This study aimed to evaluate the in vitro antifungal activity of terpinen-4-ol, tyrosol, and β-lapachone against strains of Coccidioides posadasii in filamentous phase (n=22) and Histoplasma capsulatum in both filamentous (n=40) and yeast phases (n=13), using the broth dilution methods as described by the Clinical and Laboratory Standards Institute, to determine the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of these compounds. The mechanisms of action of these compounds were also investigated by analyzing their effect on cell membrane permeability and ergosterol synthesis. The MIC and MFCf these compounds against C. posadasii, mycelial H. capsulatum, and yeast-like H. capsulatum, were in the following ranges: 350–5720μg/mL, 20–2860μg/mL, and 40–1420μg/mL, respectively for terpinen-4-ol; 250–4000μg/mL, 30–2000μg/mL, and 10–1000μg/mL, respectively, for tyrosol; and 0.48–7.8μg/mL, 0.25–16μg/mL, and 0.125–4μg/mL, respectively for β-lapachone. These compounds showed a decrease in MIC when the samples were subjected to osmotic stress, suggesting that the compounds acted on the fungal membrane. All the compounds were able to reduce the ergosterol content of the fungal strains. Finally, tyrosol was able to cause a leakage of intracellular molecules.
Coccidioidomycosis and histoplasmosis are systemic mycoses reported in the Americas, especially in the United States, Mexico, and Brazil. These diseases are caused by the dimorphic fungi Coccidioides spp. and Histoplasma capsulatum var. capsulatum, respectively. In Brazil, coccidioidomycosis caused by the species C. posadasii has only been reported in the Northeast, while histoplasmosis has been reported throughout the country with a 40% lethality rate when associated with AIDS.1,2 The treatment of these diseases involves azoles in cases with mild to moderate symptoms and amphotericin B in severe cases.3,4 Although the standard antifungal therapies are effective for treating these mycoses, some disadvantages have been observed for these therapies, such as nephrotoxicity caused by amphotericin B,5 the high cost of the lipid formulations of amphotericin B,6 and the fact that only 50–60% of patients with coccidioidomycosis respond to the treatment with fluconazole and itraconazole7 and 56–70% of patients with histoplasmosis respond to the treatment with ketoconazole.8 Hence, a search for new therapeutic strategies against these pathogens is of great importance.
Terpinen-4-ol is a terpene alcohol that exhibits in vitro antifungal properties against Candida spp., Cryptococcus neoformans, Malassezia spp., Rhodotorula spp., Trichosporon spp., Aspergillus spp., Penicillium spp., and dermatophytes.9–12 Tyrosol is a phenolic compound with antioxidant properties and an autoregulatory molecule of C. albicans13,14 which has in vitro antifungal properties against Candida spp.15 β-Lapachone is a quinone derived from lapachol with antifungal properties against Candida spp., Cryptococcus neoformans, and dermatophytes.16–19 In the light of the earlier studies, this investigation aimed at evaluating the in vitro inhibitory effects of these compounds and investigating the mechanism of action of these compounds against the fungal strains, C. posadasii and H. capsulatum.
Materials and methodsMicroorganismsTwenty-two strains of C. posadasii in the filamentous phase (18 clinical, 3 environmental, and 1 animal) and 40 strains of H. capsulatum in the filamentous stage (38 clinical and 2 animal) were used for this study. Among the H. capsulatum strains, 13 were also evaluated in the yeast phase. All the fungal strains were obtained from the fungal collection of the Specialized Medical Mycology Center (CEMM, Federal University of Ceará, Brazil). The procedures for identification of the fungi included the classic mycological analysis, as described by Brilhante et al.20 All the procedures were performed in a class II biological safety cabinet in a biosafety level 3 laboratory.
