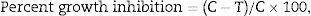The antagonistic potential of Trichoderma strains was assayed by studying the effect of their culture filtrate on the radial growth of Sclerotium rolfsii, the causal agent of chickpea collar rot. Trichoderma harzianum-1432 (42.2%) and Trichoderma atroviride (40.3%) were found to be strong antagonists. To enhance their antagonistic potential, mutagenesis of these two selected strains was performed. Two mutants, Th-m1 and T. atroviride m1, were found to be more effective than their parent strains. The enzymatic activities of the selected parent and mutant strains were assayed, and although both mutants were found to have enhanced enzymatic activities compared to their respective parent strains, Th-m1 possessed the maximum cellulase (5.69U/mL) and β-1,3-glucanase activity (61.9U/mL). Th-m1 also showed high competitive saprophytic ability (CSA) among all of the selected parent and mutant strains, and during field experiments, Th-m1 was found to successfully possess enhanced disease control (82.9%).
Chickpea (Cicer arietinum) collar rot (Sclerotium rolfsii Sacc.) is one of the most devastating soil-borne diseases of fungal origin and results in a 10–30% yield loss annually, depending on disease severity. The chemical control of soil-borne pathogens provides a certain degree of control but can simultaneously have adverse effects on the environment affecting beneficial soil microorganisms. Therefore, the biological control of plant pathogens by microorganisms has been considered a more natural and environmentally acceptable alternative to existing chemical treatment methods.1,2Trichoderma spp. are free-living fungi that are common in soil and root ecosystems and have now been established to be opportunistic, avirulent plant symbionts and parasites of several soil-borne phytopathogens.3 Depending on the strain, the use of Trichoderma in agriculture can provide numerous advantages: (1) the colonization of the rhizosphere (rhizosphere competence), allowing rapid establishment within stable microbial communities in the rhizosphere; (2) the control of pathogenic and competitive/deleterious microflora through a variety of mechanisms; and (3) improvements in plant health.
In the present study, attempts were made to develop an improved strain of Trichoderma for the better management of this disease.
Material and methodsOrigin, isolation and maintenance of Trichoderma strainsThe sampling sites selected for the isolation of Trichoderma from soil were in the areas where the disease caused by S. rolfsii was either very low or non-existent in the presence of susceptible hosts. The soil samples were transferred to the laboratory and air-dried at room temperature. Trichoderma species were isolated on a selective medium [(TSM) Elad, 1981]. Approximately 20mL of TSM medium was poured into Petri dishes and allowed to solidify. The serial dilution method was employed to isolate Trichoderma from soil samples. One gram of dried soil was added to 9mL of sterilized distilled water and was serially diluted to a dilution factor of 104. Thereafter 200-μL aliquots of soil suspension were spread on TSM. The plates were incubated at 25±2°C. The colonies appearing on the medium were transferred to PDA. The cultures of Trichoderma were also maintained on PDA slants at 4°C for further study. The identification of Trichoderma isolates was made using the taxonomic keys of Rifai (1969), Bisset (1984, 1991a,b and 1992), Kubicek and Harman (1998) and Nagamani et al. (2002).
Effect of culture filtrates of the selected Trichoderma strains on S. rolfsiiThe selected Trichoderma spp. and S. rolfsii were grown on PDA medium in Petri dishes at 25±2°C for 4 days. Two equal size blocks (5mm each) of Trichoderma species, cut from the actively growing margins of 4-day-old cultures, were inoculated separately into 250-mL Erlenmeyer flasks each containing 100-mL sterilized potato dextrose broth in triplicate. After 10 days of incubation at 25±2°C, the static cultures were filtered through Whatman filter paper number 44 and then through a Seitz filter (G 4) attached to a vacuum pump to obtain cell-free culture filtrates. Three concentrations of the culture filtrates (10, 20 and 40%) were used for this study. Five-millimeter agar blocks of actively growing colonies from 5-day-old S. rolfsii cultures were cut from the colony margin and inoculated at the center of a Petri dish separately containing PDA medium and the culture filtrate. The control set was made by pouring 20mL of PDA medium only in sterilized Petri dishes. The inoculated Petri dishes were incubated at 25±2°C and the radial colony growth was measured after 4 days of incubation. The percent inhibition in the radial growth of the colony was calculated using the following formula:
where C, growth in control and T, growth in treatment.Generation of mutant strains through N′-methyl-N′-nitro-N′-guanidine (NTG) treatmentThe method of Chadegani and Ahmadjian4 was followed for mutagenesis of T. harziaunum-1432 and T. atroviride, which exhibited maximum activity against the pathogen during screening, using N′-methyl-N′-nitro-N′-guanidine (NTG). Spore suspensions from a 10-day-old culture of the selected Trichoderma isolates were prepared in 5mL of sterile 0.1M sodium citrate buffer (pH 5.5), filtered through cheese cloth, centrifuged twice at 10,000rpm and subsequently washed with the same buffer. After the second washing, the pellets were resuspended in 5mL of sodium citrate buffer and the spore concentration was adjusted to 1×105 spores/mL using hemocytometer. A stock solution of NTG (1mg/mL) was prepared in sodium citrate buffer immediately before the treatment and the final concentration used was 50μg/mL of spore suspension. The NTG-treated spores were incubated at 37°C in a shaking water bath for 45–90min to achieve 5–10% viability. At selected intervals, mutagenesis was stopped by passing the entire 4-mL sample through a 0.45-μm Millipore filter, washing the spores twice with 0.1M phosphate buffer, and finally resuspending the spores in the same buffer. The spores treated with NTG were inoculated on minimal medium for colony forming units. The sensitivity of wild-type isolates of Trichoderma to fungicide was tested by amending the culture medium with increasing concentrations of the fungicide.
