Twelve isolates of Trichoderma spp. isolated from tobacco rhizosphere were evaluated for their ability to produce chitinase and β-1,3-glucanase extracellular hydrolytic enzymes. Isolates ThJt1 and TvHt2, out of 12 isolates, produced maximum activities of chitinase and β-1,3-glucanase, respectively. In vitro production of chitinase and β-1,3-glucanase by isolates ThJt1 and TvHt2 was tested under different cultural conditions. The enzyme activities were significantly influenced by acidic pH and the optimum temperature was 30°C. The chitin and cell walls of Sclerotium rolfsii, as carbon sources, supported the maximum and significantly higher chitinase activity by both isolates. The chitinase activity of isolate ThJt1 was suppressed significantly by fructose (80.28%), followed by glucose (77.42%), whereas the β-1,3-glucanase activity of ThJt1 and both enzymes of isolate TvHt2 were significantly suppressed by fructose, followed by sucrose. Ammonium nitrate as nitrogen source supported the maximum activity of chitinase in both isolates, whereas urea was a poor nitrogen source. Production of both enzymes by the isolates was significantly influenced by the cultural conditions. Thus, the isolates ThJt1 and TvHt2 showed higher levels of chitinase and β-1,3-glucanase activities and were capable of hydrolyzing the mycelium of S. rolfsii infecting tobacco. These organisms can be used therefore for assessment of their synergism in biomass production and biocontrol efficacy and for their field biocontrol ability against S. rolfsii and Pythium aphanidermatum infecting tobacco.
In the recent years, biological control of plant disease, especially soil-borne plant pathogens and nematodes by microorganisms, has been considered to be a more natural and environmentally acceptable alternative to the existing chemical treatment methods.1 The genus Trichoderma is the most common saprophytic fungi in the rhizosphere; it is found in almost any soil. The mycoparasitic ability of Trichoderma species against some economically important aerial and soil-borne plant pathogens2 and nematodes3 allows the development of biocontrol strategies. The biocontrol efficacy of Trichoderma depends largely on the physical, chemical and biological condition of soil. There have been numerous recent attempts to use Trichoderma spp. on soil-borne pathogens such as Sclerotium, Fusarium, Pythium and Rhizoctonia species on different crops.2,4
Successful biological control systems commonly employ naturally occurring, antagonistic microorganisms that can effectively reduce activities of plant pathogens.5 Cook6 suggested that microorganisms isolated from the root or rhizosphere of a specific crop may be better adapted to that crop and may provide better control of diseases than organisms originally isolated from other plant species. Species of the genus Trichoderma have a wide biotechnological interest; however, their use as biocontrol agents requires a comprehensive analysis of the biological principles of their action.7 The antagonistic abilities of Trichoderma spp. are a combination of several mechanisms, including direct mycoparasitism, which involves the production of cell-wall-degrading enzymes (CWDE).8,9Trichoderma spp. are frequently associated with both biocontrol activity and promotion of plant and root growth.10,11 Screening of diverse population of biocontrol agents is an important requirement for developing efficient biocontrol agents. Therefore, it is imperative to index biocontrol agents prevailing in the area concerned. Tobacco is one of the important quality-conscious commercial crops grown in India. The presence of fungal diseases in tobacco and its economical consequences require the use of many fungicides. Sclerotium rolfsii and Pythium aphanidermatum cause serious diseases in tobacco nurseries, leading to death of seedlings. There is no resistance to S. rolfsii and P. aphanidermatum in the available cultivars. The objective of this study was to investigate the effect of in vitro cultural conditions on the production of chitinase and β-1,3-glucanase enzymes by 2 out of 12 selected Trichoderma isolates collected from tobacco rhizosphere from different regions.
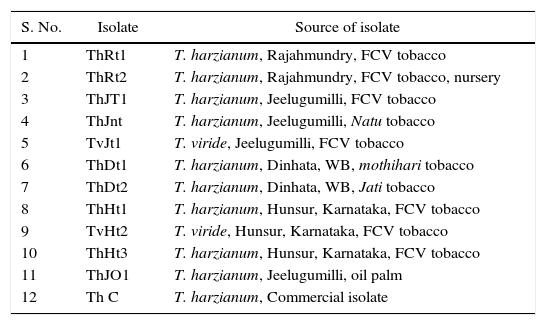
Materials and methodsSource of organismsTrichoderma isolates were obtained from the Division of Plant Protection, CTRI, Rajahmundry, and their places of isolation were given in Table 1. The 12 isolates were maintained on potato dextrose agar at 25±2°C.
Trichoderma isolates and their places of isolation.
| S. No. | Isolate | Source of isolate |
|---|---|---|
| 1 | ThRt1 | T. harzianum, Rajahmundry, FCV tobacco |
| 2 | ThRt2 | T. harzianum, Rajahmundry, FCV tobacco, nursery |
| 3 | ThJT1 | T. harzianum, Jeelugumilli, FCV tobacco |
| 4 | ThJnt | T. harzianum, Jeelugumilli, Natu tobacco |
| 5 | TvJt1 | T. viride, Jeelugumilli, FCV tobacco |
| 6 | ThDt1 | T. harzianum, Dinhata, WB, mothihari tobacco |
| 7 | ThDt2 | T. harzianum, Dinhata, WB, Jati tobacco |
| 8 | ThHt1 | T. harzianum, Hunsur, Karnataka, FCV tobacco |
| 9 | TvHt2 | T. viride, Hunsur, Karnataka, FCV tobacco |
| 10 | ThHt3 | T. harzianum, Hunsur, Karnataka, FCV tobacco |
| 11 | ThJO1 | T. harzianum, Jeelugumilli, oil palm |
| 12 | Th C | T. harzianum, Commercial isolate |
The Trichoderma isolates were grown on buffered minimal synthetic medium (MSM)12 containing the following components (in grams per litre): MgSO4·7H2O, 0.2; K2HPO4, 0.9; KCl, 0.2; NH4NO3, 1.0; FeSO4·7H2O, 0.002; MnSO4, 0.002; and ZnSO4, 0.002. The medium was supplemented with the appropriate carbon source (0.2%) and the pH was set to 6.0. Fifty mL of medium was dispensed into 250mL Erlenmeyer flasks and sterilized at 1.2kg/cm−1. The flasks were inoculated (triplicate sets) with inoculum (5mm disc) of actively growing mycelial mat from PDA plate and were incubated at 28±2°C for 6 days with shaking at 110rpm for 12h in a day. Culture filtrates were separated from mycelial mat by filtering through Whatman No. 1 filter paper and supernatant was centrifuged at 4°C for 15min at 15,000rpm and used for estimation of enzyme activity.
