This study was conducted to isolate an acid-producing, alkaliphilic bacterium to reduce the alkalinity of cement industry waste (cement kiln dust). Gram-positive isolate KG1 grew well at pH values of 6–12, temperatures of 28–50°C, and NaCl concentrations of 0–16% and thus was further screened for its potential to reduce the pH of an alkaline medium. Phenotypic characteristics of the KG1 isolate were consistent with those of the genus Bacillus, and the highest level of 16S rRNA gene sequence similarity was found with Bacillus halodurans strain DSM 497 (94.7%). On the basis of its phenotypic characteristics and genotypic distinctiveness from other phylogenetic neighbors belonging to alkaliphilic Bacillus species, the isolated strain was designated B. halodurans strain KG1, with GenBank accession number JQ307184 (= NCIM 5439). Isolate KG1 reduced the alkalinity (by 83.64%) and the chloride content (by 86.96%) of cement kiln dust and showed a potential to be used in the cement industry for a variety of applications.
Microorganisms have been isolated from a variety of environments but are most abundant in moderate environments. Several environments can be considered extreme, including environments with a low or high pH, a low or high temperature, a high salinity, the presence of organic solvents, heavy metals, etc.1 The microorganisms inhabiting extreme environment are termed “extremophiles”. Extremophiles have gained considerable momentum in research due to their ability to grow under extreme conditions and to abate environmental pollution.2–4
Alkaliphilic bacteria have many potential applications in the biotechnology due to their enzymes, including proteases, cellulases, and xylanases, which are stable at high pH (>9.5) and temperature (>50°C).5 The enzymatic activities enable the bacteria to adapt to alkaline environments, biodegrade certain xenobiotics, produce several metabolites, and detoxify detrimental metal compounds.6
Most of the alkaliphilic bacteria studied belong to the genus Bacillus; they may have different characteristics depending on the strain,7 and live in a variety of habitats such as soda lakes, deserts, and arid soils.8 At least 26 alkaliphilic and alkalitolerant Bacillus species have been identified to date,9 which constitute the sixth rRNA group in the genus Bacillus.10
The outer cell layers of the alkaliphile can maintain the intracellular pH values near neutral in an alkaline environment at pH 10–13. The presence of Na+ ions in the growth medium plays a pivotal role in the adaptation of alkaliphilic Bacillus species to high pH values. Various studies have reported that Bacillus strains show variations in NaCl requirements due to species-specific differences in halotolerance.7 Guffanti et al.11 observed that the total membrane cytochrome content was considerably higher in cells grown at pH 10.5 than in those grown at pH 7.5, exhibiting the largest pH-dependent difference. The growth pH also resulted in different protein profiles.
Most alkaliphiles have been isolated from neutral environments, although their counts are higher in samples from alkaline environments.1 The frequency of alkaliphiles in the environment, especially in ordinary neutral soil samples, has been shown to be 102–105 per gram of soil, which corresponds to 1/10–1/100 of the population of neutrophiles. For isolation of alkaliphilic microorganisms in vitro, the sample can be enriched with different substrates such as peptones, glucose, bile salts, casamino acids and casein.12 An alkaline medium has to be used by adjusting pH to around 10 with sodium carbonate (Na2CO3) and/or Borax–NaOH, Na2HPO4/NaOH, or KCl/NaOH buffer systems, with buffering capacities over a pH range of 9–12 in various media.
Since pH is an important parameter of industrial waste and needs to be controlled, several environmental authorities such as the Environmental Protection Agency (EPA) in the US and the Central Pollution Control Board in India have set guidelines directing that the pH of the effluent or waste discharged by industries should be in the range of 6.5–8.5. Various chemicals such as hydrochloric acid,2 sulfuric acid,13 phosphoric acid14 and carbon dioxide15 are available to neutralize highly alkaline industrial waste byproducts and wastewater. However, these chemicals are difficult to handle as they are hazardous and corrosive, and large quantities of acid would be required to neutralize alkaline wastes, which is economically not feasible and has a dangerous effect on the health of workers as well as on industrial processes.