Effect of culture filtrate of the parent and mutant strains of Trichoderma strains on S. rolfsii radial growthThe effect of culture filtrate of the parent and mutant Trichoderma strains on the radial growth of S. rolfsii was assayed using the method described in the “Material and methods” section.
Effect on the cellulase and β-1,3-glucanase activity of the selected Trichoderma strainsThe selected parent and mutant strains of Trichoderma were cultured at 30°C on a synthetic medium (SM medium). Flasks containing 50mL of liquid SM medium were inoculated with 2 blocks (5mm) of mycelia discs cut from the actively growing cultures of the selected mutant and parent strains of Trichoderma. The glucose in the medium was substituted with selected carbon sources (0.2%, v/v) and nitrogen with selected nitrogen sources (0.1%, v/v). Cultures were incubated at 30°C in a rotary shaker at 120rpm for 4 days and then centrifuged at 15,000×g at 4°C for 10min. The supernatant was assayed for chitinase and β-1,3-glucanase activity by the method described as follows.
β-1,3-Glucanase assayβ-1,3-Glucanase was assayed by measuring the release of reducing sugar with DNS.5 One milliliter of enzyme sample was incubated with 1mL of 0.2% laminarin in 50mM sodium acetate buffer (pH 4.8) at 50°C for 1h. Two milliliters of copper reagent was then added, and the reaction mixture was boiled for 10min in a water bath. The tubes were cooled, and 2mL of arsenomolybdate reagent was added and vertexed; the final volume was adjusted to 25mL with distilled water. The solution was centrifuged at 10,000rpm for 5min and supernatant aliquots were assayed for their absorbance at 500nm. The amount of reducing sugars released was calculated from a glucose standard curve. One unit of β-1,3-glucanase activity was defined as the amount of enzyme that catalyzed the release of 1.0μmol of glucose equivalents per min during the hydrolysis reaction.
Cellulase assayCellulase activity was assayed by measuring the release of reducing sugar with DNS.6 The assay mixture contained 1mL of 0.5% cellulose (Sigma Co.) suspended in 50mM citrate phosphate buffer (pH 4.8) and 1mL of culture filtrates of different Trichoderma strains in 15-mL test tubes. The reaction mixture was incubated for 30min at 50°C and then centrifuged at 12,000rpm for 5min at 4°C. The reaction was arrested by adding 3mL of 1% DNS (dinitrosalicylate) reagent in 1M NaOH and followed by heating for 10min at 100°C to develop a red-brown color. While it was hot, 1mL of 40% Rochelle salt (potassium sodium tartrate) was added to stabilize the color. Blanks were generated the same way using distilled water in place of culture filtrate. After cooling the mixture to room temperature in a water bath, the absorbance was measured with a spectrophotometer (Systronics spectrophotometer) at 540nm. The glucose content was obtained using a glucose standard prepared by the same procedure. One unit of cellulase activity was defined as the amount of enzyme in 1mL of the reaction mixture that released 1μmol of reducing sugar under assay conditions.
Competitive saprophytic ability (CSA) of the selected Trichoderma strainsThis experiment was performed following the Cambridge method,7 later modified by Ahmad and Baker.8 A pure inoculum of S. rolfsii was prepared in sterilized 500-mL conical flasks each containing 200g of acid-washed sand+3% maize meal. Each flask was inoculated with three blocks of S. rolfsii culture (5-mm diameter). The moisture level of the sand-maize meal was maintained at 25%, and the pH was adjusted to 6.5. The flasks were incubated at 25±2°C for 10 days. The parent and mutant strains of Trichoderma strains were grown on PDA. The plates were incubated at 25±2°C for 5 days. The plates were then flooded with sterile distilled water, and the conidia were gently freed from the culture plate with the help of brush. The suspension was sieved through four layers of cheese cloth and then centrifuged at 2500×g for 15min. The number of conidia in the suspension was counted with a hemocytometer, and their numbers were then adjusted to the desired level. The S. rolfsii inoculum-soil mixture was mixed with freshly harvested conidia of parent and mutant strains of Trichoderma at rates of 101, 102, 103 and 104 conidial suspension in 0.1mL per gram of soil. No conidia were added to the controls. The soil was mixed thoroughly by hand and was filled in 500mL flasks. Twenty pieces (2cm in length and 0.2–0.5cm in diameter) of sterilized chickpea roots were buried in 500-mL conical flasks containing 250g of inoculum mixture of antagonist and pathogen for each dilution. The flasks were incubated at 25±2°C for four weeks, and then, root bits were taken out and washed 10–12 times with sterilized distilled water. The washed root pieces were then surface-sterilized with 0.1% sodium hypochlorite solution and 5% ethanol for 5min and again washed thoroughly with sterilized distilled water. The root pieces were then soaked between the folds of sterilized blotting papers and transferred to Petri dishes containing 20mL TSM (Trichoderma selective medium). Five root pieces were placed onto the medium in each Petri dish. The experiments were set in three replicates for each dilution. All of the Petri dishes were incubated at 22± 2°C for 6 days, and the percent colonization of root pieces by the test antagonist was recorded.