Preparation of S. rolfsii cell wallsThe cell walls of S. rolfsii were prepared by inoculating 100mL of PD medium in 250mL Erlenmeyer flasks with actively growing mycelium of S. rolfsii disc (0.5mm). The inoculated flasks were incubated at 25±3°C for 7 days. The mycelium was then collected by filtration through Whatman No. 1 filter paper, washed with distilled water and homogenized in liquid nitrogen and the powder was stored in −80°C. At the time of use the mycelial powder is homogenized with distilled water and centrifuged at 6000rpm for 10min and the pellet was dried in oven and used as carbon source.
Estimation of enzyme activityFor estimation of chitinase and β-1,3-glucanase activities, the isolates were grown on MSM containing chitin for chitinase and laminarin for β-1,3-glucanase activity.
Effect of pH on enzyme activityTo study the effect of pH of the growth medium on the enzyme activities, the pH of media containing chitin and laminarin for activities of chitinase and β-1,3-glucanase, respectively, was adjusted with KOH and HCl to 3, 4, 5, 5.5, 6, 6.5, 7, 8 and 9.
Effect of incubation temperature on enzyme activitiesTo study the effect of temperature during the growth on the enzyme activities of chitinase and β-1,3-glucanase, the isolates were grown in MSM with chitin (pH 6.0) and laminarin (pH 5.8), respectively, by incubating the flasks at 20, 25, 30, 35 and 40°C for 6 days.
Effect of carbon and nitrogen sources on enzyme activitiesThe isolates were grown in MSM supplemented with different carbon sources (0.2%). Chitin, cell walls of S. rolfsii, glucose, fructose, sucrose, starch, galactose, maltose, succinic acid and citric acid were used as carbon sources. The effect of nitrogen sources on enzyme activities was tested in MSM containing different nitrogen sources. Ammonium nitrate, sodium nitrate, sodium nitrite, ammonium sulphate, urea, calcium ammonium nitrate, glycine and glutamic acid were used as nitrogen sources along with chitin for chitinase and laminarin for β-1,3-glucanase. To study the effect of carbon sources on repression of enzyme activity, isolates were grown in MSM with 0.2% of chitin along with 0.2% of glucose, fructose, sucrose, starch, galactose, maltose, succinic acid or citric acid for chitinase activity and 0.2% laminarin along with 0.2% of carbon sources for β-1,3-glucanase as in the case of chitinase.
Assay of enzyme activitiesβ-1,3-glucanase (EC 3.2.1.58) activity was estimated according to the method of Elad et al.13 The reaction mixture contained 0.5mL culture filtrate, 1mL citrate buffer (pH 4.5, 0.1M) and 0.5mL laminarin. Test tubes containing the reaction mixture were incubated at 40°C for 1h and kept in boiling water bath for 5min to stop the reaction. 2mL of dinitrosalicylic acid reagent (1% solution of dinitrosalicylic acid in 0.7M NaOH) was added to the reaction mixture and kept in boiling water bath for 15min. After cooling to room temperature, the absorbance of the reaction mixture was measured at 575nm and the amount of glucose released was estimated from standard curve prepared with glucose. The enzyme activity was expressed as nkat/mL (nmol/s). Chitinase activity (EC 3.2.1.14) was estimated12 by incubating the reaction mixture containing 0.5mL colloidal chitin, 1mL McIlvaine's buffer (pH 4) and 0.5mL culture filtrate at 37°C for 2h. At the end of incubation, 3mL of potassium ferricyanide reagent (0.05% potassium ferricyanide in 0.05% sodium carbonate) was added and incubated in boiling water bath for 15min. The amount of N-acetyl glucosamine released was estimated by measuring the absorbance at 420nm and comparing with the standard curve prepared with N-acetyl glucosamine. The activity of the enzyme was expressed in pkat/mL (pmol/s).
The data in all the above experiments were analyzed using SAS 9.3. The data were expressed as mean±SD (n=3). Different letters in each column represent significant differences (p<0.05).
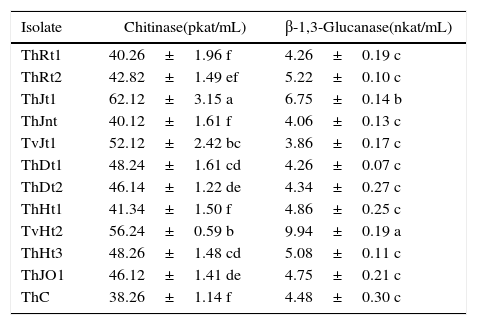
ResultsThe production of chitinase was studied by growing the Trichoderma isolates in minimal synthetic medium (MSM) containing 1% colloidal chitin as carbon source. The isolate ThJt1 showed maximum and significantly higher chitinase activity compared to other isolates (Table 2). The isolate TvHt2 was the second highest producer of chitinase, with production significantly higher than those of other isolates except ThJt1. The isolates ThDt1 and ThDt2 collected from Dinhata were on a par in chitinase activity.
Activities of extracellular enzymes produced by different Trichoderma isolates.
| Isolate | Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) |
|---|---|---|
| ThRt1 | 40.26±1.96 f | 4.26±0.19 c |
| ThRt2 | 42.82±1.49 ef | 5.22±0.10 c |
| ThJt1 | 62.12±3.15 a | 6.75±0.14 b |
| ThJnt | 40.12±1.61 f | 4.06±0.13 c |
| TvJt1 | 52.12±2.42 bc | 3.86±0.17 c |
| ThDt1 | 48.24±1.61 cd | 4.26±0.07 c |
| ThDt2 | 46.14±1.22 de | 4.34±0.27 c |
| ThHt1 | 41.34±1.50 f | 4.86±0.25 c |
| TvHt2 | 56.24±0.59 b | 9.94±0.19 a |
| ThHt3 | 48.26±1.48 cd | 5.08±0.11 c |
| ThJO1 | 46.12±1.41 de | 4.75±0.21 c |
| ThC | 38.26±1.14 f | 4.48±0.30 c |
The different letters in the columns represent significant differences (p<0.05).