During the manufacturing of cement clinker, an alkaline waste byproduct known as cement kiln dust (CKD) is generated and collected from electrostatic precipitators and baghouses. The chemical composition of CKD may vary, depending on the type of raw material and the cement manufacturing process. During clinker production in the kiln, volatile sulfates, alkalis (K2O and Na2O) and chlorides are preferentially drawn toward CKD. In 2013, the Indian cement industry generated approximately 42–56 million metric tons of cement kiln dust, which is estimated to account for 15–20% of clinker production.16 The major part of it is dumped on an open land as landfill material, because, due to its high alkalinity, CKD is not reused in the cement kiln. Gebhardt17 and Mohamed and El-Gamal18 treated alkaline CKD with carbon dioxide gas to remove the alkalinity, but these methods are highly expensive and laborious. A better alternative to the use of a chemical process is biological treatment of alkaline wastes using alkaliphiles, which grow well at high pH.
The potential of alkaliphilic bacteria for wastewater treatment was reported by Tanabe et al.19 Ntougias et al.20 reported that large bacterial populations existed in alkaline olive mill wastes, and facultative alkaliphilic bacteria such as Exiguobacterium sp. were capable of lowering pH of highly alkaline wastewater from 12.0 to 7.5.6 These studies suggested that alkaliphilic bacteria could degrade pollutants under highly alkaline conditions, and their significant advantage was that they were not easily contaminated by neutrophilic microorganisms. However, reports on the application of alkaliphilic bacteria for treatment of solid alkaline wastes are very rare. Thus, in the present study we undertook the task of isolating and identifying new alkaliphilic bacteria from soil. One of the isolates, designated strain KG1 and belonging to the genus Bacillus, grew well at very high pH values (∼11–12) and can potentially be used for alkalinity removal for proper disposal and reuse of cement kiln dust.
Materials and methodsIsolation of alkaliphilic bacteriaAlkaliphilic bacterial strains were isolated from the rhizospheric soil of a citrus plant (pH 8.2), which is a habitat for a variety of microorganisms.1,8 An enrichment medium (10g glucose, 10g peptone, 5g yeast extract, 1g K2HPO4, and 15g bacteriological agar per 1000mL of water, pH 10.5 adjusted with 1N NaOH) was used for microbial isolation. A sterile glucose solution was added to the medium aseptically. Soil samples were collected randomly from a location at Thapar University, Patiala, India, and 10g were suspended in 100mL of sterilized water. The suspension was serially diluted, and 100-μL aliquots were then spread on agar plates and incubated at 35±2°C for 48h. Microbial colonies were selected on the basis of colony morphology and color. Single isolated colonies were then picked and re-streaked on agar plates with the same medium until pure colonies were obtained. The plates were stored at 4°C for further use.
The pure isolates were screened for their tolerance to pH 11 and 12 on a minimal medium (M9), since components of the enrichment medium precipitate at such high pH values. M9 medium contained (per 1000mL): 10g sucrose, 2.5g K2HPO4, 2.5g KH2PO4, 1g (NH4)2HPO4, 2g MgSO4·7H2O, 0.01g FeSO4·7H2O, and 0.007g MnSO4·4H2O, and pH was adjusted using KCl–NaOH buffer instead of NaOH to avoid precipitation or turbidity of the medium. The colonies isolated on the enrichment medium were then subcultured and adapted to M9 medium (pH 11–12) and stored at 4°C for further use.
Screening of the isolates for reducing the alkalinity of cement kiln dustScreening of the isolates was done on the basis of their capability to reduce the pH of the alkaline liquid minimal and enrichment media (pH ∼12). Acid production by the microbial isolates was monitored by decrease in pH at regular intervals for up to 5 days (CyberScan pH 510 pH meter). Each treatment was done in triplicates.