Biological control of collar rot of chickpea in the fieldThe parent strains of T. harzianum-1432 and T. atroviride and their most potent mutants, Th-m1 and T. atroviride m1, were used for field experiments. The plot size (1.5m×1.5m) was prepared and used for each combination in triplicate. All of the plots were infected with the pathogen by amending 150g of pure inoculum prepared on barley grains, and the plots were left for 20 days to allow the pathogen to establish itself in the soil. Then, 450g of antagonists prepared on barley grains were also amended in field soil and left for 10 days. Surface-sterilized seeds of the susceptible variety of chickpea were sown at a rate of 100 seeds per plot. The development of disease was monitored regularly, and complete disease appearance was noted after 14 days of sowing. The percent mortality and percent disease control were calculated using the following formulae:
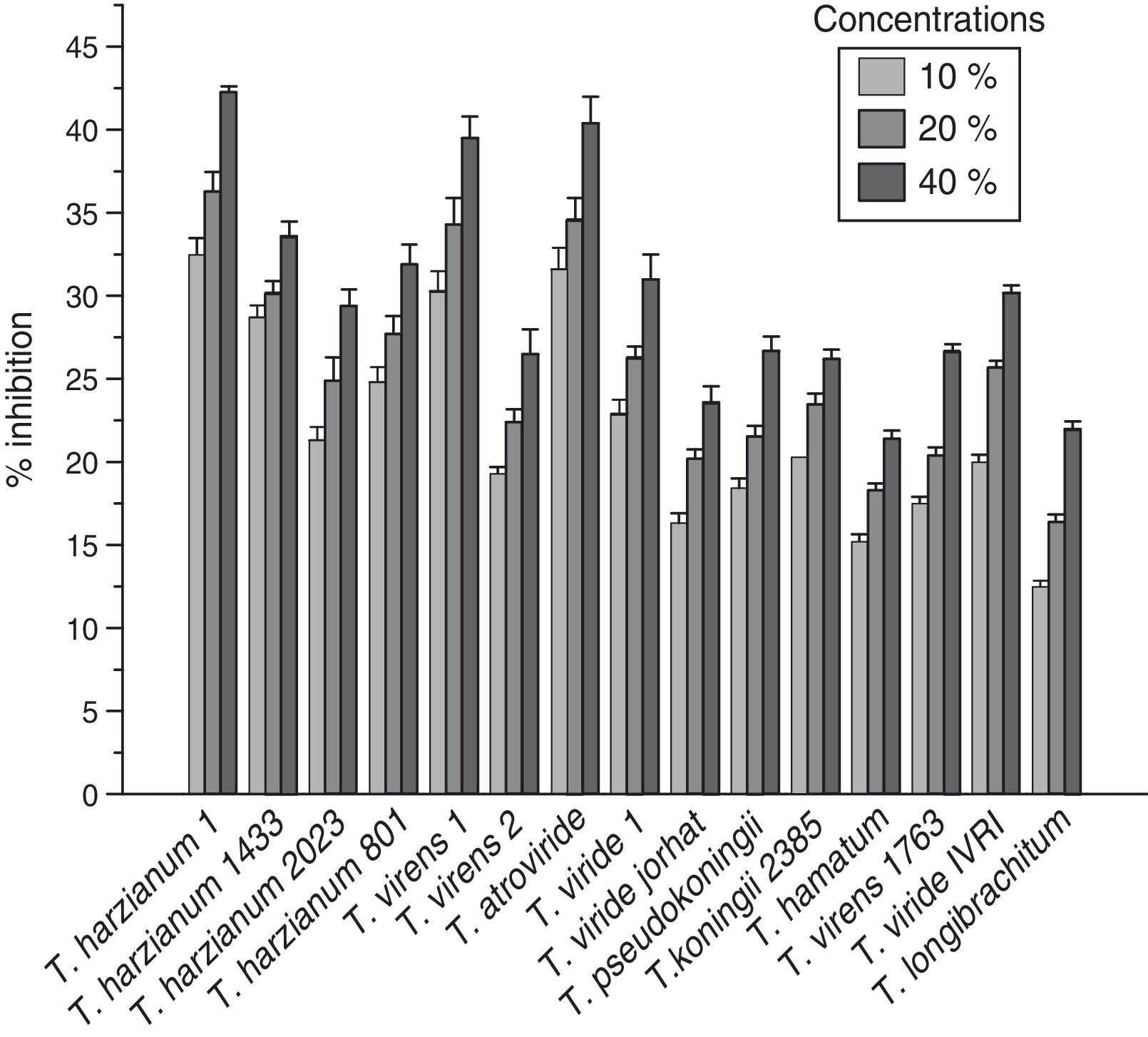
ResultsThe pure culture of the pathogen isolated from the infected field of chickpea suffering from collar rot disease and the different strains of Trichoderma were maintained on PDA medium at 25±2°C. Fig. 1 shows the effect of different concentrations of culture filtrates of potent Trichoderma species on the percent inhibition of S. rolfsii radial growth. Three concentrations of culture filtrates (10%, 20%, and 40%) were used in this study. The inhibition rates of S. rolfsii radial growth varied at every concentration of culture filtrate for all of the Trichoderma used. Fig. 1 shows that the maximum inhibition of radial growth of S. rolfsii by all strains was found at the 40% concentration of culture filtrate, compared to the 10% and 20% culture filtrate concentrations. It is evident that the maximum percent inhibition of S. rolfsii was found at 40% T. harzianum-1432 (42.2%) followed by T. atroviride (40.3%).
