This study compares patients with and without non-viral microbial keratitis in relation to sociodemographic variables, clinical aspects, and involved causative agent. Clinical aspects, etiology and therapeutic procedures were assessed in patients with and without keratitis that were diagnosed in an Eye Care Center in Campo Grande, MS, Brazil. Patients were divided into two groups: (a) cases: 64 patients with non-viral microbial keratitis diagnosed at biomicroscopy; and (b) controls: 47 patients with other eye disorders that were not keratitis. Labor activity related to agriculture, cattle raising, and contact lens use were all linked to keratitis occurrence (p<0.005). In patients with keratitis, the most common symptoms were pain and photophobia, and the most frequently used medicines were fourth-generation fluoroquinolones (34.4%), amphotericin B (31.3%), and natamycin (28.1%). Microbial keratitis evolved to corneal perforation in 15.6% of cases; transplant was indicated in 10.9% of cases. Regarding the etiology of this condition, 23 (42.2%) keratitis cases were caused by bacteria (Pseudomonas aeruginosa, 12.5%), 17 (39.1%) by fungi (Fusarium spp., 14.1% and Aspergillus spp., 4.7%), and 4 (6.3%) by Acanthamoeba. Patients with keratitis present with a poorer prognosis. Rapid identification of the etiologic agent is indispensable and depends on appropriate ophthalmological collection and microbiological techniques.
Microbial keratitis is an infectious corneal disease associated with potential vision impairment and blindness. It is one of the primary indications for corneal transplants in Brazil and around the world. The widespread use of contact lenses, corneal surface diseases, trauma, and eye surgery have been described as major risk conditions for its occurrence.1–4 Complications associated with contact lens wear have been observed worldwide, with the highest incidence occurring in developed countries and populations of higher socioeconomic status.5,6
Studies have shown that the etiology of microbial keratitis varies according to geographic region, economic activity, and climatic differences. Thus, it is believed that prior knowledge of the epidemiological characteristics of a given region, combined with clinical suspicion, can guide empirical therapy.3 For instance, ocular trauma caused by vegetable matter has been shown to be responsible for keratitis occurrence, particularly in low-income countries and areas that feature agricultural economies.1,4
Due to the aggressiveness of various etiologic agents, early diagnosis and treatment are essential to prevent complications, such as endophthalmitis, corneal transplants, and vision loss. Although not always available, the use of laboratory tests plays an important role in cases of microbial keratitis when assisting the ophthalmologist in determining the optimal therapeutic approach.7–9
This study compared patients with and without non-viral microbial keratitis in relation to sociodemographic variables, clinical aspects, and involved etiologic agents to better understand the dynamics of this infection, which features rapid clinical progression and high morbidity.
MethodsThis was a case–control study conducted at an eye institute in the city of Campo Grande, Mato Grosso do Sul state, Brazil, which provides services to patients from the capital and from the countryside. Patients that were over 18 years old with suspected eye infection, and who attended the eye institute from 2009 to 2013, submitted to biomicroscopy and biological material collection for the laboratory examination, were included in this study.
The patients were divided into two groups: cases – patients with clinical manifestations and a diagnosis of keratitis, as verified by the presence of epithelial defects and epithelial or stromal infiltration, and attested in biomicroscopy; and controls – patients with eye diseases that were not keratitis, suspected conjunctivitis, Chlamydia trachomatis infection, endophthalmitis, blepharitis, dacryocystitis, and other diseases that are not located in the cornea.
The study variables were as follows: sex, age, work activity, the presence of comorbidities, contact lens wear, previous surgery, ocular trauma, concurrent eye injuries, symptoms, clinical signs, clinical specimens sent for laboratory examination, microbiological examination results, pre- and post-treatment following microorganism identification, and clinical evolution. Microbiological analysis included research and culture for bacteria, fungi, and Acanthamoeba.
Corneal scraping was obtained with a sterile blade and plated directly onto different culture media, included Sabouraud agar, 5% sheep blood agar, and thioglycolate broth. Blood agar underwent incubation at 35°C for a period of 24–36h and Sabouraud agar underwent incubation at 25°C, for a month.