A promising isolate was selected and used for the treatment of CKD. The CKD collected from electrostatic precipitators at a cement plant was fine powdered and gray-black in color. The chemical composition of the sample was analyzed by energy dispersive X-ray spectrometry (EDX; JEOL JSM-6510 LV, USA) (Table 1). The CKD was mixed with the isolated bacterial strain designated KG1 at different OD values (OD value of 1.0≈108 cells), ranging from 0.6 to 1.0, and a ratio of CKD to culture equal to 4:1. The treatment mixture (in triplicate) was incubated at 35°C for 20 days, and sufficient moisture was maintained so that CKD was in the form of moist powder, not slurry or liquid mixture. A sucrose solution (10%) was added as a carbon source for the growth of the bacterial strain only once during the treatment, after 5 days of incubation. Aeration was provided by manual mixing during the treatment. As a control treatment, CKD was incubated under similar conditions without bacteria. After 20 days of incubation, samples were taken from the different treatments, mixed with water (1:10) in conical flasks, shaken (130rpm for 1h) to generate a leachate, and analyzed for alkalinity and chloride content, along with the control treatment.21 The pH values of the leachates from the different treatments were also measured using a pH meter (CyberScan pH 510). To confirm a decrease in alkalinity, a bacterial treated sample was analyzed by EDX for a change in the chemical composition and by X-ray diffraction (XRD) for a change in alkali phases (such as arcanite). All samples were air-dried and ground to a fine powder before EDX and XRD analyses.
Chemical composition of cement kiln dust without and with bacterial treatment.
| Chemical composition (compound %) | CKD control | CKD + strain KG1 |
|---|---|---|
| CaCO3 (C) | 21.56 (100) | 39.40 (+82.75) |
| MgO (Mg) | 0.69 (100) | 0.58 (−15.94) |
| Al2O3 (Al) | 2.38 (100) | 2.19 (−7.98) |
| SiO2 (Si) | 13.17 (100) | 10.97 (−16.70) |
| SO3 (S) | 1.13 (100) | 0.82 (−27.43) |
| K2O (MAD 10 feldspar) | 1.12 (100) | 0.59 (−47.32) |
| Ca (wollastonite) | 55.78 (100) | 48.19 (−13.61) |
| Fe2O3 | 2.62 (100) | 2.41 (−8.01) |
| CuO | 0.89 (100) | 0.80 (−10.11) |
| ZnO | 0.66 (100) | 0.64 (−3.03) |
| Equivalent alkali (Na2O+K2O) | 0.74 (100) | 0.39 (−47.30) |
The values in parentheses denote the percentage of compound increased or decreased compared to the control. The values were statistical significant at p<0.0001 using two way ANOVA.
For EDX analysis, samples were mounted on brass stubs using carbon tape. The XRD analysis was carried out using the PANalytical X’Pert Pro system (Netherlands). Random mounted powder specimens were scanned from 10 to 60°2Θ. Interpretation of the X-ray patterns was carried out by matching the peaks to the patterns reported in the literature and by using standard JCPDS cards, a database of X-ray powder diffraction patterns maintained by the International Center for Diffraction Data.
Characterization and identification of strain KG1Characterization of the strain was performed using standard biochemical tests22 and 16S rRNA-based phylogenetic analysis. Cell morphology was examined under a compound microscope (Nikon E100). Gram staining was performed as described by Cappuccino and Sherman.23 Biochemical tests, namely, starch and gelatin hydrolysis, H2S production, methyl red-Voges Proskauer, citrate utilization, catalase activity, and carbohydrate utilization, were performed according to Aneja.22 Growth experiments at different pH values (5–12), NaCl concentrations (0–20%), and temperatures (20–55°C) were conducted in both enrichment broth and minimal broth media. The efficient acid-producing alkaliphilic bacterial strain was then presumptively identified as per Sneath,24 Cowman and Steel's manual25 and Yoon et al.26
Sequencing of the 16S rRNA gene was performed by Xcelris Labs Limited, Ahmedabad (India). DNA was isolated from bacterial strain KG1 using the QIAamp DNA Purification Kit (Qiagen). The 16S rRNA gene was amplified by PCR with universal bacterial primers 8F (AGA GTT TGA TCC TGG CTC AG) and 1492R (ACG GCT ACC TTG TTA CGA CTT). The amplified PCR product was purified using the Qiagen MinElute Gel Extraction Kit according to the manufacturer's protocol. Sequencing of the purified 16S rRNA gene product was performed using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) as recommended by the manufacturer and an ABI 3730xl Genetic Analyzer (Applied Biosystems, USA). The 16S rRNA gene sequence of strain KG1 was compared to the corresponding neighbor sequences from the GenBank database using the BLAST tool at the National Center for Biotechnology Information server (http://www.ncbi.nlm.nih.gov). Multiple alignments of the strain KG1 sequence with those from related Bacillus species were performed using the MultAlin program,27 and a phylogenetic tree was constructed by the neighbor-joining method.28 Evolutionary distance matrices for the neighbor joining method were calculated using the algorithm of Kimura's29 two-parameter model. The topology of the phylogenetic tree was evaluated by performing a bootstrap analysis with 1000 replicates. The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequences of strain KG1 and related Bacillus species are shown in Fig. 5.