The most effective antagonists tested, T. harzianum-1432 and T. atroviride, were selected for the generation of mutant strains to enhance the efficacy of anti-S. rolfsii antagonism. The radial growth of wild-type isolates was inhibited by approximately more than 90% at 50ppm of carbandazim in the medium. The treatment of spores with NTG was much more stable than with UV irradiation and its resultant isolates. These mutagenized strains possessed significant levels of carbandazim tolerance and a higher growth rate than their parent strains. Ultimately, 2 isolates, one derived from T. harzianum-1432 and one derived from T. atroviride, were obtained after a series of 11-to-13 serial transfers on media with increasing concentrations of fungicide up to 100ppm. Tolerant isolates were capable of growing at fungicide concentrations lethal to wild-type isolates and were stable following culture on unamended medium. Tolerance was also found to be unaltered when isolates were retrieved from long-term storage cultures in the absence of fungicide (PDA slants covered with paraffin oil). These mutants were named Th-m1 and T. atroviride m1. They showed different and characteristic morphological features as well as specific growth patterns, which differed from their parent strains.
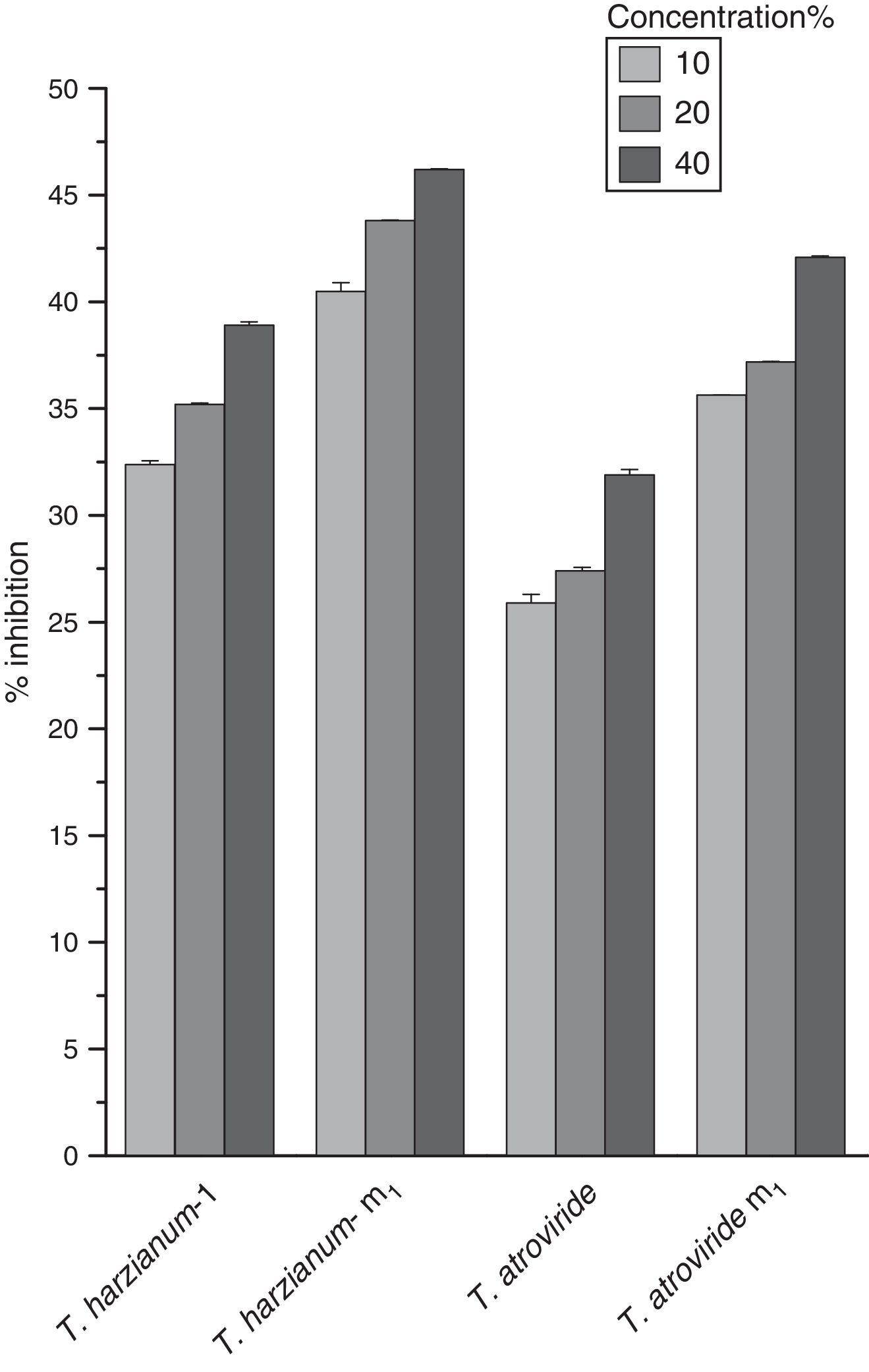
The effect of different culture filtrate concentrations of Trichoderma parent and mutant strains on the percent inhibition of S. rolfsii radial growth is shown in Fig. 2. As the concentration of culture filtrates was increased (20–40%), the percent inhibition also increased. It is evident from Fig. 2 that Th-m1 showed maximum percentage of S. rolfsii inhibition at all three concentrations tested in this study compared with other mutant and parent strains tested.