Antimicrobial agentsFor the assays, terpinen-4-ol, tyrosol, and β-lapachone (all from Sigma Chemical Corporation, USA) were used. The traditional antifungal drugs, amphotericin B (AMB) (Sigma Chemical Corporation, USA) and itraconazole (ITC) (Janssen Pharmaceutica, Belgium) were used as control drugs. The stock solutions of terpinen-4-ol, ITC, and AMB were prepared in 100% dimethyl sulfoxide (DMSO); β-lapachone was dissolved in 80% DMSO; and tyrosol was dissolved in sterile distilled water.15,21,22 All the stock solutions were stored at −20°C until use. Serial dilutions of each compound were prepared in RPMI 1640 medium (Sigma Chemical Corporation, USA), supplemented with l-glutamine, buffered to pH 7.0 using 0.156M MOPS (Sigma Chemical Corporation, USA). DMSO was included in the assays as control to confirm that the DMSO used to dilute the compounds did not interfere with fungal growth.20
Preparation of inoculum for antifungal susceptibility assaysThe strains of C. posadasii were grown on potato agar and incubated for 7 days at room temperature (25–28°C). To prepare the inoculum, 2mL of sterile saline were added to each culture, and the surface of the mycelium was scraped with a microbiological loop. The suspensions were transferred to sterile tubes and allowed to stand for 5min. The supernatant was read in a spectrophotometer at a wavelength of 530nm, and its transmittance was set to 95%. The suspensions containing arthroconidia and hyphal fragments were diluted to 1:10 with RPMI 1640 medium to obtain inocula containing approximately 1×103–5×103CFU/mL.20
H. capsulatum strains in filamentous form were grown on brain heart infusion (BHI) agar (Himedia, India) at 28°C for 7 days. The inoculum was prepared as described earlier. H. capsulatum strains in the yeast phase were grown in Sabouraud agar or BHI agar supplemented with 10% sheep blood and incubated for 7 days at 35°C. Then, an aliquot of the fungal colony was transferred to 2mL of sterile saline. The absorbance of supernatant was measured in a spectrophotometer at a wavelength of 530nm, and its transmittance was set to 95%. The suspensions containing arthroconidia and hyphal fragments were diluted to 1:10 with RPMI 1640 medium to obtain inocula containing approximately 1×103–5×103CFU/mL.20
Antifungal susceptibility testThe susceptibility of C. posadasii strains to the compounds being tested was determined through the broth macrodilution method, according to the M38-A2 protocol standardized by the CLSI.23 The susceptibility of H. capsulatum to the compounds was determined by the broth microdilution method, according to the M27-A3 protocol standardized by the CLSI.24 The concentrations of the tested compounds for C. posadasii strains were as follows: Terpinen-4-ol (350–5720μg/mL), tyrosol (250–4000μg/mL), β-lapachone (0.48–7.8μg/mL), AMB (0.0625–1μg/mL), and ITC (0.0625–1μg/mL). The concentrations of the compounds being tested against H. capsulatum strains (in both phases) were as follows: Terpinen-4-ol (10–5720μg/mL), tyrosol (3.9–2000μg/mL), β-lapachone (0.0312–16μg/mL), AMB (0.0039–2μg/mL), and ITC (0.00195–1μg/mL).
The MIC for AMB was defined as the lowest concentration of drug capable of inhibiting 100% of fungal growth, while for the other compounds, MICs were defined as the lowest concentration of compounds capable of inhibiting 80% of fungal growth, when compared to the drug-free growth control.20 All the tests were performed in duplicate. The results obtained were visually examined after 48h (for C. posadasii), 4 days (for H. capsulatum in the yeast phase), and 7 days (for H. capsulatum in the filamentous phase) of incubation at 35°C. For quality control of the antifungal susceptibility tests, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included.20
The minimum fungicidal concentration (MFC) of the compounds against C. posadasii and H. capsulatum was defined as the lowest concentration that completely inhibited fungal growth, after seeding 100μL of the fungal suspension, at concentrations of compound just above the MIC, on potato agar and incubating for 6–7 days at 35°C.
