresh plates and incubated overnight at 37°C for 24h prior to use. The organisms were harvested and a cell suspension prepared in sterile phosphate buffered saline (PBS) at 520nm to an optical density of 1.5. From this cell suspension, 0.5mL was added to tubes containing 2mL of PBS (control) and 2mL of PBS/antifungal agent ×2 MIC (test). This gave a cell suspension of 106cellsmL−1 in each assay tube. The tubes were then incubated at 37°C for a period of 30min. Following this limited exposure, the drugs were removed by two cycles of dilution with sterile PBS and centrifugation for 10min at 3000×g (gravity). Afterwards the supernatant was completely decanted and the pellets were re-suspended in 2.5mL of sterile PBS. This procedure has been used previously for drug removal and has shown to reduce the concentration of the drug as much as 10,000 fold, thereby minimizing any carry-over effect of the drug following its removal.7,9–12 Viable counts of the control and the test were performed after drug removal. As the procedure of drug removal effectively eliminated any carry-over effect there was virtually no difference on the viable counts of the control and the tests following exposure to already diluted sub-therapeutic concentrations of the drug. In addition, under Gram-stain, Gram-positive yeast cells were seen when Candida were observed under light microscopy before brief exposure to these antifungals and did not reveal any observable changes soon after removal of these drugs (i.e. before the phospholipase production assays were performed) compared to the cells observed before the exposure.
Phospholipase production assay (egg yolk agar method)C. albicans isolates were screened for phospholipase activity by growing the isolates on egg yolk medium and measuring the size of the zone of precipitation (i.e. production of phospholipase) as done in previous studies.7,10,13,14 The egg York medium consisted of Sabouraud's dextrose agar, 13.0g; NaCl, 11.7g; CaCl2, 0.11g; and 10% sterile egg yolk emulsion in 184mL of distilled water. The egg yolk emulsion was centrifuged at 500×g for 10min at room temperature, and 20mL of the supernatant was added to the cooled sterilized medium. A 10μL suspension of 107 yeast cells (drug-exposed and control) per mL in PBS was plated on the surface of egg yolk medium in a 90mm diameter petridish and left to dry at room temperature. The plates were then incubated at 37°C for 72h. The phospholipase activity (Pz index) of the isolates was interpreted positive when a precipitation zone was visible around the growth and the value of phospholipase production was determined by the ratio of the diameter of the colony plus the precipitation zone to the diameter of the colony as done previously.7,10,13
All experiments were repeated on three separate occasions with duplicate determinations on each occasion.
Statistical analysisThe data obtained from the phospholipase assay was analyzed using ANOVA Dunnett's t-tests, which treat one group as a control (unexposed to drugs), and compare the other group (exposed to drugs) against it. In addition the variations between the effects of antifungal drugs from each other on the suppressive effect on phospholipase production was analyzed using Tukey–Kramer Multiple Comparative tests. A p value of <0.05 was considered statistically significant.
ResultsAll C. albicans isolates were susceptible to the antifungal drugs tested. The MIC (μg/mL) values of all C. albicans isolates for nystatin obtained from the broth dilution technique ranged from 0.78 to 1.56. The MIC values obtained by E-tests for amphotericin B was between 0.004 and 0.19 and for caspofungin it ranged from 0.004 to 0.125. Ketoconazole elicited a MIC of 0.004–0.032 while it ranged between 0.047 and 0.125 for fluconazole.