The production of β-1,3-glucanase by Trichoderma isolates was assessed by growing the isolates in MSM with laminarin (1%) as carbon source. The isolate TvHt2 showed maximum and significantly higher β-1,3-glucanase activity when compared to all other isolates (Table 2). The isolate ThJt1 showed the second highest activity of β-1,3-glucanase. All other isolates showed β-1,3-glucanase activity but they were all on a par. Isolate TvHt2 showed nearly 61.66% higher β-1,3-glucanase activity when compared to the isolate TvJt1. The isolates ThDt1 and ThDt2 from Dinhata showed similar activity of β-1,3-glucanase.
To study the effect of cultural conditions on enzyme production, the isolates ThJt1 and TvHt2 were selected based on their superiority in the production of extracellular enzymes and inhibition of Sclerotium and Pythium mycelial growth (data not shown).
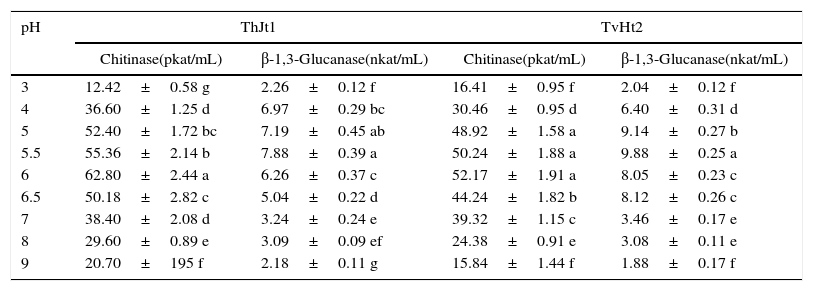
Effect of pH on extracellular enzyme activities of Trichoderma isolatesChitinase activity increased significantly with increase in pH from 3 to 6; and on further increase in pH, the activity decreased significantly in both isolates of ThJt1 and TvHt2 (Table 3). The isolate ThJt1 showed maximum chitinase activity at pH 6 (62.80pkat/mL), which was significantly higher than those at other pH levels. The chitinase activity decreased by 39.24% and 67.03% with increase in pH from 6 to 7 and 6 to 9, respectively. The isolate TvHt2 showed maximum β-1,3-glucanase activity at pH 5.5. The β-1,3-glucanase activity of isolate TvHt2 increased by 79.35% with increase in pH from 3 to 5.5 and significantly decreased by increasing pH from 5.5 to 6 (Table 3). With increase in pH from 5.5 to 9, the β-1,3-glucanase activity was decreased by 80.79%. The isolate ThJt1 showed 16.92% higher chitinase activity compared to the isolate TvHt2, whereas the isolate TvHt2 showed 20.24% higher β-1,3-glucanase activity compared to the isolate ThJt1.