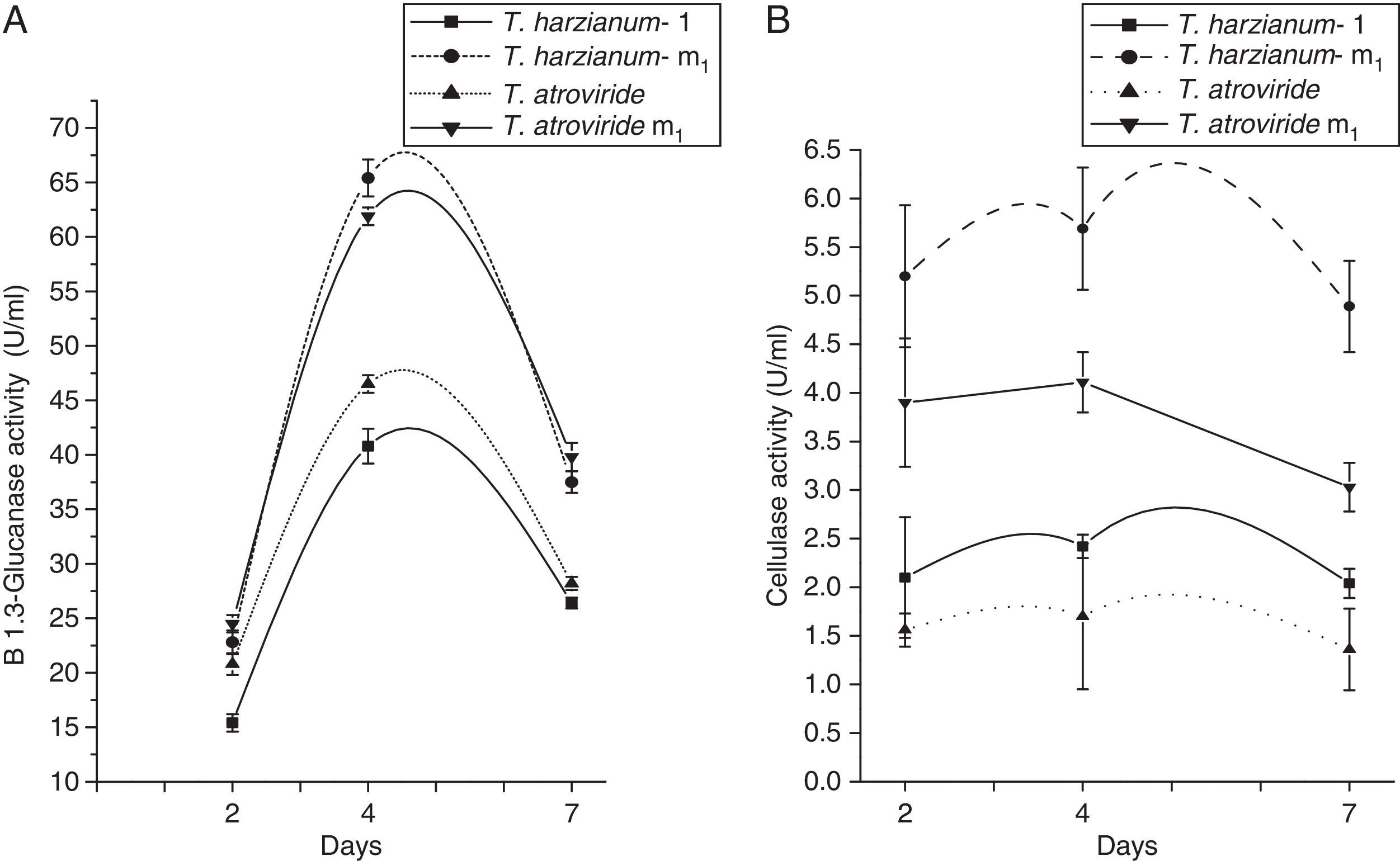
The production of β-1,3-glucanase by mutant strains compared to their respective parent after 2, 4 and 7 days of incubation has been presented in Fig. 3A. It is evident from this figure that the β-1,3-glucanase activity of each strain increased as the days passed, but after 4 days of incubation, they follow the same pattern as in cellulase activity. The maximum enzyme activity was shown by Th-m1 (61.9U/mg) followed by T. atroviride m1 (46.5U/mg) in 4 days of growth under the same growth conditions.