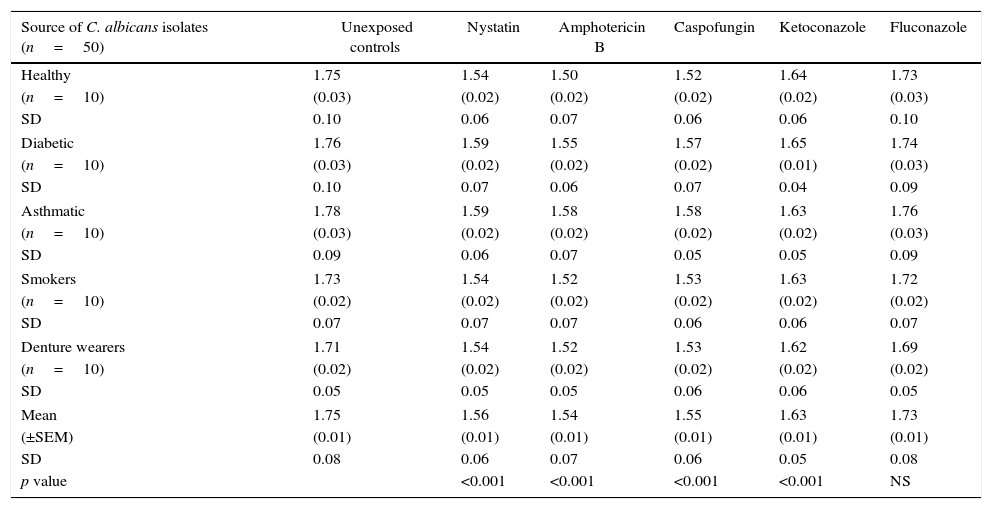
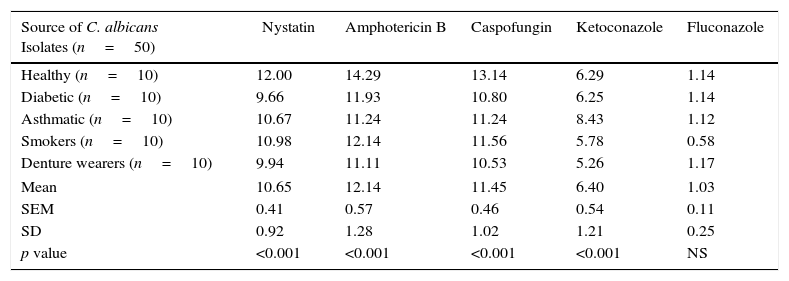
Mean phospholipase production (as Pz index) of the 50 C. albicans isolates, following limited exposure to five different antifungal drugs, drug removal and subsequent production of phospholipase activity is shown in Table 1. Mean phospholipase production of the unexposed isolates was 1.75, whereas this figure following exposure to nystatin, amphotericin B, caspofungin, ketoconazole and fluconazole was 1.56, 1.54, 1.55, 1.64 and 1.73, respectively. Hence, as shown in Table 2, a significant reduction of phospholipase activity was seen following exposure to nystatin, amphotericin B, caspofungin and ketoconazole, with a mean percentage reduction of 10.65%, 12.14%, 11.45% and 6.40%, respectively. Overall suppressive effect on the phospholipase production following exposure to fluconazole was not significant in comparison to the unexposed control.
Mean (±SEM) phospholipase activity (Pz index) of 50 oral C. albicans isolates following 1h exposure and subsequent removal of five different antifungal drugs. Each value indicates the mean (±SEM) phospholipase activity of 10 oral C. albicans obtained either from healthy individuals, diabetic patients, asthmatic patients, smokers or denture wearers. SEM, standard error of mean; SD, standard deviation.
| Source of C. albicans isolates (n=50) | Unexposed controls | Nystatin | Amphotericin B | Caspofungin | Ketoconazole | Fluconazole |
|---|---|---|---|---|---|---|
| Healthy | 1.75 | 1.54 | 1.50 | 1.52 | 1.64 | 1.73 |
| (n=10) | (0.03) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) |
| SD | 0.10 | 0.06 | 0.07 | 0.06 | 0.06 | 0.10 |
| Diabetic | 1.76 | 1.59 | 1.55 | 1.57 | 1.65 | 1.74 |
| (n=10) | (0.03) | (0.02) | (0.02) | (0.02) | (0.01) | (0.03) |
| SD | 0.10 | 0.07 | 0.06 | 0.07 | 0.04 | 0.09 |
| Asthmatic | 1.78 | 1.59 | 1.58 | 1.58 | 1.63 | 1.76 |
| (n=10) | (0.03) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) |
| SD | 0.09 | 0.06 | 0.07 | 0.05 | 0.05 | 0.09 |
| Smokers | 1.73 | 1.54 | 1.52 | 1.53 | 1.63 | 1.72 |
| (n=10) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) |
| SD | 0.07 | 0.07 | 0.07 | 0.06 | 0.06 | 0.07 |
| Denture wearers | 1.71 | 1.54 | 1.52 | 1.53 | 1.62 | 1.69 |
| (n=10) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) |
| SD | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 |
| Mean | 1.75 | 1.56 | 1.54 | 1.55 | 1.63 | 1.73 |
| (±SEM) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) |
| SD | 0.08 | 0.06 | 0.07 | 0.06 | 0.05 | 0.08 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | NS | |
Summary of the mean percentage suppression of phospholipase production in 50 oral C. albicans isolates following 1h exposure and subsequent removal of five different antifungal drugs. Each value indicates the mean percentage reduction of phospholipase production in 10 oral C. albicans obtained from either healthy individuals, diabetic patients, asthmatic patients, smokers or denture wearers. SEM, standard error of mean; SD, standard deviation.