Effect of pH on activities of chitinase and β-1,3-glucanase of Trichoderma isolates ThJt1 and TvHt2.
| pH | ThJt1 | TvHt2 | ||
|---|---|---|---|---|
| Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | |
| 3 | 12.42±0.58 g | 2.26±0.12 f | 16.41±0.95 f | 2.04±0.12 f |
| 4 | 36.60±1.25 d | 6.97±0.29 bc | 30.46±0.95 d | 6.40±0.31 d |
| 5 | 52.40±1.72 bc | 7.19±0.45 ab | 48.92±1.58 a | 9.14±0.27 b |
| 5.5 | 55.36±2.14 b | 7.88±0.39 a | 50.24±1.88 a | 9.88±0.25 a |
| 6 | 62.80±2.44 a | 6.26±0.37 c | 52.17±1.91 a | 8.05±0.23 c |
| 6.5 | 50.18±2.82 c | 5.04±0.22 d | 44.24±1.82 b | 8.12±0.26 c |
| 7 | 38.40±2.08 d | 3.24±0.24 e | 39.32±1.15 c | 3.46±0.17 e |
| 8 | 29.60±0.89 e | 3.09±0.09 ef | 24.38±0.91 e | 3.08±0.11 e |
| 9 | 20.70±195 f | 2.18±0.11 g | 15.84±1.44 f | 1.88±0.17 f |
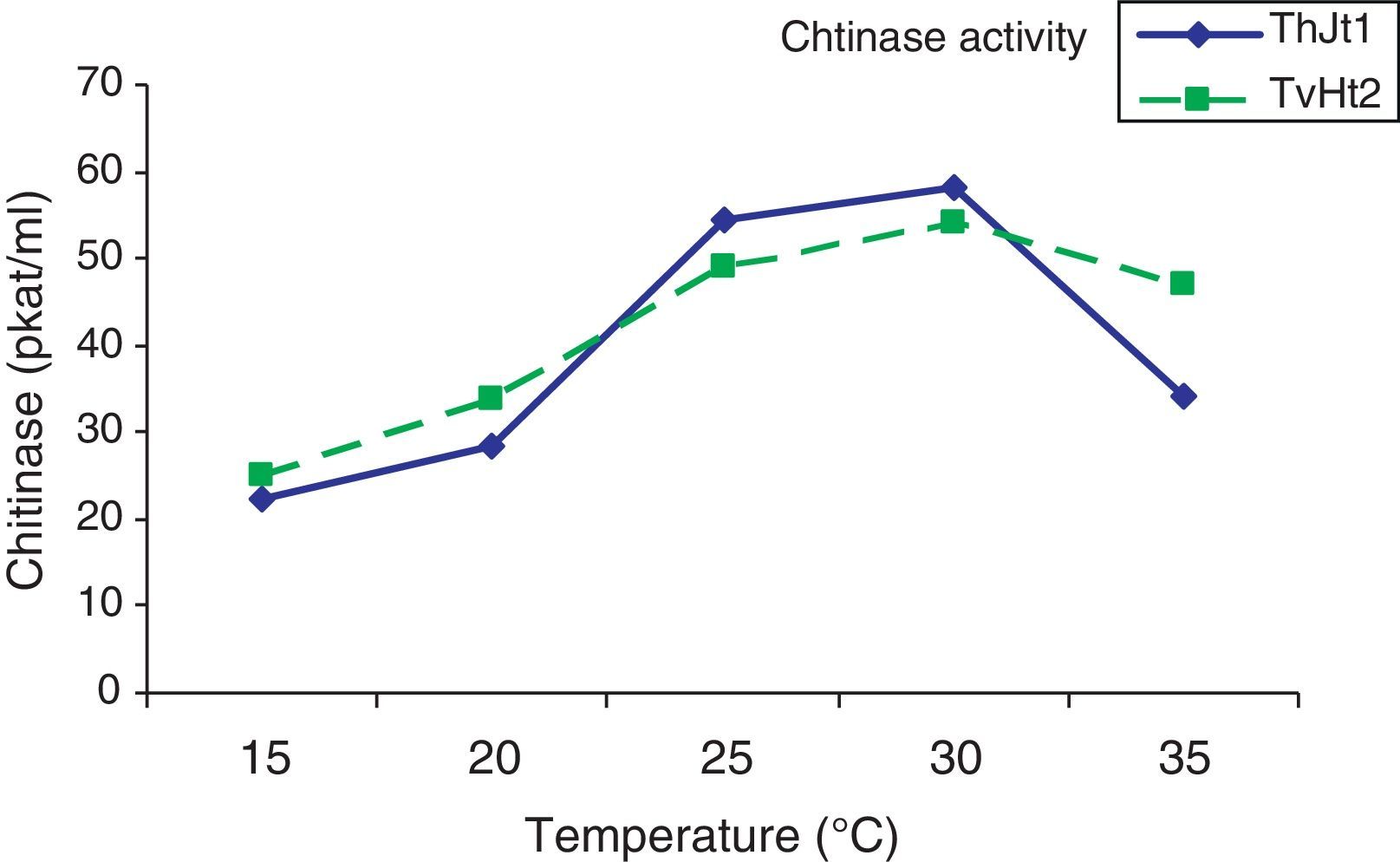
The chitinase activity of both isolates increased significantly with increase in incubation temperature from 15 to 30°C (Fig. 1). The chitinase activity of isolate ThJt1 was maximum at 30°C but it was on a par with the activity at 25°C and significantly higher than at 15°C and 20°C. With increase in temperature from 30°C to 35°C, the chitinase activity significantly decreased by 41.19%.
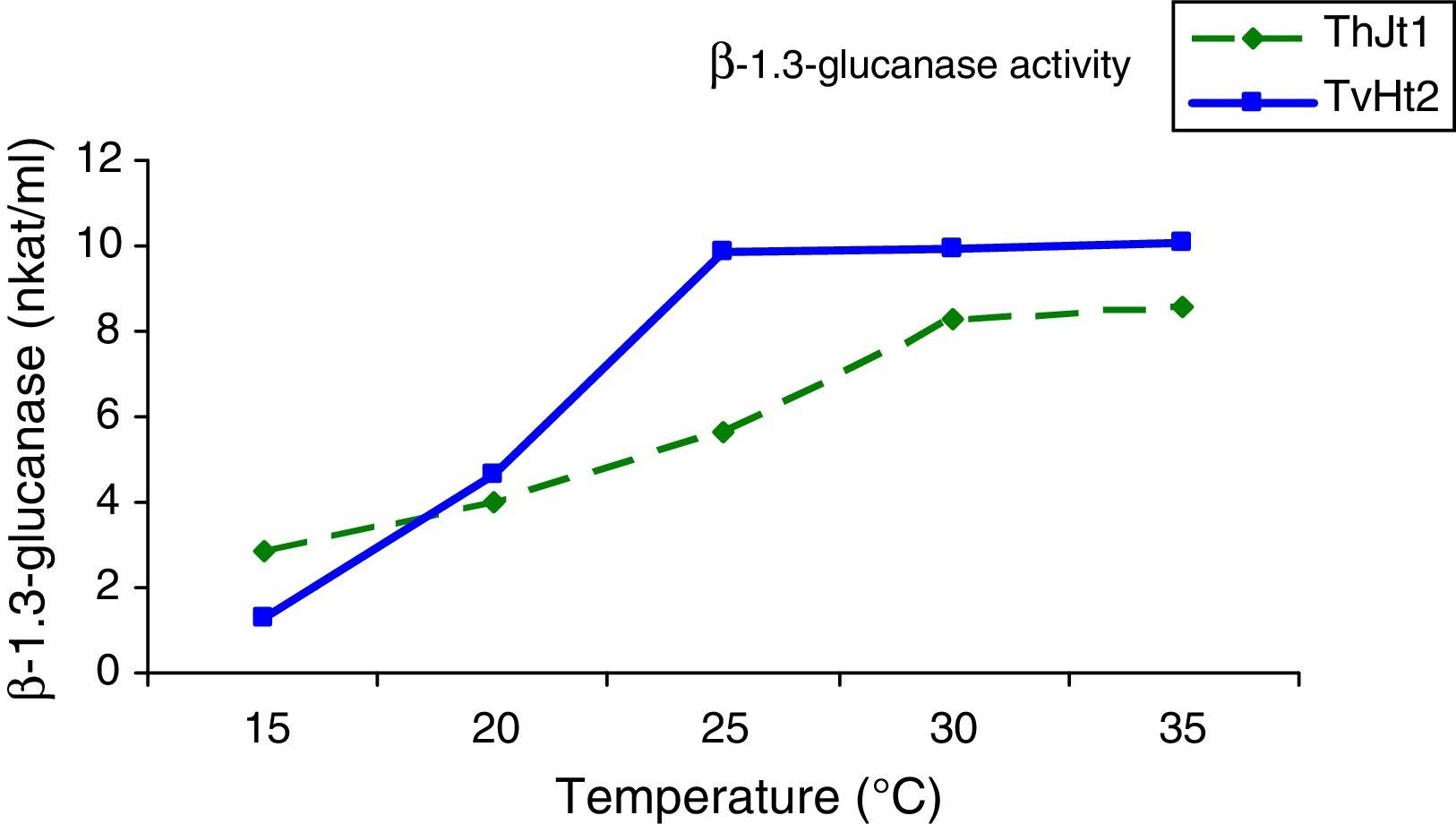
The β-1,3-glucanase activity increased significantly with increase in incubation temperature from 15 to 25°C; and on further increase in temperature from 25 to 35°C, there was non-significant increase in activity (Fig. 2). The activities of β-1,3-glucanase at the incubation temperatures of 25, 30 and 35°C were on a par. The β-1,3-glucanase activity increased by 87.25% with increase in temperature from 15 to 35°C (Fig. 2).