Inhibitory effect of the compounds under osmotic stressThe ability of the compounds to alter the permeability of the fungal membrane was also evaluated by macrodilution and microdilution. The assay was performed with 12 strains of C. posadasii (filamentous phase), 8 strains of H. capsulatum (filamentous phase), and 8 strains of H. capsulatum (yeast phase). Osmotic stress was induced by adding 0.1M NaCl to RPMI 1640 medium (pH 7.0) as described by Brilhante et al.1 The compounds were used below inhibitory concentrations. The results were visually determined at the end of the above mentioned incubation period at 35°C.1 The concentration of NaCl incorporated into RPMI broth was determined based on the results of susceptibility of fungal strain to salt, in which concentrations ≤0.175M did not interfere with fungal growth for both species compared with the drug-free and salt-free growth control.
Effect of the compounds on cell membrane permeabilityThis test was performed, in order to determine the ability of these compounds to alter the permeability of the fungal membrane, causing leakage of intracellular molecules, as described by Cordeiro et al.2 Ten strains of C. posadasii (filamentous phase), eight strains each of the filamentous phase and yeast phase of H. capsulatum were used. These strains were exposed to different concentrations (MIC and MIC/2) of the compounds as previously determined in the macrodilution and microdilution assays. Growth control (without drugs) and blank solution (culture medium control) were also included in the tests. AMB (negative control) and isoniazid (positive control) were used as the controls.2 One milliliter from each tube containing the fungal inoculum exposed to the compound was transferred to sterile microcentrifuge tubes and centrifuged for 15min (13,400×g). Of the supernatant, 70μL was removed from each tube and diluted 1:10 in sterile distilled water. The release of UV-absorbing molecules was analyzed by spectrometry at 260nm for nucleic acids and 280nm for proteins.
Ergosterol quantificationCellular ergosterol was quantified as described by Brilhante et al.1 The extraction was performed using ten strains of C. posadasii strains (filamentous phase), eight strains each of the filamentous phase and yeast phase of H. capsulatum. Ergosterol was extracted after the exposure of the strains to inhibitory and subinhibitory concentrations of the compounds being tested, itraconazole (positive control drug), and amphotericin B (negative control drug), using macrodilution and microdilution techniques. Six different concentrations of the compounds were tested. The ranges of concentration tested against C. posadasii were as follows: Terpinen-4-ol (10–1420μg/mL), tyrosol (15–2000μg/mL), β-lapachone (0.03–3.9μg/mL), AMB (0.003–0.5μg/mL), and ITC (0.003–0.5μg/mL). The ranges of concentration tested against H. capsulatum (in both phases) were as follows: Terpinen-4-ol (0.6–350μg/mL), tyrosol (0.4–500μg/mL), β-lapachone (0.003–1μg/mL), AMB (0.001–1μg/mL), and ITC (0.00006–0.5μg/mL). The fungal pellet for each concentration was exposed to 0.5mL of 20% (w/v) KOH, 60% (v/v) EtOH solution and incubated at 95°C for 1h in a water bath. Then, 0.6mL of hexane was added to isolate the sterols. The solutions were centrifuged for 5min (10,000×g). Then, the top layer of hexane was transferred to microtubes, and 0.5mL of hexane was added. The quantification of ergosterol was performed in a UV-visible spectrophotometer at λ=295.10nm and compared with a predetermined standard curve. Ergosterol extracted from the strains of C. posadasii and H. capsulatum grown in drug-free RPMI medium was quantified and treated as a positive control.1
Statistical analysisThe results of the assays performed in this study were analyzed using the Student's t-test and ANOVA, using the post hoc Fisher's LSD test. In all the cases, p<0.05 was considered statistically significant.