Other ocular specimens were obtained with cotton swab applicators and seeded onto brain-heart infusion broth (BHI), chocolate agar and 5% sheep blood agar or as requested by the specialist physician. Chocolate and blood agars underwent incubation at 35°C for a period of 24–36h, and then at 25°C for up to more weeks.
The corneal smear exhibit little adherence to glass microscope slides. They should be fixed in flame for later staining with Gram's stain, or they should be previously fixed in methanol, for May Grünwald–Giemsa's stain 3,17 It is recommended that one slide be initially stained with Gram and then observed microscopically, due to the small amount of material obtained during the collection. An observer with this type of sample can visualize bacteria, yeasts, filamentous fungi, Acanthamoeba, and the presence of small oval spore microsporidia. Gram's stain was the first performed and observed for all specimens.
With a second reserved slide featuring a corneal scraping sample, the researcher can choose either another stain or a more appropriate methodology, as guided by the observation of the first, such as May Grünwald–Giemsa, modified Ziehl–Neelsen's stain, electron microscopy, and others.
To verify the possible associations between the study variables, the chi-squared test or Fisher's exact test were used at a 5% significance level. To estimate the odds ratios (OR) adjusted with the respective 95% confidence intervals, logistic regression was used, in which variables that were <20% significant were preselected first, and were subsequently excluded by backward selection, in order to detect potentially important confounders. The programs used were: EPI INFO version 7 (Centers for Diseases Control and Prevention, Atlanta, GA, USA) and BioEstat 5.3 (Mamirauá Society, Belém, Pará, Brazil).
This study was approved by Brazil's Ministry of Health Platform, under the CAAE 22284913.1.0000.0021 protocol.
ResultsSixty-four patients with keratitis (cases) and 47 without keratitis (controls) were studied. The data in Table 1 show that there were no associations between keratitis occurrence and the following variables: sex, age, the presence of comorbidities, previous eye surgery (up to 3 years prior to the investigation), injuries, or concomitant clinical aspects (glaucoma, ocular neoplasia, blepharitis, lagophthalmos, entropion, conjunctiva injury, and post-surgical infection). Conversely, there was an association between the development of keratitis and agricultural- and cattle raising-related labor activity, prior ocular trauma, and the use of contact lenses. However, there was no statistically significant difference in the proportion of patients exhibiting improper lens wear and care in the groups with and without keratitis (Table 1).
Association between keratitis occurrence and the study variables.
| Variables | Keratitis (n=64) | Without keratitis (n=47) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | |||||
| Male | 36 | 56.2 | 22 | 46.8 | 0.325a |