| Source of C. albicans Isolates (n=50) | Nystatin | Amphotericin B | Caspofungin | Ketoconazole | Fluconazole |
|---|---|---|---|---|---|
| Healthy (n=10) | 12.00 | 14.29 | 13.14 | 6.29 | 1.14 |
| Diabetic (n=10) | 9.66 | 11.93 | 10.80 | 6.25 | 1.14 |
| Asthmatic (n=10) | 10.67 | 11.24 | 11.24 | 8.43 | 1.12 |
| Smokers (n=10) | 10.98 | 12.14 | 11.56 | 5.78 | 0.58 |
| Denture wearers (n=10) | 9.94 | 11.11 | 10.53 | 5.26 | 1.17 |
| Mean | 10.65 | 12.14 | 11.45 | 6.40 | 1.03 |
| SEM | 0.41 | 0.57 | 0.46 | 0.54 | 0.11 |
| SD | 0.92 | 1.28 | 1.02 | 1.21 | 0.25 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | NS |
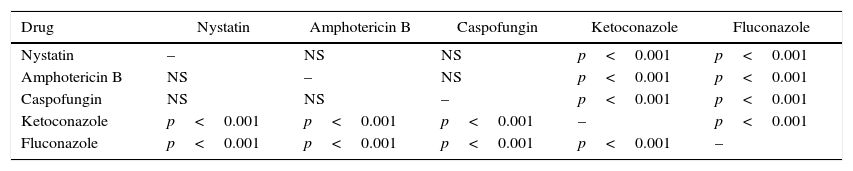
As shown in Table 3, analysis of the variation between the five drugs on the suppression of phospholipase production using Tukey–Kramer multiple comparative tests revealed that the suppressive effect elicited by the polyene and echinocandin antifungal agents were significantly higher and statistically different from that of the two azole drugs (p<0.001). In addition, the suppressive effect elicited by the two azoles was also statistically different from each other (p<0.005). However, the suppressive effect elicited by the two polyenes and the echinocandin agent was not significantly different.
Statistical analysis by Tukey–Kramer multiple comparison test on the comparison of the relative efficacy of five different agents in reducing phospholipase production of C. albicans isolates.
| Drug | Nystatin | Amphotericin B | Caspofungin | Ketoconazole | Fluconazole |
|---|---|---|---|---|---|
| Nystatin | – | NS | NS | p<0.001 | p<0.001 |
| Amphotericin B | NS | – | NS | p<0.001 | p<0.001 |
| Caspofungin | NS | NS | – | p<0.001 | p<0.001 |
| Ketoconazole | p<0.001 | p<0.001 | p<0.001 | – | p<0.001 |
| Fluconazole | p<0.001 | p<0.001 | p<0.001 | p<0.001 | – |
NS, difference on the adhesion suppressive effect between the drugs was not significant.
A wide array of exquisite and specific attributes is required for a pathogenic organism to penetrate the eukaryotic epithelial cell barrier of the human host. It is well known that the filamentous candidal hyphae are critical in this process and these together with the extracellular phospholipases of the fungus facilitate the yeast invasion of the host tissues. Clearly, therefore, both physical or enzymatic activity or a combination of both are associated with the pathogenesis of candidal disease. As phospholipids are a foremost constituent of the host cell envelope, enzymes capable of hydrolyzing phospholipids i.e. phospholipases, are likely to play a critical role in host cell invasion. By cleaving phospholipids, candidal phospholipases undermine the membrane and cell lysis is the end result.15
Amongst the various Candida species, C. albicans is by far the major aetiological agent associated with oral candidosis and its superior phospholipase activity in comparison to other non-albicans Candida species, is considered to be one reason for its relatively high virulence amongst its peers.7,15
There is scarcity of data on the impact of antimycotic agents on phospholipase production of oral C. albicans. Hence, we attempted to study the impact of polyenes (nystatin and amphotericin B), echinocandins (caspofungin) and azoles (ketoconazole and fluconazole) on phospholipase production of oral C. albicans obtained from different patient groups. Our data indicate that the, exposure of oral C. albicans isolates to sub-MIC concentrations of all the tested drugs significantly suppressed phospholipase production with the exception of fluconazole.
