Carbapenem-resistant Acinetobacter baumannii infection is a concern in developing countries due to high incidence, few therapeutic options, and increasing costs.
ObjectiveCharacterize and analyze the antibiotic susceptibility patterns of carbapenem-resistant A. baumannii isolates and evaluate clinical data of meningitis and bacteremia caused by this microorganism.
MethodsTwenty-six A. baumannii isolates from 23 patients were identified by MALDI-TOF and automated methods and genotyped using pulsed field genotyping electrophoresis. Clinical data and outcomes were evaluated. Susceptibility of isolates to colistin, tigecycline, meropenem, imipenem, and doxycycline was determined.
ResultsMortality due to A. baumannii infections was 73.91%; all patients with meningitis and 7/8 patients with ventilator-associated pneumonia died. All isolates were susceptibility to polymyxin (100%; MIC50, MIC90: 1μg/mL, 1μg/mL) and colistin (100%; MIC50, MIC90: 2μg/mL, 2μg/mL), and 92% were susceptible to tigecycline (MIC50, MIC90: 1μg/mL, 1μg/mL) and doxycycline (MIC50, MIC90: 2μg/mL, 2μg/mL). blaOXA-23 was identified in 24 isolates. Molecular typing showed 8 different patterns: 13 isolates belonged to pattern A (50%).
ConclusionCarbapenem-resistant A. baumannii infections mortality is high. Alternative antimicrobial therapy (doxycycline) for selected patients with carbapenem-resistant A. baumannii infection should be considered.
Acinetobacter baumannii healthcare associated infections (HAIs) are considered a serious public health problem owing to their prevalence and mortality in developing countries.1,2 The susceptibility of A. baumannii to the most commonly used antibiotics has decreased.3
The treatment of carbapenem-resistant A. baumannii (CR-AB) has been a challenge because carbapenem is the first option in most hospitals for empirical treatment of HAI. Consequently, older drugs with increased toxicity, including polymyxins and aminoglycosides, have been used based solely on empirical evidence.4 Doxycycline is an oral tetracycline with activity against A. baumannii and this drug can achieve therapeutic levels in cerebrospinal fluid when adequate dosage is used.5 Tigecycline is another option approved for intra-abdominal and soft tissue infections that has also been used extensively for the treatment of multidrug resistant infections at other anatomic locations. Unfortunately, clinical and antimicrobial susceptibility data are rarely evaluated together, so the true efficacy of non-carbapenem antibiotics against CR-AB is unknown. The aims of this study were to characterize the molecular types of A. baumannii isolates from cerebrospinal fluid and blood culture and to evaluate clinical data and susceptibility patterns of these isolates to treatment with polymyxin, colistin, tigecycline, doxycycline, meropenem, and imipenem.
Material and methodsStudy settingA transversal study was carried out at Hospital Universitario Evangelico de Curitiba (HUEC), a tertiary-care, trauma reference hospital, in Southern Brazil, with a total number of 660 beds. The study included patients admitted from April 2010 through April 2013. This study was approved by the Ethics Committee of FEPAR and FPP (311.769).
Clinical dataData were collected from medical charts and/or hospital computer system databases. The clinical data evaluated included age, gender, site of infection, comorbidities, admission at intensive care unit, antimicrobial therapy, and outcome. Antimicrobial therapy was included in the analysis when it was started within 72h of infection diagnosis. Infections where classified according to CDC criteria.6 Thirty-day mortality was also analyzed.
Microbiological studiesTwenty-six isolates of A. baumannii from 23 patients admitted at HUEC from April 2010 through April 2013 were evaluated. Nineteen isolates were obtained from blood culture and 7 from cerebrospinal fluid culture. All isolates were identified using the Vitek2® Compact System (bioMérieux, Durham, USA) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) using Biotyper 2.0 software (Bruker151 Daltonik Inc, Bremen, Germany).