| Female | 28 | 43.8 | 25 | 53.2 | |
| Age group | |||||
| From 18 to 20 years old | 6 | 9.4 | 4 | 8.5 | 0.193b |
| From 21 to 40 years old | 34 | 53.1 | 18 | 38.3 | |
| From 41 to 60 years old | 14 | 21.9 | 15 | 31.9 | |
| From 61 to 85 years old | 10 | 15.6 | 10 | 21.3 | |
| Labor activity | |||||
| No information | 1 | 1.6 | 1 | 2.1 | <0.001a |
| Retired/homemaker/student | 23 | 35.9 | 19 | 40.4 | |
| Farming | 21 | 32.8 | 1 | 2.1 | |
| Service provision/commerce/industry | 19 | 29.7 | 26 | 55.3 | |
| Comorbidity typec | |||||
| Diabetes | 10 | 15.6 | 5 | 10.6 | 0.448c |
| Hypertension | 2 | 3.1 | 1 | 2.1 | 1.000c |
| Anemia | 2 | 3.1 | – | – | 0.507c |
| Cancer | 2 | 3.1 | – | – | 0.507c |
| Heart disease | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Autoimmune disease | 1 | 1.6 | – | – | 1.000c |
| Sinusitis | 1 | 1.6 | – | – | 1.000c |
| Hypothyroidism | – | – | 1 | 2.1 | 0.423c |
| Contact lenses use | |||||
| Yes | 25 | 39.1 | 4 | 8.5 | <0.001b |
| No | 39 | 60.9 | 43 | 91.5 | |
| Inadequate contact lenses use | |||||
| Yes | 11 | 17.2 | 8 | 17.0 | 0.982c |
| No | 53 | 82.8 | 39 | 83.0 | |
| Previous ocular surgery (up to 3 years) | |||||
| Yes | 4 | 6.3 | 4 | 8.5 | 0.720c |
| No | 60 | 93.7 | 43 | 91.5 | |
| Trauma typec | |||||
| With vegetable/wood/land/animal | 19 | 29.7 | – | – | <0.001b |
| Metal object | 3 | 4.7 | – | – | 0.261c |
| Eye scratching | 1 | 1.6 | – | – | 1.000c |
| Injuries or concomitant clinical aspectsc | |||||
| Conjunctival and intraepithelial neoplasia | 8 | 12.5 | 1 | 2.1 | 0.076c |
| Blepharitis | 2 | 3.1 | – | – | 0.507c |
| Lagophthalmos | 1 | 1.6 | – | – | 1.000c |
| Glaucoma | 1 | 1.6 | – | – | 1.000c |
| Iris lesion in childhood | – | – | 1 | 2.1 | 0.423c |
| Entropion | – | – | 1 | 2.1 | 0.423c |
| Post-surgical infectious process | – | – | 1 | 2.1 | 0.423c |
| Conjunctival injury | – | – | 1 | 2.1 | 0.423c |
Note: The “no information” category, when present, was removed from the statistical calculation.
In 64 patients with keratitis, the most frequently reported symptoms were pain (n=47; 73.4%) and photophobia (n=27; 42.2%). Among the 47 patients without keratitis, the main complaints were burning and tearing (n=17; 36.2%), conjunctival redness (n=15; 31.9%), and sensation of sand or a foreign body in the eyes (n=13; 27.7%).
The main clinical specimens sent for microbiological examination were obtained by corneal scraping (n=47; 73.4%) and via contact lenses (n=14; 21.9%), while in patients without a keratitis diagnosis (n=47), conjunctival discharge (n=36; 76.6%) and tarsal–conjunctival scraping (n=10; 21.3%) were the most frequently used methods.
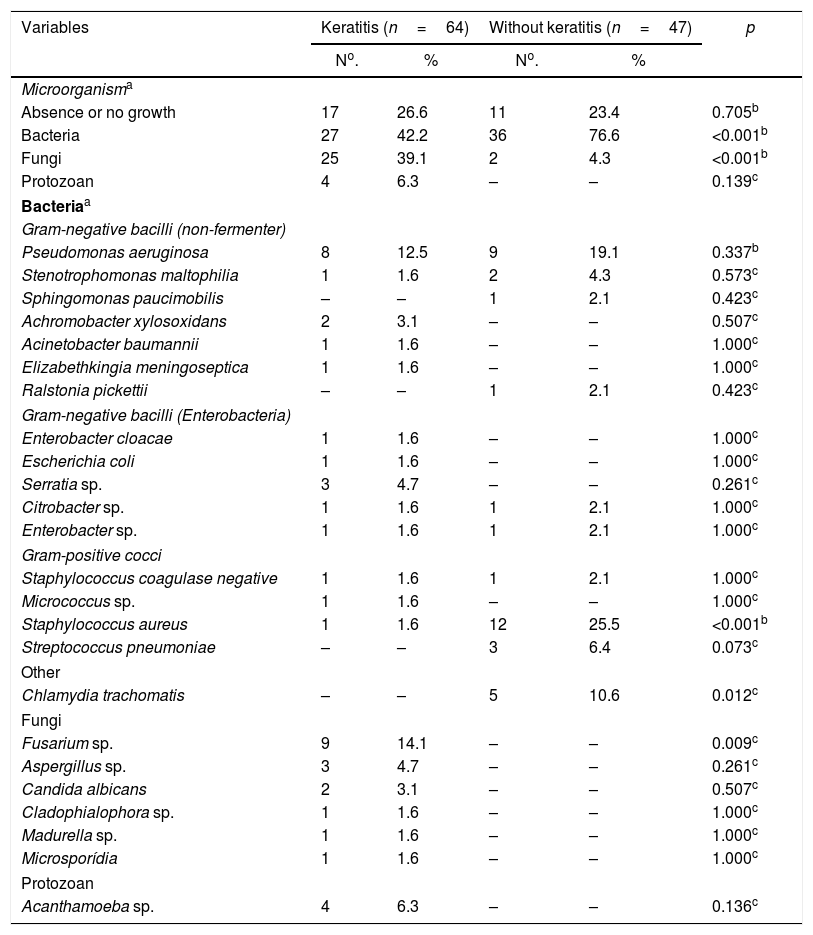
Regarding the etiology of infectious processes, when comparing cases and controls, it was observed that there were more cases of bacterial infection (n=36; 76.6%) in the group without keratitis (n=47) and a higher percentage of fungal infection (n=25; 39.1%) in patients with keratitis (n=64). The list of identified microbial agents is shown in Table 3.