Fig. 3B shows the production of cellulase by Trichoderma parent and mutant strains at 2, 4 and 7 days. The maximum cellulase activity was recorded for all strains used in the study 4 days after the inoculation of spores. The rate at which the enzyme appeared in the culture medium was higher in the case of Th-m1 (5.69U/mL) followed by T. atroviride m1 (4.11U/mL).
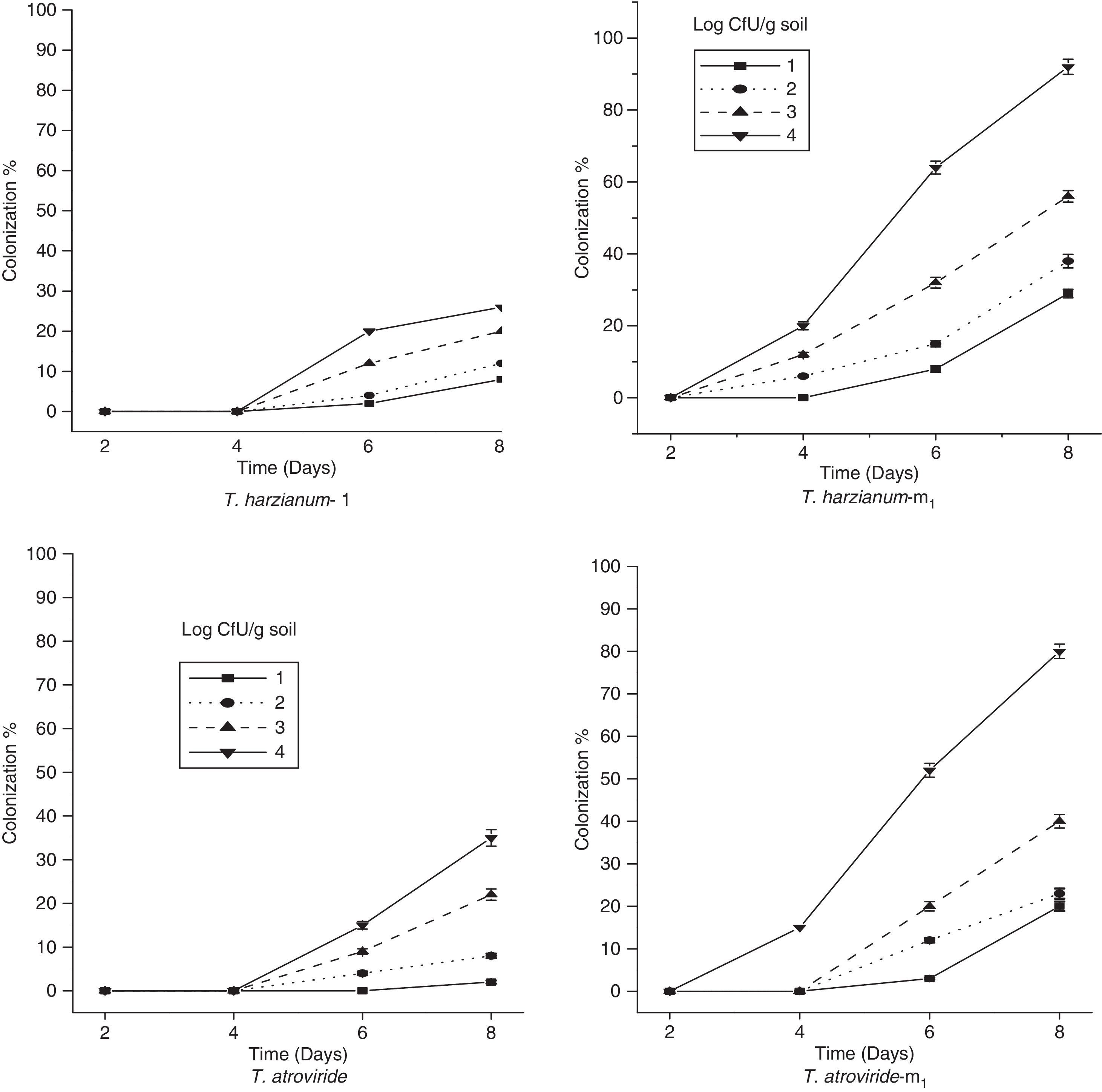
The percent colonization of chickpea root segments by selected parent and mutant strains of Trichoderma is shown in Fig. 4. The data revealed that when root segments were removed after 2, 4 and 6 days of incubation from the soil infested with the conidia of Trichoderma strains, the two parent strains, T. harzianum-1432 and T. atroviride, were recovered from root segments less frequently than the mutant strains used in the study and were slow to colonize root segments at higher population densities. However, the mutant T. atroviride m1 was isolated from the root pieces only at higher population densities. From Fig. 4, it is clear that Th-m1 showed a significantly higher percent colonization than the other mutant strain at all population densities on all studied days.