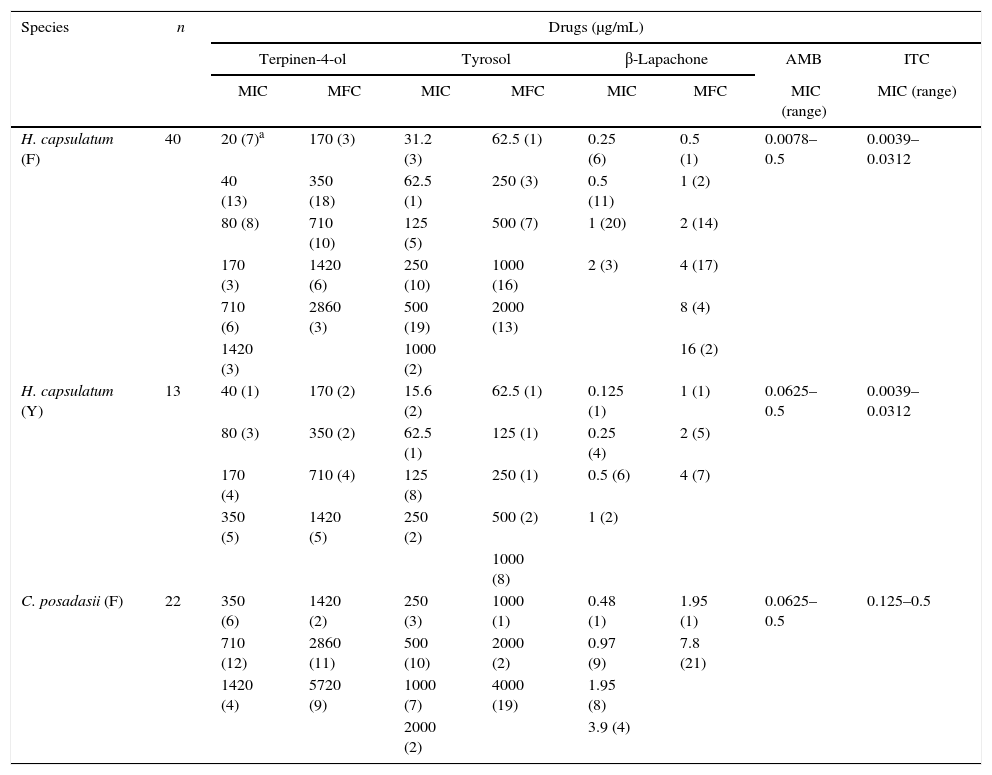
ResultsIn vitro susceptibility testingThe MICs and MFCs of the compounds determined against C. posadasii were as follows: 350≤MIC≤1420μg/mL and 1420≤MFC≤5720μg/mL for terpinen-4-ol; 250≤MIC≤2000μg/mL and 1000≤MFC≤4000μg/mL for tyrosol; and 0.48≤CIM≤3.9μg/mL and 1.95≤MFC≤7.8μg/mL for β-lapachone (Table 1).
Minimum inhibitory and minimum fungicidal concentrations for terpinen-4-ol, tyrosol, β-lapachone and control drugs against C. posadasii and H. capsulatum strains.
| Species | n | Drugs (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Terpinen-4-ol | Tyrosol | β-Lapachone | AMB | ITC | |||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC (range) | MIC (range) | ||
| H. capsulatum (F) | 40 | 20 (7)a | 170 (3) | 31.2 (3) | 62.5 (1) | 0.25 (6) | 0.5 (1) | 0.0078–0.5 | 0.0039–0.0312 |
| 40 (13) | 350 (18) | 62.5 (1) | 250 (3) | 0.5 (11) | 1 (2) | ||||
| 80 (8) | 710 (10) | 125 (5) | 500 (7) | 1 (20) | 2 (14) | ||||
| 170 (3) | 1420 (6) | 250 (10) | 1000 (16) | 2 (3) | 4 (17) | ||||
| 710 (6) | 2860 (3) | 500 (19) | 2000 (13) | 8 (4) | |||||
| 1420 (3) | 1000 (2) | 16 (2) | |||||||
| H. capsulatum (Y) | 13 | 40 (1) | 170 (2) | 15.6 (2) | 62.5 (1) | 0.125 (1) | 1 (1) | 0.0625–0.5 | 0.0039–0.0312 |
| 80 (3) | 350 (2) | 62.5 (1) | 125 (1) | 0.25 (4) | 2 (5) | ||||
| 170 (4) | 710 (4) | 125 (8) | 250 (1) | 0.5 (6) | 4 (7) | ||||
| 350 (5) | 1420 (5) | 250 (2) | 500 (2) | 1 (2) | |||||
| 1000 (8) | |||||||||
| C. posadasii (F) | 22 | 350 (6) | 1420 (2) | 250 (3) | 1000 (1) | 0.48 (1) | 1.95 (1) | 0.0625–0.5 | 0.125–0.5 |
| 710 (12) | 2860 (11) | 500 (10) | 2000 (2) | 0.97 (9) | 7.8 (21) | ||||
| 1420 (4) | 5720 (9) | 1000 (7) | 4000 (19) | 1.95 (8) | |||||
| 2000 (2) | 3.9 (4) | ||||||||
F, filamentous phase; Y, yeast phase; n, number of strains; MIC, Minimum Inhibitory Concentration; MFC, Minimum Fungicidal Concentration.