Microorganisms identified in groups with and without keratitis.
| Variables | Keratitis (n=64) | Without keratitis (n=47) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Microorganisma | |||||
| Absence or no growth | 17 | 26.6 | 11 | 23.4 | 0.705b |
| Bacteria | 27 | 42.2 | 36 | 76.6 | <0.001b |
| Fungi | 25 | 39.1 | 2 | 4.3 | <0.001b |
| Protozoan | 4 | 6.3 | – | – | 0.139c |
| Bacteriaa | |||||
| Gram-negative bacilli (non-fermenter) | |||||
| Pseudomonas aeruginosa | 8 | 12.5 | 9 | 19.1 | 0.337b |
| Stenotrophomonas maltophilia | 1 | 1.6 | 2 | 4.3 | 0.573c |
| Sphingomonas paucimobilis | – | – | 1 | 2.1 | 0.423c |
| Achromobacter xylosoxidans | 2 | 3.1 | – | – | 0.507c |
| Acinetobacter baumannii | 1 | 1.6 | – | – | 1.000c |
| Elizabethkingia meningoseptica | 1 | 1.6 | – | – | 1.000c |
| Ralstonia pickettii | – | – | 1 | 2.1 | 0.423c |
| Gram-negative bacilli (Enterobacteria) | |||||
| Enterobacter cloacae | 1 | 1.6 | – | – | 1.000c |
| Escherichia coli | 1 | 1.6 | – | – | 1.000c |
| Serratia sp. | 3 | 4.7 | – | – | 0.261c |
| Citrobacter sp. | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Enterobacter sp. | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Gram-positive cocci | |||||
| Staphylococcus coagulase negative | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Micrococcus sp. | 1 | 1.6 | – | – | 1.000c |
| Staphylococcus aureus | 1 | 1.6 | 12 | 25.5 | <0.001b |
| Streptococcus pneumoniae | – | – | 3 | 6.4 | 0.073c |
| Other | |||||
| Chlamydia trachomatis | – | – | 5 | 10.6 | 0.012c |
| Fungi | |||||
| Fusarium sp. | 9 | 14.1 | – | – | 0.009c |
| Aspergillus sp. | 3 | 4.7 | – | – | 0.261c |
| Candida albicans | 2 | 3.1 | – | – | 0.507c |
| Cladophialophora sp. | 1 | 1.6 | – | – | 1.000c |
| Madurella sp. | 1 | 1.6 | – | – | 1.000c |
| Microsporídia | 1 | 1.6 | – | – | 1.000c |
| Protozoan | |||||
| Acanthamoeba sp. | 4 | 6.3 | – | – | 0.136c |
In patients with bacterial keratitis, bacterial infections were particularly caused by Pseudomonas aeruginosa. There was no difference in the percentage of infection by P. aeruginosa between groups, but there was a higher infection percentage by Staphylococcus aureus (25.5%) and Chlamydia trachomatis (10.6%) in patients without keratitis (n=47) (Table 3). In patients with keratitis, fungal infections were mainly caused by Fusarium spp. and Aspergillus spp., while in the control group, no fungal agents were identified (Table 3).