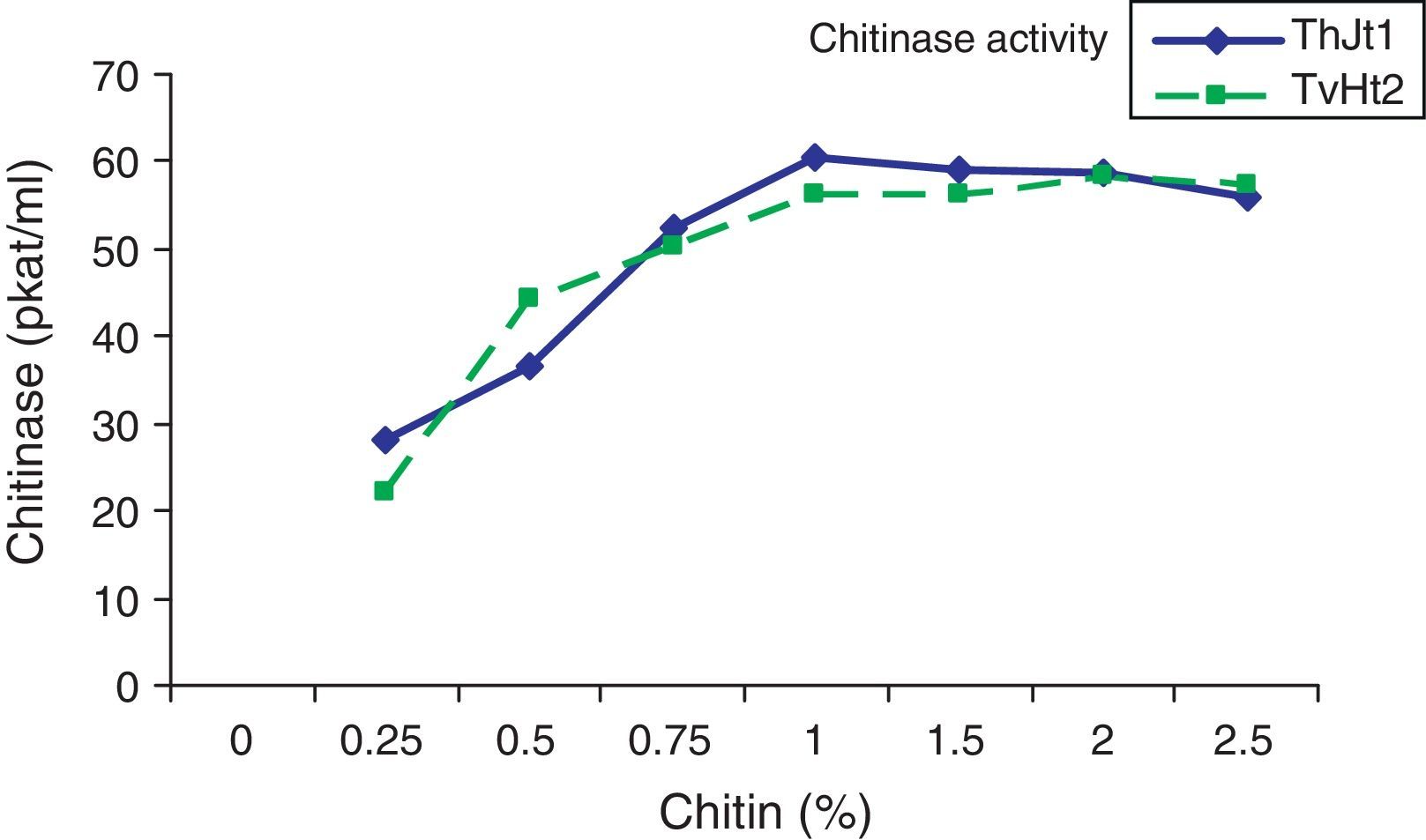
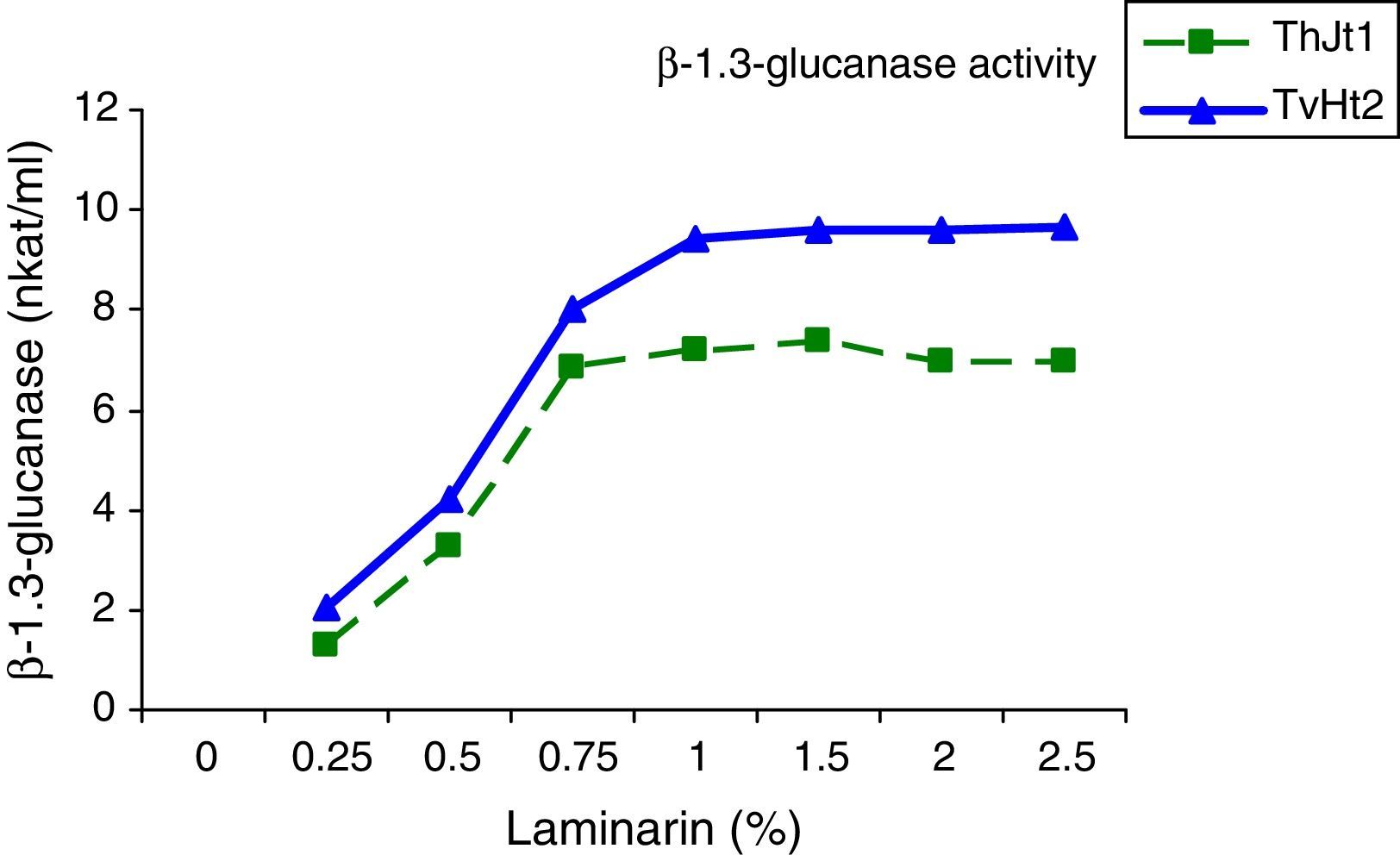
Effect of chitin/laminarin content on extracellular enzyme activities of Trichoderma isolatesThe effect of different levels of chitin on chitinase and laminarin on β-1,3-glucanase activity was studied by incubating the isolates in MSM with different concentrations of chitin/laminarin as carbon sources. The chitinase activity of isolate ThJt1 increased significantly with increase in chitin content from 0.25% to 1% and showed maximum activity with 1% chitin (Fig. 3). The chitinase activity remained the same when the chitin content increased from 1% to 2% and the activity was decreased marginally with increase in chitin content from 2% to 2.5%. The chitinase activity increased by 53.30% with increase in chitin content in the medium from 0.25% to 1%, whereas it decreased by 17.26% with increase in chitin content from 1% to 2.5%. The isolate TvHt2 showed maximum chitinase activity with 1% chitin in the medium; the activity remained the same with 1.5% and 2.5% of chitin (Fig. 4). The β-1,3-glucanase activity of the isolate TvHt2 increased with increase in laminarin content and was maximum at 1%, remaining the same with 1.5%, 2.0% and 2.5% of laminarin in the medium.
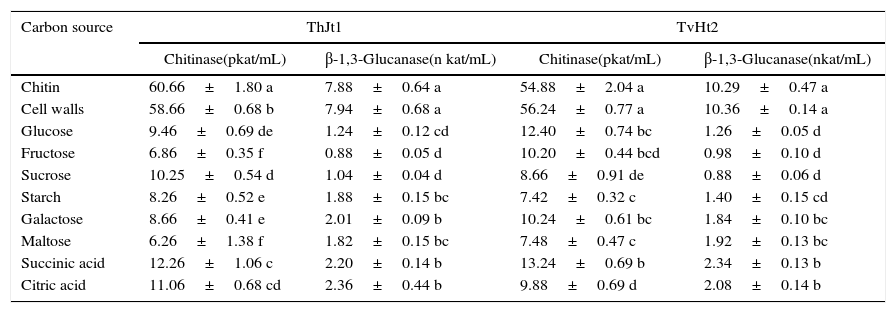