The MICs and MFCs determined against H. capsulatum in the filamentous phase were as follows: 20≤MIC≤1420μg/mL and 170≤MFC≤2860μg/mL for terpinen-4-ol; 30≤MIC≤1000μg/mL and 60≤MFC≤2000μg/mL for tyrosol; and 0.25≤MIC≤1μg/mL and 0.5≤MFC≤16μg/mL for β-lapachone (Table 1).
For H. capsulatum in the yeast phase, the following MICs and MFCs were obtained: 40≤MIC≤350μg/mL and 170≤MFC≤1420μg/mL for terpinen-4-ol; 10≤MIC≤250μg/mL and 60≤MFC≤1000μg/mL for tyrosol, and 0.125≤MIC≤1μg/mL and 1≤MFC≤4μg/mL for β-lapachone.
The MIC values determined for the control drugs (AMB and ITC) were in agreement with the intervals found in previous tests against C. posadasii and H. capsulatum (Table 1). The standard strains, C. parapsilosis and C. krusei used as control exhibited MIC values for AMB and ITC that were in agreement with CLSI guidelines.
Osmotic stressThe MICs determined for the compounds tested with RPMI+NaCl were statistically lower than those obtained using the standard RPMI against C. posadasii and H. capsulatum strains. There was a significant reduction in the range of 4–64 times in the MIC values of terpinen-4-ol (p=0.0000), tyrosol (p=0.0001), and β-lapachone (p=0.0012) and the control drug, ITC (p=0.0003) (Table 2).
MIC values (mean) for terpinen-4-ol, tyrosol and β-lapachone and the control drug itraconazole in the absence and presence of osmotic stress against C. posadasii and H. capsulatum strains.
| Species | n | Drugs (μg/mL) – mean of MIC values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Terpinen-4-ol | Tyrosol | β-Lapachone | Itraconazole | ||||||
| RPMI | RPMI+NaCl | RPMI | RPMI+NaCl | RPMI | RPMI+NaCl | RPMI | RPMI+NaCl | ||
| C. posadasii (F) | 12 | 768 | 133 | 958 | 87 | 2 | 0.5 | 0.2 | 0.01 |
| H. capsulatum (F) | 8 | 65 | 9 | 253 | 32 | 0.6 | 0.1 | 0.1 | 0.009 |
| H. capsulatum (Y) | 8 | 176 | 22 | 89 | 9 | 0.4 | 0.06 | 0.02 | 0.001 |
F, filamentous phase; Y, yeast phase; n, number of strains; MIC, Minimum Inhibitory Concentration. Reduction of MIC values: Terpinen-4-ol (p=0.0000), Tyrosol (p=0.0001), β-lapachone (p=0.0012) and Itraconazole (p=0.0003).