Antibiotic susceptibility testsMinimal inhibitory concentration (MIC) of colistin, doxycycline, imipenem, meropenem, polymyxin B and tigecycline was estimated by the agar dilution method according to the CLSI, M7-A9 approved standard.7 Polymyxin B and colistin were also tested by a broth microdilution method according to CLSI M7-A9.8 Interpretation of MIC was performed according to CLSI, M100-S24,9 except for tigecycline.10 The MIC50 and MIC90 were calculated for both the agar and broth methods. We also compared broth and agar dilution for colistin and polymyxin using a concordance level of ±≤1log2.11
Molecular detection of oxacillinase genesTo determine oxacillinase-encoding genes, polymerase chain reaction (PCR) was performed for all isolates by using the following primers: blaOXA-23 (F-5′ GATCGGATTGGAGAACCAGA 3′; R-5′ ATTTCTGACCGCATTTCCAT 3′), blaOXA-51 (F-5′ TAATGCTTTGATCGGCCTTG 3′; R-5′ TGGATTGCACTTCATCTTGG 3′), blaOXA-58 (F-5′ AAGTATTGGGGCTTGTGCTG 3′; R-5′ CCCCTCTGCGCTCTACATAC 3′), blaOXA-143 (F-5′ TGGCACTTTCAGCAGTTCCT 3′; R-5′ TAATCTTGAGGGGGCCAACC 3′). PCR was performed in a final volume of 25μL with a final DNA concentration of 100ng/mL. The amplification conditions were 94°C for 25s, followed by 52°C for 40s and 72°C for 50s.12
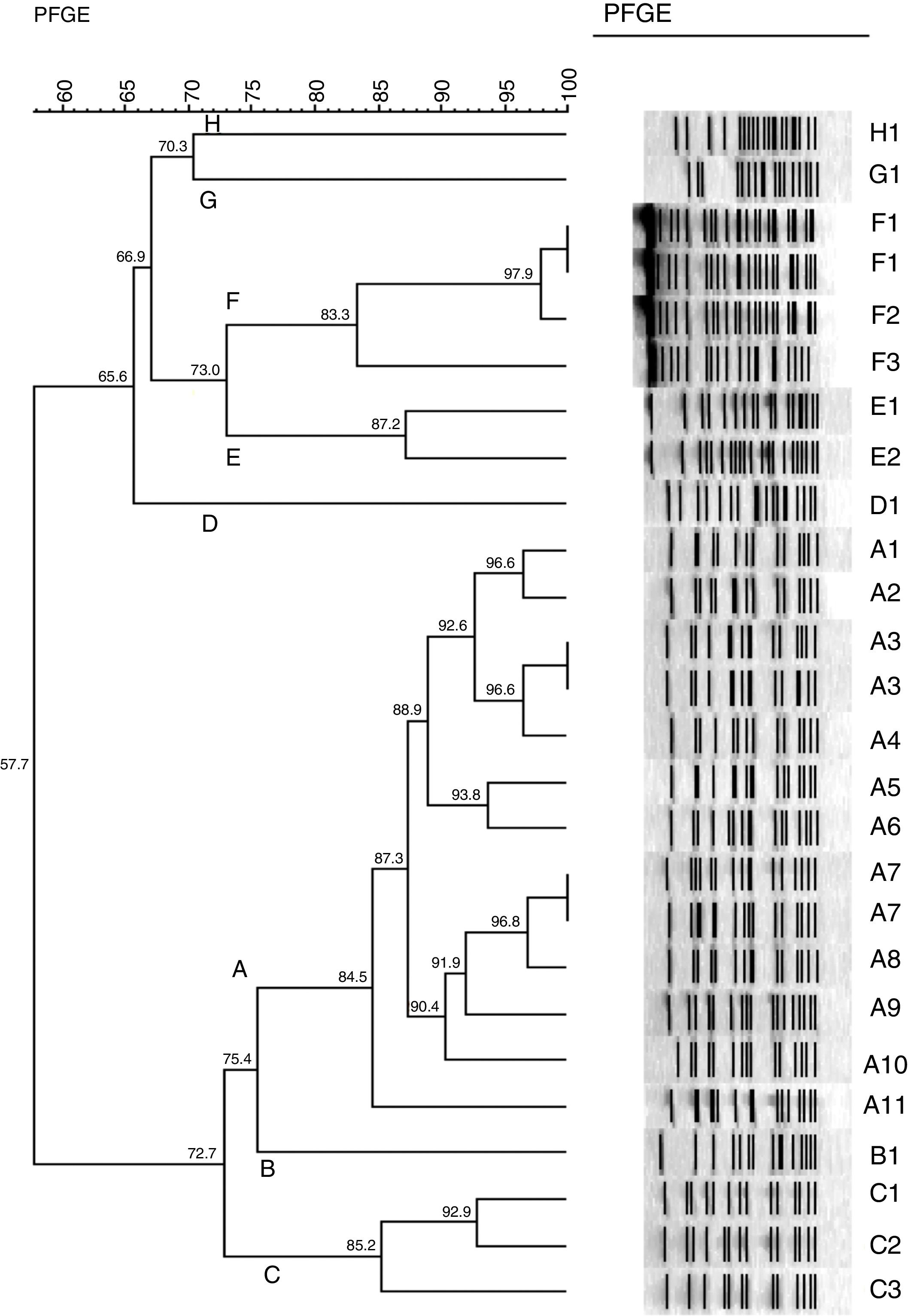
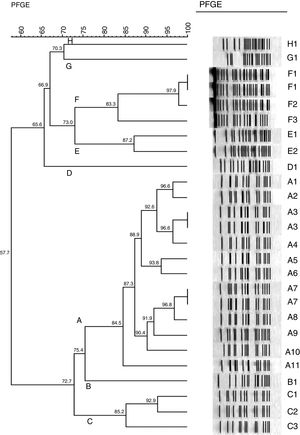