Statistical analysisStatistical analysis of the data (t-test and analysis of variance) was performed using the GraphPad Prism (version 5) software, and the significance level was set at p<0.05.
Results and discussionEight bacterial isolates were obtained from the soil, of which three isolates (strains KG1, KG4 and KG5) were able to grow at pH 12. Among the three isolates, strain KG1 showed better growth in culture broth at higher pH values (11 and 12) than the other two strains as well as the ability to tolerate and grew at high pH values by adjusting its cell metabolism, which is essential for survival.30 Few studies have reported the use of buffer systems such as phosphate buffer11,31 and carbonate buffer11,30 to increase pH of the growth medium, but no work has been reported on the use of KCl–NaOH buffer as a buffer system for increasing pH of the medium. The pH of the KCl–NaOH buffer system is around 13, so it can be used for preparing media with a high pH value such as 12.
Morphological, biochemical and phylogenetic characterization revealed that all the isolates were related to members of the genus Bacillus, and thus they were designated as Bacillus sp. strains KG1, KG4 and KG5. Strain KG1 was selected on the basis of its potential to neutralize the alkaline medium and reduce alkalinity and chloride levels more efficiently than the other two strains.
Acid production by bacterial strain KG1 resulted in a significant (p<0.0001) reduction of alkalinity in both minimal and enrichment media (Fig. 1). In the minimal medium, an overall change in 3.95 pH units was recorded after 3 days compared to 4th and 5th day of treatment. A similar trend of decreasing pH during 3 days of incubation was also observed in the enriched medium. This reduction of pH supported the results of acid production by Bacillus sp. strain KG1. Sucrose was the sole carbon source in the minimal medium. It was reported to be efficiently utilized by various Bacillus strains, resulting in a decrease in the pH of the medium.1 The change in pH could be attributed to the production of organic acids in the medium by enzymes, and these results were in close agreement with the findings of Paavilainen et al.32 Since the minimal medium is an economical and defined medium compared to enrichment media, it was used for subculturing of the isolate in this study.
According to the guidelines of several environmental agencies, the pH of industrial wastes should be neutral before their discharge to the environment as this is an important parameter to control surface and groundwater pollution. The removal of the CKD alkalinity using alkaliphilic bacterial strains is an eco-friendly method and was a challenging task for this study, since no study has been performed until now to treat alkaline solid industrial wastes with biological organisms. Fig. 2 represents the alkalinity reduction of cement kiln dust after treatment with strain KG1 at different cell densities (OD 0.6–1.0). The alkalinity of the control CKD leachate was 1467mg/L, whereas it was reduced by 83.64% after treatment with strain KG1 at an OD value of 0.8. The results exhibited a significant (p<0.05; t-test at 95% confidence limits) reduction in the alkalinity of the CKD leachate compared to the control. Alkalinity and pH are two different measurable parameters of leachate or water analysis, and they are often confused. The measurement of pH indicates acidity, alkalinity or neutrality of a leachate or water, while the measurement of alkalinity provides a buffering capacity, i.e., how much alkalinity is present in a sample as alkalinity ranges from above pH 4.0 to 14.0, and determines the form of alkalinity such as bicarbonate, carbonate or hydrate alkalinity. If pH is above 8.3, then alkalinity is due to carbonates, whereas if pH is above 4.3 then the alkalinity is due to bicarbonates. In this study, the pH value of the control CKD was 12.15, and after bacterial treatment (at OD 0.8) the pH value was reduced to 8.72. As per the WHO, US EPA and Indian Standards Institute (ISI), the pH and alkalinity of drinking water should be in the ranges from 6.5 to 8.5 and 200 to 600mg/L, respectively. After treating the CKD with bacterium KG1 (OD 0.8), the pH value was 8.72, which is slightly on a higher side, whereas the alkalinity was 240mg/L, i.e., within the desirable limit, according to the WHO, US EPA and ISI drinking water guidelines. Thus, after bacterial treatment CKD can easily be used in landfilling or reutilized in different applications to reduce surface and groundwater pollution.