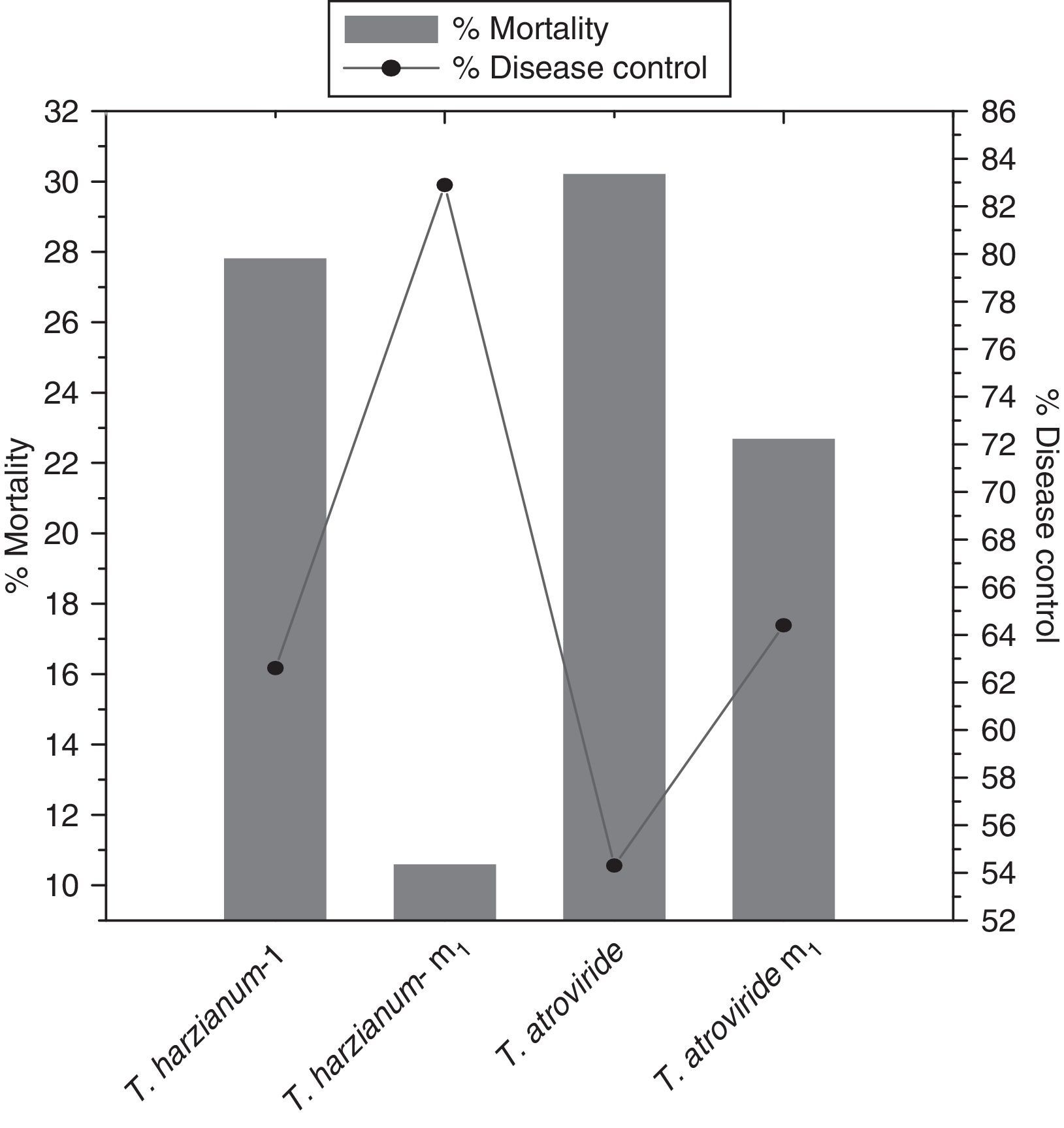
Results presented in Fig. 5 show that Th-m1 possessed the maximum percent of disease control (82.9%) and minimum percent mortality (10.6%). The two parent strains, Th-1432, T. atroviride and the mutant T. atroviride m1, were found less significant to control the disease than Th-m1.
DiscussionThe pathogen S. rolfsii, which responsible for chickpea collar rot, is globally distributed and is more competitive for nutrients than most other soil microorganisms. The biocontrol of this pathogen through Trichoderma species has proven to be a good alternative because of their high reproductive capacity, ability to survive under unfavorable conditions, efficiency in the utilization of nutrients, capacity to modify the rhizosphere, strong aggressiveness against phytopathogenic fungi, and efficiency in promoting plant growth and defense mechanisms.
Culture filtrates have been used in the present study to demonstrate the possible presence and role of fungal metabolites in the process of Trichoderma species antagonist behavior.9,10 Three concentrations of culture filtrates (10%, 20%, and 40%) were used in this study. Fifteen strains of Trichoderma species were screened against S. rolfsii in vitro and were found to produce varying degrees of inhibition on S. rolfsii radial colony growth. Only two strains were found effective (Fig. 1). The maximum percentage of S. rolfsii inhibition was found at the 40% concentration of T. harzianum-1432 (42.2%), which was followed by T. atroviride (40.3%) (Fig. 1). The inhibition of S. rolfsii radial growth by all strains was found to be higher at 40% concentration than at the 10% and 20% culture filtrate concentrations. It has been reported that the production of metabolites from different Trichoderma strains depends on ecological factors, so the strains showed varying effect on pathogens.11T. harzianum-1432 and T. atroviride appeared to be strong antagonists against the pathogen. The antagonism of Trichoderma species against several pathogens has been reported.12–14
It has been shown that not all Trichoderma species possess the mechanisms and characteristics deemed necessary for optimum biocontrol. Very often, those strains that have the capacity to produce enzymes and antibiotics that are associated with biocontrol are not the ones that have good storage qualities or function well at temperatures and moisture levels where pathogen flourish. Additionally, effective pathogen control may require 105–106 propagules to be present in one gram of soil in the case of Trichoderma. The presence of such high amounts of conidia is undesirable from an ecological, economic, and public health perspective. To solve this problem, one of the important aims of the present investigation was to develop a biocontrol strain that had improved biocontrol potential and increased tolerance to carbandazim.