Pulsed field gel electrophoresis (PFGE)All 26 isolates were genotyped by PFGE and DNA was prepared as described previously.13 The entire chromosomal DNA of the strains was digested with 30U of ApaI (Fermentas – Life Science, Glen Burnie, MD). PFGE was performed by using a clamped homogeneous electric field electrophoresis (CHEF) DRIII apparatus (Bio-Rad Laboratories, Hercules, USA). The conditions employed were as follows: temperature of 12°C, voltage of 6V/cm, run time of 30h, and switch time of 5–35s. The pattern of bands was interpreted according to previously described criteria.14 PFGE clustering was determined by using the unweighted-pair group method with arithmetic averages (UPGMA) and by using Dice's coefficient. The tolerance was set at 1%. Identical isolates were assigned the same capital letter. Those with 80% similarity were assigned as a subtype of the major type, followed by an Arabic number (e.g. A1, A2, A3, A4).
ResultsPatient dataTwenty-six isolates from 23 patients were evaluated. The median age of patients was 42 years (10–60 years). All patients were hospitalized in the intensive care unit when clinical samples were collected. Isolates were obtained from blood culture or cerebrospinal fluid. Samples from other body sites (sputum or bronchoalveolar lavage, urine or tissue biopsy) were not evaluated. Clinical characteristics are presented in Table 1.