According to data from Table 4, 38 (59.4%) patients with keratitis (n=64) and 43 (91.5%) patients without keratitis (n=47) had not used any medicine prior to specimen collection for the microscopic examination. There was greater medicine use in patients with keratitis, and the most commonly used were the fourth-generation fluoroquinolone (n=16; 25.0%) and aminoglycosides (n=11; 17.2%).
Medicines used before and after sample collection for microscopic examination in groups with and without keratitis.
| Variables | Keratitis (n=64) | Without keratitis (n=47) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Medicines before collectiona | |||||
| None | 38 | 59.4 | 43 | 91.5 | <0.001b |
| Fourth-generation fluoroquinolone | 16 | 25.0 | 1 | 2.1 | 0.002b |
| Aminoglycosides | 11 | 17.2 | – | – | 0.002c |
| Acyclovir | 4 | 6.3 | – | – | 0.136c |
| Amphotericin B | 3 | 4.7 | – | – | 0.261c |
| Third-generation cephalosporin | 2 | 3.1 | 2 | 4.3 | 1.000c |
| Ciprofloxacin | 2 | 3.1 | – | – | 0.507c |
| Corticoid | 2 | 3.1 | – | – | 0.507c |
| Sulfamethoxazole trimethoprim F | 1 | 1.6 | – | – | 1.000c |
| Natamycin | 1 | 1.6 | – | – | 1.000c |
| Chloramphenicol | 1 | 1.6 | – | – | 1.000c |
| Decadron | 1 | 1.6 | – | – | 1.000c |
| Propamidine Isethionate | 1 | 1.6 | – | – | 1.000c |
| Therapeutic lenses | 1 | 1.6 | – | – | 1.000c |
| Ophthalmic lubricant | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Doxycycline | – | – | 3 | 6.4 | 0.073c |
| Bevacizumab | – | – | 1 | 2.1 | 0.423c |
| Vancomycin | – | – | 1 | 2.1 | 0.423c |
| Medicines after collectiona | |||||
| Fourth-generation fluoroquinolone | 26 | 40.6 | 9 | 19.2 | 0.042b |
| Amphotericin B | 20 | 31.3 | – | – | <0.001b |
| Aminoglycosides | 18 | 28.1 | 15 | 31.9 | 0.666b |
| Natamycin | 18 | 28.1 | – | – | <0.001b |
| Ophthalmic lubricant | 9 | 14.1 | 7 | 14.9 | 0.902b |
| Ciprofloxacin | 8 | 12.5 | 10 | 21.3 | 0.215b |
| Ketoconazole | 5 | 7.8 | – | – | 0.071c |
| Acyclovir | 4 | 6.3 | – | – | 0.136c |
| Biguanide | 4 | 6.3 | – | – | 0.136c |
| Atropine | 2 | 3.1 | – | – | 0.507c |
| Fluconazole | 2 | 3.1 | – | – | 0.507c |
| Mebendazole | 1 | 1.6 | – | – | 1.000c |
| Azithromycin | 1 | 1.6 | 5 | 10.6 | 0.081c |
| Chloramphenicol | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Itraconazole | 1 | 1.6 | 1 | 2.1 | 1.000c |
| Propamidine isethionate | 1 | 1.6 | – | – | 1.000c |
| Corticoid | 1 | 1.6 | – | – | 1.000c |
| Doxycycline | – | – | 5 | 10.6 | 0.012c |
| Third-generation cephalosporin | – | – | 4 | 8.5 | 0.030c |
| Dexamethasone | – | – | 2 | 4.3 | 0.177c |
| Vancomycin | – | – | 2 | 4.3 | 0.177c |
| Sulfamethoxazole trimethoprim F | – | – | 1 | 2.1 | 0.423c |
| Ciprofloxacin hydrochloride 0.3% with dexamethasone 0.1% | – | – | 1 | 2.1 | 0.423c |
Regarding the use of medicine following the microscopic examination (Table 4), in patients with keratitis (n=64), there was a higher use of fourth-generation fluoroquinolone (n=26; 40.6%), amphotericin B (n=20, 31.3%), and natamycin (n=18; 28.1%); in patients without keratitis (n=47), doxycycline (n=5; 10.6%) and third-generation cephalosporins (n=4; 8.5%) were most frequently used.