The select group of agents we chose to study, exerts their antifungal activity through different pharmacodynamics mechanisms. Polyenes, for instance bind to the sterol components in the yeast cell wall and make it more permeable.1,6 It has also been advocated that the formation of sterols or their precursors may also be repressed by the polyenes.1,6 Furthermore, studies have shown internally collapsed cells with an intact cell wall leaving “ghosts-like” cells and deflated Candida cells following exposure to sub-cidal concentrations of polyenes.11 Caspofungin, on the contrary, acts by inhibiting the enzyme responsible for biosynthesis of β-1,3 glucan resulting in disturbing the integrity of the fungal cell wall.16 In addition caspofungin has shown excellent growth-inhibitory activity against Candida at low concentrations.16 As for ketoconazole, it alters the fungal cell membranes by blocking the 14 α-demethylation step in the biosynthesis of ergosterol. The consequent depletion of ergosterol and accumulation of 14 α-methyl-sterols leads to alterations in a number of membrane-associated functions.1,6 Considering the foregoing wide range pharmacodynamic properties of the agents we have studied it is tempting to speculate that the structural changes of the cell wall as well as the metabolic effects elicited by the drugs would all contribute to the suppression of yeast phospholipase activity we have described in the current study. In this context it is noteworthy that a previous study has also implied that the drug-induced metabolic changes in suppressing phospholipase production persisted into the subsequent progeny of daughter yeasts and not the parent generation of cells exposed to antifungals, due to the fact that phospholipase activity is determined after 72h of incubation.7 The current results also tend to augment and confirm these previous observations.
The significant difference between polyenes and echinocandins with that of azoles on the suppressive effect on phospholipase production of Candida as seen in this study may well be due to the differences in the pharmacodynamics of the antimycotic groups. Polyenes and echinocandins are fungicidal against C. albicans isolates whereas azoles are fungistatic.1,6,16 This could be the main reason for the exquisite sensitivity of the cell-surface structures of C. albicans to nystatin, amphotericin B and caspofungin compared to the azoles. In addition to the aforementioned difference, a significant difference in the suppressive effect on phospholipase production was also seen between the two azoles. Though fluconazole is remarkably more effective than ketoconazole in the management of candidal infection, its growth inhibitory activity against C. albicans isolates in vitro is much less than that of ketoconazole.6,9,17 Furthermore, there could be subtle changes in the pharmacodynamics between the two azoles, ketoconazole being a imidazole and fluconazole being a triazole. Therefore, though not conclusive, it is likely that the differences in the mode of activity between the two azoles may be the reasons for fluconazole not eliciting a significant suppression of phospholipase production of C. albicans isolates tested.
When the phospholipase production of C. albicans isolates obtained from different patient groups were compared following exposure to antifungal drugs as well as of the unexposed control isolates there was no significant difference amongst the isolates, indicating that the phospholipase production of all the isolates were quite uniform with or without exposure to the drugs, at least in this study.
Taken together, the foregoing data contributes further to the understanding of the pharmacodynamics of these antimycotics in relation extracellular phospholipase production of C. albicans, which frequently colonizes and cause oral disease in diabetics, asthmatics using steroid inhalers, smokers and denture wearers. While the precise clinical significance of these antimycotics in modulating Candida phospholipase production cannot be ascertain, it could be argued that these drugs are capable disarming Candida virulence by suppressing phospholipase production, in addition to their other antifungal pharmacodynamics. To our knowledge this is the first study to document the impact of antimycotic agents with a wider array of pharmacodynamics properties (i.e. polyene, echinocandin, imidazole and triazole) on phospholipase production, of oral C. albicans isolates obtained from a diverse group of individuals from a single geographic locale. The exact cellular mechanisms underlying our observations, however, remain to be elucidated.
ConclusionsBrief and transient exposure to sub-therapeutic concentrations of antimycotics may modulate phospholipase production of C. albicans isolates in vitro, thereby possibly quelling its pathogenicity.
Conflicts of interestThe authors declare no conflicts of interest.
The work was supported by Kuwait University Research Grant No. DB 01/14. Technical advice and support on laboratory procedures in carrying out this project given by Ms. Rachel Chandy of Health Sciences Center, Kuwait University, Kuwait and Dr. Sumedha Jayathilake, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka are thankfully acknowledged. Advice from Dr. Prem Sharma, Faculty of Medicine, Kuwait University, Kuwait on data analysis is also very much appreciated.