The results showed that despite the possible effects of all the compounds on the permeability of the fungal membrane, only tyrosol was able to cause leakage of intracellular molecules in C. posadasii and H. capsulatum strains. Tyrosol caused the leakage of nucleic acids leading to a significant increase in absorbance at 260nm, reaching values significantly higher than those obtained for the fungal growth control (p=0.0000) and the control drug AMB (negative control) (p=0.0000), but similar to the absorbance values obtained for isoniazid (positive control) (p>0.05) at MIC and MIC/2 (Fig. 1). Tyrosol also caused leakage of proteins with absorbance values (at 280nm) significantly higher than those obtained for the fungal growth control (p=0.0000), the control drug AMB (negative control) (p=0.0000), but similar to the values obtained for the positive control drug isoniazid (p=0.0000) at MIC and MIC/2 (Fig. 1). The values obtained with other compounds at 260nm and 280nm had no significant differences, when compared to the fungal growth control and AMB (p>0.05), and were lower than those of isoniazid (p=0.0000), indicating no evidence of leakage of molecules (Fig. 1).
Effect of terpinen-4-ol (TER), tyrosol (TIR), β-lapachone (B-LAP), amphotericin B (AMB, control drug), and isoniazid (INH, positive control drug) at MIC and MIC/2 (μg/mL) on the permeability of the cell membrane of the ten strains of C. posadasii strains (1a–b), eight strains of filamentous (F) H. capsulatum strains (1c–d) and eight yeast strains (Y) of H. capsulatum (1e–f) resulting in the leakage of nucleic acids (260nm) (1a, 1c, 1e) and proteins (280nm) (1b, 1d, 1f). These values represent the mean±standard error of the absorbance values obtained for all tested strains. Control: fungal growth without drug.
The results showed that the exposure of strains of C. posadasii and H. capsulatum to sub-inhibitory concentrations of terpinen-4-ol, tyrosol, and β-lapachone resulted in a decrease in the amount of ergosterol extracted from fungal cells compared to the fungal growth control (p=0.0000) (Fig. 2). Higher the concentration of the compounds, lower is the amount of ergosterol extracted from the fungi. Similar results were observed for ITC (positive control), with no significant difference between the results obtained for the compounds and the control drug, ITC (p>0.05) (Fig. 2).
Quantification of ergosterol from the ten strains of Coccidioides posadasii (2a), eight filamentous (F) strains of Histoplasma capsulatum (2b), and eight yeast (Y) strain of H. capsulatum (2c) after exposure to sub-inhibitory concentrations of terpinen-4-ol (TER), tyrosol (TIR), β-lapachone (B-LAP), itraconazole (ITC, control drug), and amphotericin B (AMB, control drug). These values represent the average results for all strains tested for each species. Control: fungal growth control without drug.
The availability of a small number of antifungal drugs, the associated side effects of amphotericin B (i.e., nephrotoxicity), the high cost of the lipid formulations of amphotericin B, and the possible failure of some individuals to respond to the treatment with azoles may complicate the treatment of these mycoses. Hence, there is an urgent need to find new drugs or compounds possessing antifungal properties.5–8,25 Several studies have contributed to the development of new agents and to expand the list of antifungal drugs, such as terpinen-4-ol, tyrosol, and β-lapachone. The decision to include these compounds in the present work was motivated by their antimicrobial properties observed in other studies, especially against some species of yeasts and filamentous fungi. In addition, there are no reports available on the effects of these compounds against the dimorphic fungi, C. posadasii and H. capsulatum.
It was observed that the MIC values obtained for terpinen-4-ol against C. posadasii and the filamentous and yeast phases of H. capsulatum were comparable to those reported by Hammer et al.9 against Candida spp., Saccharomyces cerevisiae, Rhodotorula mucilaginosa, Trichosporon spp., Aspergillus spp., Penicillium spp., and dermatophytes of the genera, Trichophyton, Epidermophyton, and Microsporum (MIC=0.008–0.25%) and the findings of Marcos-Arias et al.15 against Candida spp. (MIC=0.06–0.5%).
In the case of tyrosol, the MIC values obtained against C. posadasii and H. capsulatum in both the filamentous and yeast phases showed concentrations lower than the levels reported in literature, such as the findings of Marcos-Arias et al.15 against Candida spp. (MIC=0.5–8%).
In the case of β-lapachone, the MIC values obtained against C. posadasii and the filamentous and yeast phases of H. capsulatum were similar to the data reported in other studies with naphthoquinone derivatives, such as the findings of López et al.17 against Candida spp., Cryptococcus neoformans, and dermatophytes of the genera Trichophyton and Microsporum (MICs=0.5–64μg/mL).