Alkalinity and chloride reduction in CKD as influenced by the cell density of Bacillus halodurans strain KG1. The numbers above the bars represent the percentages of alkalinity and chloride, respectively, in the treated CKD samples compared to the untreated CKD control. The differences are statistically significant (p<0.05) as found by the t-test at 95% confidence limits.
The chloride content in the control CKD leachate was 460mg/L, which is higher than the maximum permissible limit (250mg/L) provided by the EPA and WHO standards for drinking water. The CKD treated with strain KG1 at OD 0.8 registered a significant reduction (by 86.96%; p<0.05; t-test at 95% confidence limits) in the leachate chloride content, compared to the control. Chlorides are generally present in groundwater as sodium chloride or calcium chloride. Chlorides in drinking water are not of any health concern but high chloride and magnesium contents may affect the quality and taste of drinking water. An excessive chloride concentration increases the hardness of water and the level of metal corrosion in water distribution systems, which leads to an increased metal concentration in groundwater. Water with chloride in excess of 2000mg/L is not recommended for many construction purposes as it affects the solidity and strength of concrete. Water with a high chloride content is not suitable for irrigation either, as evapotranspiration tends to increase the chloride concentration and salinity in the root zone of plants and makes it difficult for crops to take up water due to the difference in osmotic pressure between the water outside the plant and within the plant cell. Therefore, analysis of the chloride content in the CKD sample before and after bacterial treatment was necessary to know whether the sample (water or leachate) was suitable for drinking, construction, irrigation or other industrial purposes. Since CKD only contains inorganic mineral salts, mainly in the form of oxides (CaO, SiO2, Al2O3, Na2O, K2O, etc.), a carbon source should be added for the growth of the isolate in CKD, and the whole environment itself acts as a growth medium for the isolate.
Preliminary studies were conducted in this work to check the growth of the isolate in a CKD leachate containing 2% glucose solution as a carbon source. An increase of 0.227 in the cell density (OD) was observed, compared to the control medium (without inoculation), which revealed that 2% glucose supported the growth of strain KG1 to some extent. The maximum reduction in alkalinity in CKD+culture (OD 0.8) may indicate that this is the optimum cell concentration for the treatment. A low concentration of cells might affect the efficiency of the reaction, whereas a high concentration might generate unfavorable conditions for the growth as there was limited nutrition for a large number of cells. It appeared that the treatment of CKD with strain KG1 at a cell density (OD) of 0.8 supported the alkalinity reduction.
EDX and XRD analyses of the CKD sample treated with a bacterial culture (OD 0.8) were also performed to confirm the decrease in alkalinity. The EDX spectrum of CKD revealed the presence of calcite (CaCO3) and quartz (SiO2) as the major components, along with peaks for Al, Mg, K, S, and O (Fig. 3). Analysis of CKD showed the absence of Na and Cl and small amounts of Fe, Cu, and Zn (Table 1).
Thus, in the control CKD sample the alkalinity was mainly due to the presence of K (K2O) and S (SO3). The compound's percent composition of K2O and SO3 in the control CKD treatment was 1.12 and 1.13, respectively. Inoculation of CKD with bacterial strain KG1 resulted in a reduction of the K2O content to 0.59, which corresponded to a 47.32% reduction compared to the control CKD (no bacterial treatment), whereas a 24.43% reduction in the SO3 content and 47.30% equivalent alkali was observed by EDX. The EDX pattern of the control CKD compared with the bacterial strain KG1-treated CKD is depicted in Fig. 3. A reduction in the K peak height (corresponds to K2O) in the treated CKD was observed, compared to the control CKD, and this confirmed a significant (p<0.0001; two-way ANOVA) reduction in alkalinity.