A summary of the main microorganisms involved in both cases and controls, and of the patients that used medicine before and after the microbiological collection, is shown in Fig. 1.
Microorganisms and medication taken prior and after to sample collection in patients with and without keratitis. “X” in both groups indicates that there were no statistically significant differences. “X” in only 1 group indicates that the frequency in that group was statistically significantly higher when compared to the other group.
In relation to the clinical evolution, in both cases and controls, all surveyed patients were medically discharged and only one patient with keratitis experienced recurrence. Patients without keratitis showed no sequelae, except for one, who had a pupil deformity.
Among those patients with keratitis (n=67), 7 (10.9%) developed corneal opacity, 10 (15.6%) had corneal perforation, 7 (10.9%) underwent corneal transplantation, and one (1.6%) underwent scleral graft. In three (4.7%) patients, evisceration was required, and in two of them, ocular prosthesis was necessary. No patients without keratitis had corneal perforation or evisceration, and they did not require corrective measures.
DiscussionAlthough sex, in this series, was not related to keratitis occurrence, epidemiological studies in southeastern Brazil showed that the highest number of keratitis cases occurred in male patients.3,10 This difference in the incidence of keratitis between sexes, would be related to socioeconomic aspects.
Previous studies showed that eye disorders mostly affected individuals between the ages of 30 and 60 years old.1,3,6,7 It is believed that the highest number of keratitis cases observed in this study was among patients aged from 21 to 40 years old, with a mean age of 31 years, which may be related to the number of contact lens wearers, likewise some authors had reported.6,11
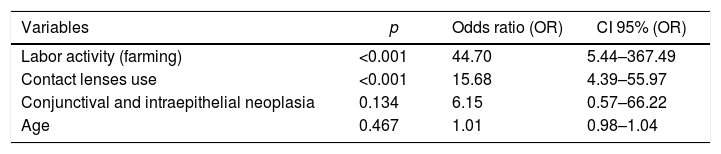
According to the multivariate analysis in the present study, it was found that the chance of developing keratitis was 16 times higher among contact lenses wearers (Table 2). Physiopathologically, contact lenses can induce corneal hypoxia, and the probability of micro-lesions and infection increases.12
Logistic regression for the factors associated with keratitis occurrence.
| Variables | p | Odds ratio (OR) | CI 95% (OR) |
|---|---|---|---|
| Labor activity (farming) | <0.001 | 44.70 | 5.44–367.49 |
| Contact lenses use | <0.001 | 15.68 | 4.39–55.97 |
| Conjunctival and intraepithelial neoplasia | 0.134 | 6.15 | 0.57–66.22 |
| Age | 0.467 | 1.01 | 0.98–1.04 |
The results obtained in this study corroborate the findings of other authors, who described a high frequency (49.3% and 64.9%) of keratitis among those who engage in farm work and other outdoor activities, respectively, as they are more likely to experience ocular trauma.3,4,10 The chance of keratitis occurrence was 45 times higher in people whose labor activity was linked to farming (Table 2). The use of safety glasses and prompt medical attention in cases of ocular trauma are measures that can reduce the incidence of keratitis among this group of workers.1
Evidence has shown that metabolic, systemic, and immunosuppressant diseases are predisposing factors for many pathologies, including ocular diseases, highlighting the existence of bacterial keratitis.2,3 However, in this study, no association was found between diabetes, other comorbidities, and keratitis occurrence.
Many reports have documented that prior eye surgery can constitute a risk factor for eye infection, with rates ranging from 1% to 35%.5,13 In this study, no association was found between previous eye surgery and keratitis occurrence, probably due to the small number of surgical procedures performed by patients with keratitis (4/64); an equal number of surgeries was also performed in the control group (4/47).