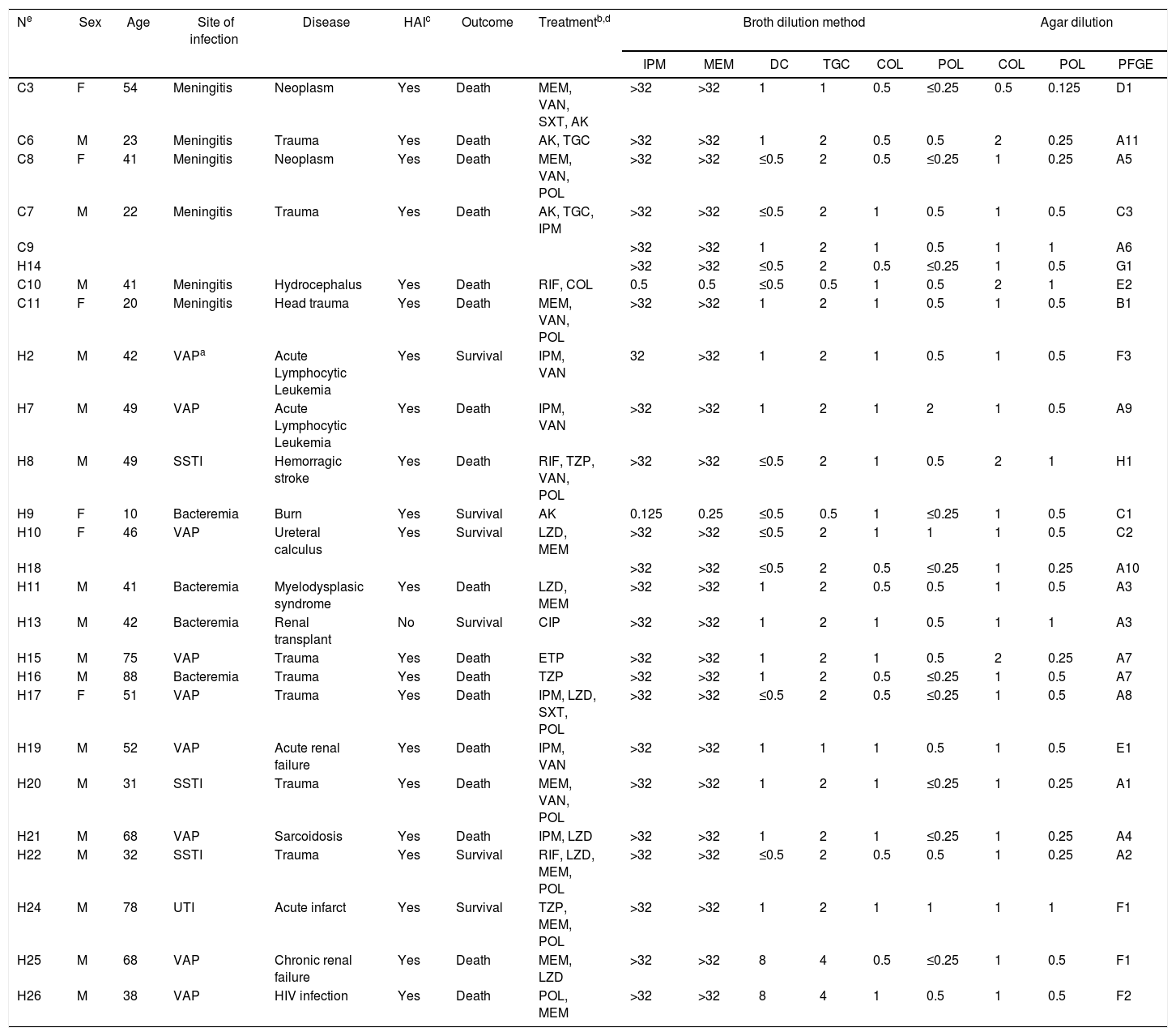
Clinical and laboratorial data from 23 patients with Acinetobacter baumannii causing infection.