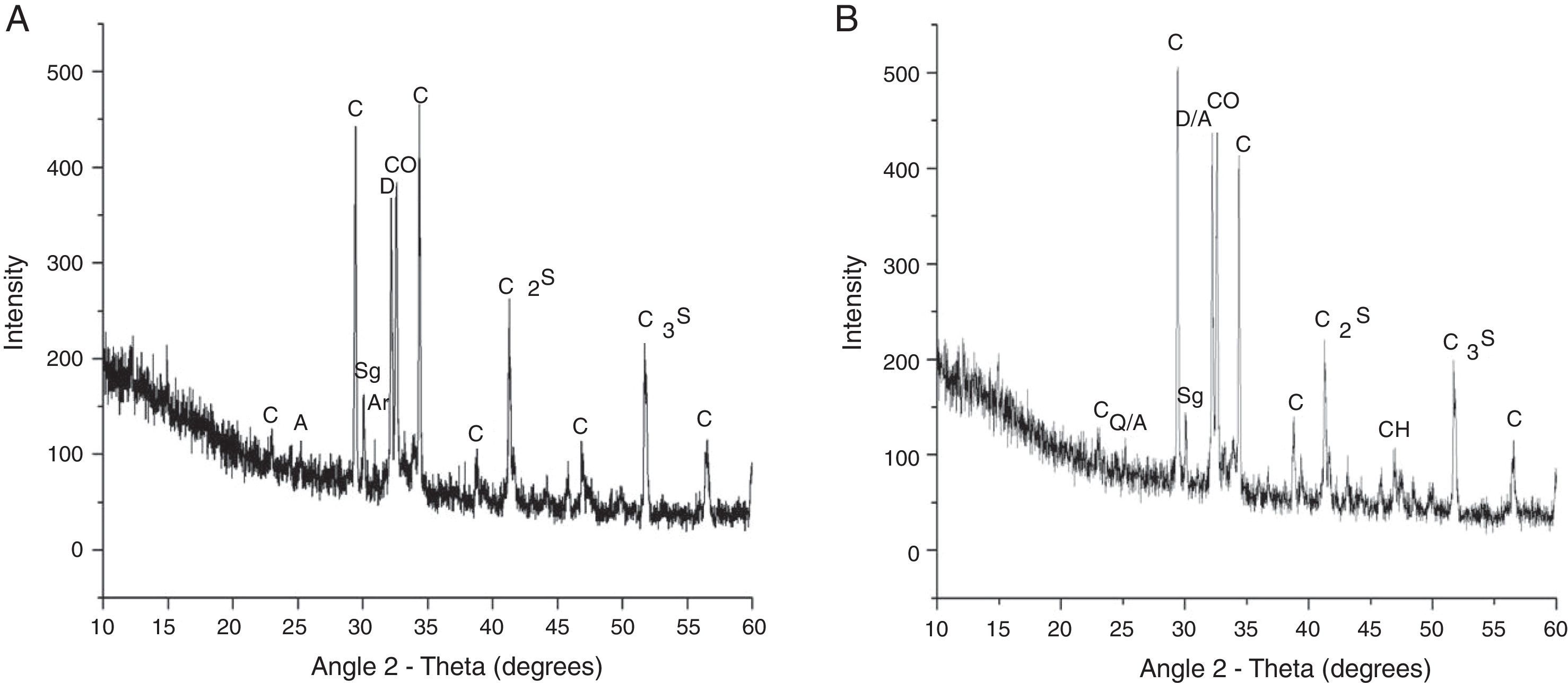
The XRD results (Fig. 4) indicated that the CKD mainly consisted of calcite (CaCO3), anhydrite (CaSO4), dolomite (CaMg(CO3)2) and free lime (CaO). Traces of crystalline alkali sulfate phases, viz., arcanites (K2SO4), and hydration product phases, viz., syngenite (K2(CaSO4)2), ettringite and calcium hydroxide (Ca(OH)2), were also observed. The absence of an arcanite phase peak (at 31°2Θ) in the bacterial-treated CKD also confirmed alkalinity reduction compared to the control (Fig. 4).