The isolate ThJt1 showed maximum chitinase activity on chitin followed by cell walls as carbon source and significantly lower activity on other carbon sources (Table 4). The lowest activity was on maltose. The chitinase activity decreased by 89.68%, 88.69% and 86.38% when maltose, fructose and sucrose were used as carbon sources, respectively, compared with the activity when using chitin. The isolate ThJt1 showed maximum β-1,3-glucanase activity on cell walls, followed by laminarin, and showed significantly lower activities on other carbon sources (Table 4). The β-1,3-glucanase activities on succinic acid- and citric acid-containing medium were on a par. Among the sugars, β-1,3-glucanase activity was maximum on the galactose and minimum on fructose. The β-1,3-glucanase activity decreased by 88.8%, 86.8% and 84.26% on fructose, sucrose and glucose, respectively, when compared with the activity when using laminarin as carbon source. The chitinase activities on glucose and on succinic acid were on a par and significantly higher than on other carbon sources. The isolate TvHt2 showed maximum β-1,3-glucanase activity on cell walls as carbon source, followed by laminarin, and showed significantly lower activity on all other carbon sources (Table 4). The β-1,3-glucanase activities on galactose, maltose, succinic acid and citric acid were on a par. The isolate TvHt2 showed the least β-1,3-glucanase activity on sucrose.