| Ne | Sex | Age | Site of infection | Disease | HAIc | Outcome | Treatmentb,d | Broth dilution method | Agar dilution | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | MEM | DC | TGC | COL | POL | COL | POL | PFGE | ||||||||
| C3 | F | 54 | Meningitis | Neoplasm | Yes | Death | MEM, VAN, SXT, AK | >32 | >32 | 1 | 1 | 0.5 | ≤0.25 | 0.5 | 0.125 | D1 |
| C6 | M | 23 | Meningitis | Trauma | Yes | Death | AK, TGC | >32 | >32 | 1 | 2 | 0.5 | 0.5 | 2 | 0.25 | A11 |
| C8 | F | 41 | Meningitis | Neoplasm | Yes | Death | MEM, VAN, POL | >32 | >32 | ≤0.5 | 2 | 0.5 | ≤0.25 | 1 | 0.25 | A5 |
| C7 | M | 22 | Meningitis | Trauma | Yes | Death | AK, TGC, IPM | >32 | >32 | ≤0.5 | 2 | 1 | 0.5 | 1 | 0.5 | C3 |
| C9 | >32 | >32 | 1 | 2 | 1 | 0.5 | 1 | 1 | A6 | |||||||
| H14 | >32 | >32 | ≤0.5 | 2 | 0.5 | ≤0.25 | 1 | 0.5 | G1 | |||||||
| C10 | M | 41 | Meningitis | Hydrocephalus | Yes | Death | RIF, COL | 0.5 | 0.5 | ≤0.5 | 0.5 | 1 | 0.5 | 2 | 1 | E2 |
| C11 | F | 20 | Meningitis | Head trauma | Yes | Death | MEM, VAN, POL | >32 | >32 | 1 | 2 | 1 | 0.5 | 1 | 0.5 | B1 |
| H2 | M | 42 | VAPa | Acute Lymphocytic Leukemia | Yes | Survival | IPM, VAN | 32 | >32 | 1 | 2 | 1 | 0.5 | 1 | 0.5 | F3 |
| H7 | M | 49 | VAP | Acute Lymphocytic Leukemia | Yes | Death | IPM, VAN | >32 | >32 | 1 | 2 | 1 | 2 | 1 | 0.5 | A9 |
| H8 | M | 49 | SSTI | Hemorragic stroke | Yes | Death | RIF, TZP, VAN, POL | >32 | >32 | ≤0.5 | 2 | 1 | 0.5 | 2 | 1 | H1 |
| H9 | F | 10 | Bacteremia | Burn | Yes | Survival | AK | 0.125 | 0.25 | ≤0.5 | 0.5 | 1 | ≤0.25 | 1 | 0.5 | C1 |
| H10 | F | 46 | VAP | Ureteral calculus | Yes | Survival | LZD, MEM | >32 | >32 | ≤0.5 | 2 | 1 | 1 | 1 | 0.5 | C2 |
| H18 | >32 | >32 | ≤0.5 | 2 | 0.5 | ≤0.25 | 1 | 0.25 | A10 | |||||||
| H11 | M | 41 | Bacteremia | Myelodysplasic syndrome | Yes | Death | LZD, MEM | >32 | >32 | 1 | 2 | 0.5 | 0.5 | 1 | 0.5 | A3 |
| H13 | M | 42 | Bacteremia | Renal transplant | No | Survival | CIP | >32 | >32 | 1 | 2 | 1 | 0.5 | 1 | 1 | A3 |
| H15 | M | 75 | VAP | Trauma | Yes | Death | ETP | >32 | >32 | 1 | 2 | 1 | 0.5 | 2 | 0.25 | A7 |
| H16 | M | 88 | Bacteremia | Trauma | Yes | Death | TZP | >32 | >32 | 1 | 2 | 0.5 | ≤0.25 | 1 | 0.5 | A7 |
| H17 | F | 51 | VAP | Trauma | Yes | Death | IPM, LZD, SXT, POL | >32 | >32 | ≤0.5 | 2 | 0.5 | ≤0.25 | 1 | 0.5 | A8 |
| H19 | M | 52 | VAP | Acute renal failure | Yes | Death | IPM, VAN | >32 | >32 | 1 | 1 | 1 | 0.5 | 1 | 0.5 | E1 |
| H20 | M | 31 | SSTI | Trauma | Yes | Death | MEM, VAN, POL | >32 | >32 | 1 | 2 | 1 | ≤0.25 | 1 | 0.25 | A1 |
| H21 | M | 68 | VAP | Sarcoidosis | Yes | Death | IPM, LZD | >32 | >32 | 1 | 2 | 1 | ≤0.25 | 1 | 0.25 | A4 |
| H22 | M | 32 | SSTI | Trauma | Yes | Survival | RIF, LZD, MEM, POL | >32 | >32 | ≤0.5 | 2 | 0.5 | 0.5 | 1 | 0.25 | A2 |
| H24 | M | 78 | UTI | Acute infarct | Yes | Survival | TZP, MEM, POL | >32 | >32 | 1 | 2 | 1 | 1 | 1 | 1 | F1 |
| H25 | M | 68 | VAP | Chronic renal failure | Yes | Death | MEM, LZD | >32 | >32 | 8 | 4 | 0.5 | ≤0.25 | 1 | 0.5 | F1 |
| H26 | M | 38 | VAP | HIV infection | Yes | Death | POL, MEM | >32 | >32 | 8 | 4 | 1 | 0.5 | 1 | 0.5 | F2 |
VAP, ventilator-associated pneumonia; UTI, urinary tract infection; SSTI, skin and soft tissue infection.