Diffraction pattern, along with crystalline phases, in the control CKD (A) and bacterial-treated CKD (B). C, calcite (CaCO3); D, dolomite (CaMg(CO3)2); A, anhydrite (CaSO4); CO, calcium oxide (CaO); Sg, syngenite (K2Ca(SO4)2); Ar, arcanite (K2SO4); C2S, dicalcium silicate; and C3S, tricalcium silicate.
The EDX and XRD analyses supported the absence of sulfate and crystalline alkaline sulfate phases in the treated powdered CKD compared to the control. The free sulfate and K2O present in the control CKD were converted after the treatment into bound forms such as anhydrites, ettringite (calcium aluminum sulfate phase) or syngenite (potassium calcium sulfate phase), which reduced the free sulfate content in CKD and thus reduced the alkalinity. These results are supported by the fact that microorganisms usually utilize carbohydrates through the fermentation and respiration processes, and the acid might be produced due to the carbohydrate metabolism. Bacterial isolates are highly diverse in their metabolism and channel a variety of materials into various central pathways (glycolysis, Kreb's cycle, etc.) of cell metabolism for complete oxidation, thereby producing an array of organic acids.30 Preliminary investigations in this work showed that direct addition of organic and inorganic acids to neutralize cement kiln dust is not feasible, since large amounts of these acids would be needed, which is not economical. Use of inorganic acids such as hydrochloric acid, sulfuric acid, etc., in the neutralization process can be hazardous and corrosive and may cause health problems to the workers. Chloride also increases the alkalinity of CKD. Reduction in the chloride content also marks an important parameter to study, as its high concentration in groundwater through leachate affects the taste of groundwater and increases its alkalinity. Excess chloride causes cardiovascular diseases. A high chloride content corrodes steel and imparts permanent hardness to groundwater. In agriculture, chloride increases salinity of soil through irrigation. In this study, chloride was reduced by approximately 86.96%, which shows that CKD can safely be reused and disposed off to landfill after bacterial treatment. Thus, the use of a biological tool for the neutralization of alkaline solid wastes is a better alternative to conventional chemical methods. The growth of the bacterium on the minimal medium also shows its economic effectiveness as a cheap growth medium, compared to ready-to-prepare enriched growth media such as nutrient broth, etc.
Characterization and identification of the bacterial strainThe phenotypic characteristics (morphological and biochemical) of strain KG1 are presented in detail in Table 2. Isolate KG1 was Gram-positive, rod-shaped, growing aerobically, able to hydrolyze starch and gelatin and was also positive in the catalase and citrate tests. Strain KG1 showed characteristic utilization of the carbohydrate substrates glucose, sucrose, fructose, maltose, xylose, mannitol and lactose. It grew well at pH 6–12, and the growth rate at pH 8–9 was better than that at acidic pH. The NaCl concentrations of 0–16 and 0–8% were found suitable for the growth of isolate KG1 in the enrichment and minimal media, respectively. The growth temperature range was 28–50°C, and the optimum growth temperature was 33–37°C.
Characteristics of Bacillus halodurans KG1 and some related Bacillus species.