Effect of carbon sources on activities of chitinase and β-1,3-glucanase of Trichoderma isolates ThJt1 and TvHt2.
| Carbon source | ThJt1 | TvHt2 | ||
|---|---|---|---|---|
| Chitinase(pkat/mL) | β-1,3-Glucanase(n kat/mL) | Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | |
| Chitin | 60.66±1.80 a | 7.88±0.64 a | 54.88±2.04 a | 10.29±0.47 a |
| Cell walls | 58.66±0.68 b | 7.94±0.68 a | 56.24±0.77 a | 10.36±0.14 a |
| Glucose | 9.46±0.69 de | 1.24±0.12 cd | 12.40±0.74 bc | 1.26±0.05 d |
| Fructose | 6.86±0.35 f | 0.88±0.05 d | 10.20±0.44 bcd | 0.98±0.10 d |
| Sucrose | 10.25±0.54 d | 1.04±0.04 d | 8.66±0.91 de | 0.88±0.06 d |
| Starch | 8.26±0.52 e | 1.88±0.15 bc | 7.42±0.32 c | 1.40±0.15 cd |
| Galactose | 8.66±0.41 e | 2.01±0.09 b | 10.24±0.61 bc | 1.84±0.10 bc |
| Maltose | 6.26±1.38 f | 1.82±0.15 bc | 7.48±0.47 c | 1.92±0.13 bc |
| Succinic acid | 12.26±1.06 c | 2.20±0.14 b | 13.24±0.69 b | 2.34±0.13 b |
| Citric acid | 11.06±0.68 cd | 2.36±0.44 b | 9.88±0.69 d | 2.08±0.14 b |
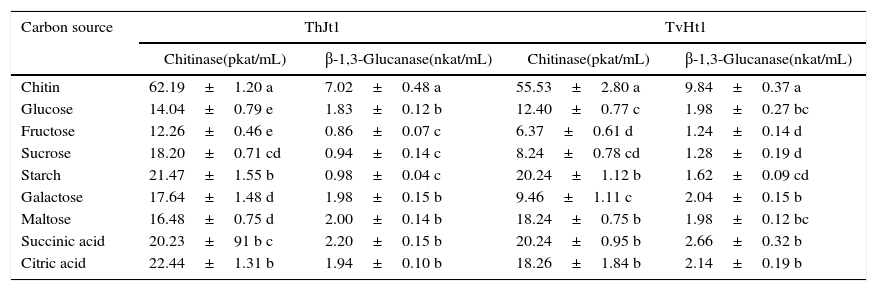
The effect of carbon sources in combination with chitin for chitinase and laminarin for β-1,3-glucanase was studied by incubating the isolates in MSM containing chitin/laminarin with different carbon sources at 1:1 ratio. The isolate ThJt1 showed maximum chitinase activity on chitin alone and the activity significantly decreased with addition of carbon sources (Table 5). The maximum suppression of chitinase activity was showed by fructose, followed by glucose. The β-1,3-glucanase activity was maximum on laminarin alone and significantly decreased with addition of other carbon sources (Table 5). The suppression of β-1,3-glucanase activity was maximum on fructose, followed by those on sucrose and starch. The β-1,3-glucanase activity was inhibited by 98.7%, 86.60% and 86.03% by fructose, sucrose and starch, respectively.
Effect of chitin+carbon sources on activities of chitinase and β-1,3-glucanase of Trichoderma isolates ThJt1 and TvHt2.
| Carbon source | ThJt1 | TvHt1 | ||
|---|---|---|---|---|
| Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | |
| Chitin | 62.19±1.20 a | 7.02±0.48 a | 55.53±2.80 a | 9.84±0.37 a |
| Glucose | 14.04±0.79 e | 1.83±0.12 b | 12.40±0.77 c | 1.98±0.27 bc |
| Fructose | 12.26±0.46 e | 0.86±0.07 c | 6.37±0.61 d | 1.24±0.14 d |
| Sucrose | 18.20±0.71 cd | 0.94±0.14 c | 8.24±0.78 cd | 1.28±0.19 d |
| Starch | 21.47±1.55 b | 0.98±0.04 c | 20.24±1.12 b | 1.62±0.09 cd |
| Galactose | 17.64±1.48 d | 1.98±0.15 b | 9.46±1.11 c | 2.04±0.15 b |
| Maltose | 16.48±0.75 d | 2.00±0.14 b | 18.24±0.75 b | 1.98±0.12 bc |
| Succinic acid | 20.23±91 b c | 2.20±0.15 b | 20.24±0.95 b | 2.66±0.32 b |
| Citric acid | 22.44±1.31 b | 1.94±0.10 b | 18.26±1.84 b | 2.14±0.19 b |
The isolate TvHt2 showed maximum chitinase activity on chitin alone and the activity decreased significantly with addition of other carbon sources (Table 5). The chitinase activity was suppressed to the maximum by fructose, followed by sucrose and galactose. The β-1,3-glucanase activity was maximum on laminarin alone and the activity decreased significantly with addition of other carbon sources (Table 5). The suppression of β-1,3-glucanase activity by the addition of other carbon sources varied from 72.96% to 87.39%.
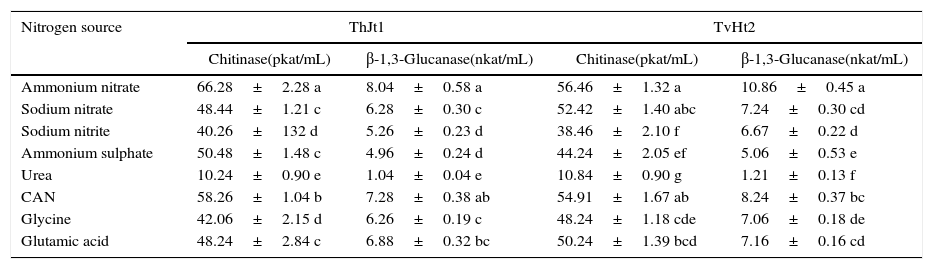
Effect of nitrogen sources on the extracellular enzyme activities of Trichoderma isolatesThe isolate ThJt1 showed maximum and significantly higher chitinase activity on ammonium nitrate, followed by calcium ammonium nitrate (Table 6). The activity of chitinase was the least on urea. Glutamic acid showed significantly higher chitinase activity compared to glycine. The isolate ThJt1 showed maximum β-1,3-glucanase activity on ammonium nitrate, which was on a par with the activity on CAN. The β-1,3-glucanase activity was the least on urea as nitrogen source. The isolate TvHt2 showed maximum chitinase activity on ammonium nitrate, which was on a par with the activities on sodium nitrate and calcium ammonium nitrate (Table 6). The β-1,3-glucanase activities on sodium nitrate, calcium ammonium nitrate, glutamic acid and glycine were on a par. The chitinase activity was the least on urea. The β-1,3-glucanase activity of the isolate TvHt2 varied from 1.21 to 10.86nkat/mL among the different nitrogen sources (Table 6).