Effective tools for strain improvement include mutagenesis.15,16 Peterson and Nevalainen,17 discussed progresses in the field of Trichoderma strain improvements. Mutation has been manipulated to improve production of antifungal metabolites and the antagonistic potential of biocontrol agents to control a broad spectrum of phytopathogens.16,18
To obtain a mutant strain that showed enhanced antagonistic capability against S. rolfsii, mutation was performed through NTG treatment. In this way, we obtained two mutants, namely Th-m1 and T. atroviride m1 that can also tolerate high levels of fungicide. The development of Trichoderma mutants for the suppression of fungal plant pathogens is an important method of strain improvement, which yields effective and reliable strains for biological control. After the development of mutants, assessing the bio-efficacy through various techniques is equally important for the suppression of the pathogen.19 The effect of different culture filtrate concentrations of the parent and mutant Trichoderma strains on the percent inhibition of S. rolfsii radial growth were also studied. It was evident from the observation that maximum percent inhibition of S. rolfsii was found at 40% for Th-m1 (46.2%) Variations among the mutants have been due to silent mutations induced during mutagenesis of wild-type Trichoderma strains to produce different levels of hydrolytic enzymes and antifungal metabolites.
The isolation of mutants showing increases in the production of several extracellular enzymes is a promising approach to improve biocontrol agents.20 Enzyme activities of the selected parent and mutant strains were therefore studied. Observations of enzyme activities were made after 2, 4 and 7 days. Trichoderma strains have been extensively studied for their ability to produce extracellular cellulolytic enzymes, namely endoglucanases, exoglucanases and cellobiase, which act synergistically to convert cellulose to glucose.21 These strains have often been mutagenized and genetically modified to obtain improved strains capable of producing higher cellulase levels.22,23
The mutant Th-m1 produced the maximum amount of cellulase in the medium when supplemented with cellulose as a substrate, whereas other Trichoderma strains released this enzyme in much lower amounts comparatively. Ahmad and Baker8 reported that mutants with higher cellulase activity than that of wild-type strains can utilize cellulose substrates on or near the root more efficiently.
Because β-1,3-glucanase is a structural component of fungal cell walls, the production of this enzyme has been reported to be an important enzymatic activity in the biocontrol of microorganisms.3 It is evident from Fig. 3B that maximum β-1,3-glucanase activity was exhibited by Th-m1 (61.9U/mL). Direct evidence for the involvement of glucanases in mycoparasitism has been demonstrated by Lorito et al.3 Many β-1,3-glucanases have been isolated, and their genes have been cloned.24
The data from the present study demonstrate that Th-m1 produces higher amount of enzymes with higher cellulase and β-1,3-glucanase activity than that of the other selected strain. These results strongly support the contention that Th-m1, as it released cellulase and β-1,3-glucanases in significant amounts, possessed stronger mycoparasitic capabilities than the other selected parent and mutant Trichoderma strains. The coordinated activity of these enzymes released by Th-m1 makes it capable of degrading different polymers, which leads to the effective breakdown of pathogen cell walls.
The extent to which an antagonist can survive in the presence of soil-borne pathogen depends on its competitive saprophytic ability, which may be assessed by the competitive saprophytic colonization of substrate units buried in graded series of inoculum dilutions of the pathogen.8 The soil moisture content plays an important role in the activity and survival of Trichoderma.25 Cumagun et al.26 also found that the higher the amount of inoculum of T. harzianum strain No. 94-016, the higher the percent colonization of rice straw in the soil. The CSA of selected Trichoderma strains was studied in the present investigation. Th-m1 possessed a significantly higher percent colonization than the other mutant strains at all population densities on all days of incubation, whereas T. atroviride-m1 were recovered only after 4 days of incubation. The parent strains Th-m1 and T. atroviride were recovered from root segments less frequently than the mutant strains and were very slow to colonize the root segments at higher population densities (Fig. 4). Th-1432 possesses higher CSA, which may be directly correlated with its higher cellulolytic activity.8
The parent and mutant strains of T. harzianum-1432 and T. atroviride were applied for field studies. When they were amended in the soil, it was observed that Th-m1 significantly suppressed the pathogen population (Fig. 5). Under field conditions, Th-m1 significantly decreased disease incidence.
The results obtained after field application of the antagonist revealed that the disease incidence was significantly reduced because of the high efficacy of the antagonist mutant strain against the pathogen by the enhanced production of extracellular enzymes.27,28,29 The disease control potential of Th-m1 may also be correlated with its high CSA. The results of biological control studies in pot and field conditions indicate that Th-m1 has the potential to serve as a biocontrol agent of chickpea collar rot.
Conflicts of interestThe authors declare no conflicts of interest.
The corresponding author is thankful to the University Grant Commission, New Delhi, India, for providing financial assistance for this work.