| Morphological, biochemical and physiological characteristics | Bacillus halodurans KG1 | Bacillus halodurans DSM 497a | Bacillus okuhidensisa,b |
|---|---|---|---|
| Gram staining | +ve | +ve | +ve (in exponential growth phase) and −ve in stationery growth phase |
| Cell shape | Small rods | Rod | Rod |
| Colony shape | Irregular | Filamentous | Smooth circular |
| Colony color | White opaque | White | Yellowish |
| Growth at: | |||
| 45°C | + | + | + |
| 50°C | + | + | + |
| pH 5 | + | ND | ND |
| pH 10 | + | V | + |
| pH 11 | + | − | + |
| pH 12 | + | ND | ND |
| Optimum pH | 8–9 | 9.0 | 10.5 |
| Tolerance of NaCl | |||
| 5% | + | + | + |
| 7% | + | + | + |
| 10% | + | + | + |
| 12% | + | + | − |
| 16% | + | − | − |
| 20% | − | ND | − |
| Methyl Red test | − | ND | ND |
| Voges–Proskauer test | + | ND | ND |
| Citrate utilization test | + | ND | ND |
| Starch hydrolysis test | + | + | + |
| Gelatin test | + | + | + |
| Catalase test | + | ND | ND |
| Lipase test | − | ND | ND |
| Carbohydrate utilization | |||
| Glucose | + | + | ND |
| Sucrose | + | + | ND |
| Lactose | + | + | + |
| Mannitol | + | ND | + |
| Xylose | + | + | + |
| Fructose | + | + | ND |
| Maltose | + | + | ND |
Characteristics are scored as: +, positive reaction; −, negative reaction; w, weak reaction; v, variable; ND, no data. For all growth assays: +, growth; −, no growth; w/+, weak growth. Superscript after the taxa denotes the reference from where the datum is obtained.
The 16S rRNA gene sequence (1408 bases) of strain KG1 was analyzed in order to determine the phylogenetic position of the isolate. It was compared for sequence similarity with sequences of previously reported strains, and a phylogenetic tree was constructed by the neighbor-joining method using the sequences of the closely related taxa, retrieved from the GenBank database (Fig. 5).
A neighbor-joining phylogenetic tree showing the relationships of bacterial strain KG1 and the type strains of closely related Bacillus species, based on 16S rRNA gene sequences. The GenBank accession numbers are given in parentheses. Bootstrap values (expressed as percentages of 1000 replications) greater than 50% are shown at the branch points. Bar, 0.02 nucleotide substitutions per site.
The results of the morphological, biochemical and phylogenetic analysis suggested that strain KG1 was a member of the sixth rRNA group10 of the genus Bacillus, which includes alkaliphiles, and formed a clade with Bacillus halodurans strain DSM 497 (AJ302709), with a bootstrap value of 99%. Pairwise sequence analysis revealed that the highest sequence similarity was with B. halodurans strain DSM 497 (94.7%), followed by Bacillus okuhidensis strain GTC 854 (93.9%), while the remaining Bacillus species with validly published names showed less than 94% similarity.
Strain KG1 could be differentiated from other phylogenetic neighbors of previously reported alkaliphilic Bacillus species on the basis of morphological, biochemical and physiological characters given in Table 2. The generally recommended and accepted criteria for delineating bacterial species state that strains with a 16S rRNA gene sequence dissimilarity greater than 3% or with DNA-DNA hybridization relatedness of less than 70% are considered to belong to separate species.35 It is recommended that bacterial strains with a difference in the 16S rRNA gene sequence of less than 3% cannot be allocated to new species without the support of DNA-DNA relatedness studies. In our study, bacterial strain KG1 showed a difference in the 16S rRNA gene sequence of 5.3% with the most closely related Bacillus species (94.7% sequence similarity with B. halodurans strain DSM 497). Thus, on the basis of morphological, biochemical, physiological and phylogenetic results, it is proposed that strain KG1 be classified as the novel type strain of a species, B. halodurans sp. strain KG1 nov. The 16S rRNA sequence of strain KG1 was submitted to GenBank and assigned accession number JQ307184. The culture is deposited at NCIM (National Collection of Industrial Microorganisms), NCL (National Chemical Laboratory), Pune (India) and assigned deposition number NCIM 5439.
ConclusionsCement kiln dust, a waste byproduct, is generated during the manufacturing of cement clinker. During clinker production in a kiln, volatile components such as sulfates, alkalis (K2O and Na2O), chlorides, etc., are drawn toward CKD and make it alkaline, thus restrict CKD reuse in a cement kiln. Huge amounts of CKD are dumped in landfill, which, due to leaching, leads to pollution of groundwater. Biological treatment using B. halodurans strain KG1 for the removal of alkalinity and chloride from CKD is a better alternative to chemical methods in terms of cost effectiveness, efficiency and non-toxicity.
Conflicts of interestThe authors declare no conflicts of interest.