It is possible that the tested compounds might exhibit a fungicidal effect because their MIC values are not much lower than their MFCs. According to Vila et al.26 when MFC values are lower than four-times the MIC values, a fungicidal effect is suggested, while a fungistatic effect is expected when MFC values are higher than four-times the MIC values.
Ergosterol is the predominant lipid molecule found in yeast cells. It regulates the fluidity and permeability of the membrane and the activity of many enzymes incorporated into this structure. Hence, it plays an important role in cell growth.27 Studies report that essential oils derived from plants and their monoterpenoid components such as terpinen-4-ol, which is a major component of the tea tree oil, have a lipophilic characteristic, likely presenting a direct action on the cell membrane structure and associated enzymes.10,15,27
It has been reported that terpenes cross the fungal cell wall and are inserted between the fatty acid chains that constitute the lipid bilayer membrane, causing changes in its fluidity and permeability. These alterations may lead to the degradation of the cell wall, reduction in adherence to host surfaces, and other effects, such as the disruption of the plasma membrane, loss of cell content, coagulation of cytoplasm, and cell lysis.15 Cox et al.28 verified the capacity of tea tree oil and its components to alter the integrity of the cell membrane of Gram-negative bacteria and the yeast C. albicans. Parveen et al.29 found that a monoterpene affects the ergosterol and lipid biosynthesis and cell wall structure of Saccharomyces cerevisiae, leading to specific changes in gene expression. The results of the present study corroborates this report, by establishing the ability of terpinen-4-ol to inhibit the synthesis of ergosterol on the tested dimorphic fungi, as shown by the reduction in the amount of ergosterol extracted from fungal cells after exposure to subinhibitory concentrations of this compound.
Currently, little is known about the mechanism of action of tyrosol on fungal cell, with no reports in the literature. However, this study was able to show the effect of tyrosol on the permeability of the cell membrane of dimorphic fungi, including the reduction of MIC values after the exposure of fungal strains to high salinity. These findings suggest the degeneration of the cell membrane. In addition, tyrosol also caused leakage of protein and nucleic acids, suggesting the effect of tyrosol on both, cellular and nuclear membrane. The results also showed the property of tyrosol to inhibit the synthesis of ergosterol, as demonstrated by the reduction in the amount of ergosterol extracted from fungal strains after exposure to high concentrations of tyrosol.
The effect of β-lapachone on the plasma membrane might be due to its lipophilic nature, which may affect the metabolic pathways and functionalities of cellular processes.17 This fact was corroborated in this study that shows that β-lapachone affects the permeability of the cytoplasmic membrane of C. posadasii and H. capsulatum, indicating that the effect is associated with the degeneration of the fungal membrane. In addition, this compound interferes with the synthesis of ergosterol, reducing the amount of ergosterol extracted from fungal cells after exposure to high concentrations of β-lapachone. This compound also has the ability to induce oxidative stress in eukaryotic cells by intracellular formation of reactive oxygen species, such as hydrogen peroxide (H2O2), the superoxide anion radical (O2−), and hydroxyl radical (OH), inducing apoptosis and cytotoxicity.16,22,29 Moreover, β-lapachone causes inhibition of the topoisomerase I and II in both prokaryotic and eukaryotic cells, which are important for the normal cellular operation, since they maintain the integrity of DNA. It is a well established fact that any functional alterations in these enzymes is sufficient to induce apoptosis.17,29,30
This study demonstrates the antifungal activity of the synthetic compounds, terpinen-4-ol, tyrosol, and β-lapachone against the dimorphic fungi, C. posadasii and H. capsulatum. It also highlights their role as potential leads for new antifungal agents for the treatment of histoplasmosis and coccidioidomycosis.
Conflicts of interestWe have no conflict of interest to declare.
This research was supported by CNPq process (303396/2014-8; 552161/2011-0) and CAPES (AE1-0052-000630100/11).