Keratitis presents with different clinical manifestations, although they are not pathognomonic of this disease. In this study, symptoms such as pain and photophobia were found to be significantly associated with keratitis, because the cornea it is a densely innervated tissue.14
In patients without keratitis, there was a higher percentage of burning, tearing, conjunctival redness, and sensations of sand or a foreign body, like in others studies.15
The etiologic identification of microbial keratitis is challenging due to the fact that it is difficult to obtain corneal specimens; there is also a lack of appropriate microbiological techniques.16 In routine eye care, collections are made with a scalpel or an insulin needle, which is slightly bent; this method requires a highly skilled ophthalmologist. Ideal corneal scrapings should be collected from the base and margin of the ulcers, using the Kimura spatula or a sterile blade, under direct vision through a slit lamp.3
Despite these difficulties, our culture-positive rate was 73.4% for keratitis cases and 76.6% for patients without keratitis. This finding, as well as our ability to identify data pertaining to the various causative agents, as obtained through microbiological tests, and represent the positive aspects of this research. Different authors obtained positivity rates that fell between 29% and 61%.3,17
Different Brazilian studies have reported a predominance of Gram-positive cocci in bacterial keratitis, with a predominance of Staphylococcus spp.3,17 In this study, a Gram-negative microorganism, Pseudomonas aeruginosa, was most frequently identified. Other studies describing the prevalence of Gram-negative bacilli have been associating its incidence with higher temperature regions and the use or wear of contact lenses.5,11,18
With respect to the fungal etiology of keratitis, Fusarium sp. was the most frequently isolated fungus, according to other authors.3,10 Among the 64 cases of keratitis investigated herein, two patients were observed with dematiaceous fungi isolates: Cladophialophora sp. and Madurella sp. These are found in the environment and are not usually isolated from keratitis samples.2,19
Moreover, of the cases examined in this report, the identification of an immunocompetent patient with microsporidial keratitis was highlighted. Reclassified as a fungus in 2001, this pathogen has been considered emergent in other countries, not only in patients with AIDS, but also in immunocompetent individuals. Microsporidia does not grow in culture media, nor is it well evidenced by staining methods used in routine laboratory settings.20–23
In suspected pictures of mild or moderate microbial keratitis, the use of fluoroquinolone monotherapy, as in this study, is being increasingly used due to its proven effectiveness when compared with the use of cephalosporins and aminoglycosides.24 The importance of microscopic examination can be observed in the analysis of pre- and post-medication sample collection. It was found that propamidine isethionate and biguanide were used following sample collections for microscopy tests of Acanthamoeba treatment. The same situation was observed for Microsporidia, in which treatment with mebendazole was also established.
Due to the morbidity of microbial keratitis, particularly given its impairment of ocular structures, more drastic measures are often required to treat this condition (corneal transplants, evisceration of the eye, grafts, and ocular prosthesis placement), as demonstrated in this study. It is believed that late diagnosis is one of the reasons why complications associated with infectious processes develop, and this can occur in regions characterized by large geographical dimensions that do not have access to treatment by specialized eye centers.
The limitations of this study are mainly associated with the fact that this study was a retrospective investigation. There were a number of issues associated with a lack of systematic and standardized records, such as the lack of more detailed data regarding the inadequate use of contact lenses. In addition, pre-treatment use of contact lenses by some patients may have affected the results of the microbiological tests.
The ocular trauma experienced in connection with labor activity can be prevented through the use of personal protective equipment. However, there are many questions regarding the use of contact lenses and the occurrence of infectious eye diseases. There is a consensus among many authors that both hard and gelatin lenses, made from materials with high oxygen permeability, adversely influence the central epithelial proliferation rates of the cornea, which indicates that the mechanical presence of a lens is enough to change the level of epithelial homeostasis when compared with individuals who do not wear contact lenses.25,26 Further studies are needed to explore the use of contact lenses, especially given their increasing popularity among youth and adolescents.
Conflicts of interestThe authors declare no conflicts of interest.