Six patients presented with nosocomial meningitis. The median age was 33 years (15–56 years) with 3 females and 3 males. All patients presented with meningitis secondary to external ventricular shunt due to head trauma or neoplasm with a mortality rate of 100%. All strains in these patients were susceptible to polymyxins (colistin and polymyxin) and doxycycline. When bacterial susceptibility to polymyxins was taken into account, 3 patients received the correct treatment. The 3 remaining patients were treated with carbapenems, and the MIC of this antibiotic was determined to be >32mg/L in all strains isolated from these patients. There was no clonality shared among the meningitis cases. In one patient with A. baumannii meningitis, the clone isolated from cerebrospinal fluid was identical to one found in blood culture and another cerebrospinal fluid sample that was obtained 5 days later (Clone A).
BacteremiaSeventeen patients presented with A. baumannii bacteremia, 4 of them primary and 13 of them secondary to infection at another site. Ventilator-associated pneumonia (VAP) with secondary bacteremia was the most common infection (9 cases), followed by secondary bacteremia caused by skin and soft tissue infection (3 cases) and urinary tract infection (1 case). The median age of patients was 50 years (10–60 year), with 14 males and 3 females. There was no clonal association with a specific site of infection. Considering the therapeutic and microbiological aspect of bacteremic patients, 7 patients received correct therapy within 72h (41.17%). The mortality of patients with A. baumannii bacteremia was 64.70%; specifically, 7 of 9 patients with VAP, and 1 of 3 with skin-soft tissue infections died. There was no correlation of survival with correct antibiotic therapy within first hours of culture sample. There was no clonality among cases of A. baumannii bacteremia.
Antimicrobial susceptibilityMore than 90% of isolates were resistant to carbapenem (MIC50 and MIC90>32μg/mL).
Isolates were susceptibility to polymyxin and colistin (MIC50 and MIC90=1μg/mL for polymyxin; MIC50=0.5μg/mL and MIC90=1μg/mL for colistin). Broth and agar dilutions for colistin and polymyxin susceptibility showed a high concordance level (100%).
Bacterial isolates also had susceptibility to tigecycline and doxycycline (MIC50 and MIC90=1μg/mL for tigecycline; MIC50 and MIC90=2μg/mL for doxycycline). Only two samples were resistant to these drugs.
PCR amplified blaOXA-51 in all isolates. The blaOXA-23 gene was amplified in all 24 isolates resistant to carbapenem, but was not detected in the two isolates susceptible to carbapenem.
PFGEMolecular typing with PFGE revealed 8 distinct patterns with 80% similarity (see dendrogram, Fig. 1). Thirteen isolates belong to pattern A (50%), including 11 subtypes. Other patterns (B, C, D, E, F, G and H) were found in 1, 3, 1, 2, 4, 1 and 1 samples, respectively. Both isolates of carbapenem-susceptible A. baumannii showed 70.3% similarity (patterns G and H)
Dendrogram of 26 isolates from 23 patients with Acinetobacter baumannii causing infection. See Table 1 to correlation of isolates and site of infection.