Effect of nitrogen sources on activities of chitinase and β-1,3-glucanase of Trichoderma isolates ThJt1 and TvHt2.
| Nitrogen source | ThJt1 | TvHt2 | ||
|---|---|---|---|---|
| Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | Chitinase(pkat/mL) | β-1,3-Glucanase(nkat/mL) | |
| Ammonium nitrate | 66.28±2.28 a | 8.04±0.58 a | 56.46±1.32 a | 10.86±0.45 a |
| Sodium nitrate | 48.44±1.21 c | 6.28±0.30 c | 52.42±1.40 abc | 7.24±0.30 cd |
| Sodium nitrite | 40.26±132 d | 5.26±0.23 d | 38.46±2.10 f | 6.67±0.22 d |
| Ammonium sulphate | 50.48±1.48 c | 4.96±0.24 d | 44.24±2.05 ef | 5.06±0.53 e |
| Urea | 10.24±0.90 e | 1.04±0.04 e | 10.84±0.90 g | 1.21±0.13 f |
| CAN | 58.26±1.04 b | 7.28±0.38 ab | 54.91±1.67 ab | 8.24±0.37 bc |
| Glycine | 42.06±2.15 d | 6.26±0.19 c | 48.24±1.18 cde | 7.06±0.18 de |
| Glutamic acid | 48.24±2.84 c | 6.88±0.32 bc | 50.24±1.39 bcd | 7.16±0.16 cd |
Several Trichoderma species reduce the incidence of soil-borne plant pathogenic fungi under natural conditions14; however, their efficacy depends largely on the physical, chemical and biological condition of soil. The results of this study show that Trichoderma isolates ThJt1 and TvHt2 produce enzymes capable of degrading chitin and β,-1,3-glucan, two major cell wall compounds of S. rolfsii and P. aphanidermatum. In the presence of chitin, laminarin and S. rolfsii cell wall fragments, Trichoderma isolates produced significant amounts of both chitinases and β-1,3-glucanases. These lytic enzymes, which are key enzymes in the lyses of cell walls of higher fungi, are produced by other organisms that are known to attack and parasitize fungi.15 The direct mycoparasitic activity of Trichoderma species has been proposed as one of the major mechanisms for their antagonistic activity against phytopathogenic fungi.16Trichoderma spp. attach to the host hyphae by coiling, hooks or appressorium-like structures and penetrate the host cell walls by secreting hydrolytic enzymes such as a basic proteinase,17 β-1,3-glucanase and chitinase.13 Chitinase and β-1,3-glucanase enzyme production was favoured by acidic pH (6.0, 5.8). Acidic pH was also reported to be an important growth parameter in the production of chitinase and β-1,3-glucanase in Trichoderma harzianum.18 Ulhoa and Peberdy19 found that the production of chitinase was markedly affected by pH, with an optimum pH of 6.0. The pH and the optimum temperature for the production of enzymes in the present investigation were supported by the findings of Jijakli and Lepoivre20 and Someshwar Bhagat and Sitansu Pan.18 These results suggest that production of both chitinases and β-1,3-glucanases may be coordinately regulated since both enzymes were influenced in the same way by similar alterations of growth parameters in the culture media.
The higher activities of chitinase and β-1,3-glucanase were observed when the medium was supplemented with chitin/dried cell walls of S. rolfsii or laminarin; the very low activities, with sugars and other carbon sources tested. The production of extracellular β-1,3-glucanases, chitinases and proteinase increases significantly when Trichoderma spp. are grown in media supplemented with either autoclaved mycelium or isolated purified host fungal cell walls.9,21 The activities of chitinase and β-1,3-glucanase decreased significantly when the medium was supplemented with other carbon sources along with chitin/laminarin, indicating the repression of enzyme production by these carbon sources. No production or low production of chitinase activity in deprived carbon sources confirms that chitinase enzyme is produced inducibly and not constitutively. The expression of these cell wall-degrading enzymes has been frequently reported to be induced by fungal cell wall components and repressed by carbon catabolite repressors such as glucose and fructose.22 The findings of El-Katatny et al.23 were in agreement with these findings and found high chitinase activity only in cultures supplied with chitin but not with other polymers such as cellulose and chitosan, which is further indicative of induction. These observations, together with the fact that chitin, β-1,3-glucan and proteins are the main structural components of most fungal cell walls,24 are the basis for the suggestion that hydrolytic enzymes produced by some Trichoderma spp. play an important role in the destruction of plant pathogens.25 Kumar and Gupta26 reported that cell walls of Macrophomina phaseolina and S. rolfsii are known to contain glucan and chitin, which should have resulted in the induction of chitinase and glucanase in mycelium-containing medium. Trichoderma spp. (especially T. harzianum and T. viride) exhibit considerable variability among strains with respect to their biocontrol activity and host range.27 Ammonium sources of nitrogen showed maximum activity of chitinase and β-1,3-glucanase compared to other sources of nitrogen and the least activity on urea. These results were supported by the report of EI-Katatny et al.,23 where it was reported that corn steep solid was the most stimulative for chitinase production, followed by (NH4)2SO4 or NH4NO3. Peptone-casein gave the least degree of enzyme activity, whereas urea gave no enzyme activity when used as a nitrogen source. Indirect support also comes from the observation that T. viride showed maximum biomass production on ammonium sulphate compared to sodium nitrate and potassium nitrate, whereas it showed the least growth on urea.28
It is widely known that environmental parameters such as abiotic (soil type, soil temperature, soil pH, water potential, and so on) and biotic factors (plant species and variety, microbial activity of the soil) as well as other factors such as method and timing of applications may have influence on the biological control efficacy of Trichoderma isolates. In the present study, the isolates ThJt1 and TvHt2 out of 12 Trichoderma isolates showed the highest enzyme activity against the S. rolfsii tested. These organisms can be used therefore for assessment of their synergism in biomass production and biocontrol efficacy and for their field biocontrol ability against S. rolfsii and P. aphanidermatum infecting tobacco.
Conflicts of interestThe authors declare no conflicts of interest.