Carbapenem-resistant A. baumannii infections are severe and result in high mortality in critically ill patients. All patients with meningitis ultimately died despite adequate therapy, a mortality rate previously described by our group.15 The outcome is not different for bacteremia, although previous studies have shown that delay in the adequate therapy can reduce the mortality.16 Carbapenem-resistant A. baumannii is becoming increasingly prevalent in Brazil, and more than 60% of strains in some areas are resistant. OXA-23 carbapenemase has been described as endemic since 2003 in Southern Brazil17 and was identified in all carbapenem-resistant A. baumannii in other publications.18 The current study confirms that this oxacillinase is prevalent and probably the main mechanism of carbapenem resistance. However, isolates from some hospitals have a different carbapenemase profile, including a low prevalence of OXA-23 in A. baumannii.19
Out of 26 isolates, 24 were susceptible to doxycycline and two isolates showed intermediate resistance. Timurkaynak et al. found a similar doxycycline susceptibility pattern in A. baumannii,20 this drug has a bactericidal effect against A. baumannii in some isolates, including carbapenem-resistant strains.21 Although minocycline has been more potent against Acinetobacter, this tetracycline is not available in Brazil.
In our study, there was a high concordance between susceptibility to colistin and polymyxin, but we did not test resistant isolates. Similar results were described by Gales et al., who reported 94.3% concordance in susceptibility to these drugs.11 Furthermore, among the 26 isolates of A. baumannii, 5 showed skipped well phenomena for colistin and 8 for polymyxin (data not shown). This phenomenon occurs when these drugs are tested in broth microdilution and is associated with hetero-resistance.22
PFGE demonstrated a predominant clone (A) with the remaining isolates characterized as related clones. The mechanism of carbapenem resistance was associated with the presence of the gene blaOXA-23, although we cannot exclude combined mechanisms such as pump efflux and porin loss.23
The question of whether to use combined therapy to treat Acinetobacter infections remains unanswered. In vitro studies are controversial and clinical studies are comprised of retrospective cohorts with small sample numbers. However, in the context of multidrug-resistant Acinetobacter, few options are available, mainly for hard-to-treat infections, like meningitis. Tigecycline has a low penetration into cerebrospinal fluid.24 Tigecycline is also contra-indicated for the treatment of primary bacteremia as peak concentration of less than 1mg/L can be achieved, an insufficient level for treatment of bloodstream infection. Furthermore, sub-therapeutic concentrations are associated with increasing resistance.25 Polymyxin is the drug primarily used for treatment of CR-AB. The use of polymyxin B is less reported than that of colistin, although this drug has shown successful results with less acute renal injury than colistin.26 However, to date, there are no reports regarding the penetration of polymyxin B, the most common polymyxin used in Brazil, in the central nervous system. Colistin has been used for the treatment of meningitis with reasonable outcomes.26 Despite the presence of a susceptibility pattern to polymyxins in most of our isolates, these drugs should be cautiously used due to increasing resistance, nephrotoxicity, and neurotoxicity. When these drawbacks are taken into consideration, doxycycline is an attractive alternative for or potential addition to other active drugs to treat nosocomial meningitis.
In summary, A. baumannii infections in our patient cohort resulted in high mortality, and carbapenem resistance was frequent in these severe infections. OXA-23 continues to be the main mechanism of resistance of A. baumannii in this region of the Brazil, with a predominant clonal pattern. Fortunately, alternative drugs are available for use under specific conditions that must be decided by experts.
Ethical approvalYes (2014 – 0118)
Informed consentN/A
FundingNone.
Conflict of interestFelipe Tuon is a CNPQ researcher.
There is not conflict of interest.