Since, there is no study reporting the mechanism of azole resistance among yeasts isolated from aquatic environments; the present study aims to investigate the occurrence of antifungal resistance among yeasts isolated from an aquatic environment, and assess the efflux-pump activity of the azole-resistant strains to better understand the mechanism of resistance for this group of drugs. For this purpose, monthly water and sediment samples were collected from Catú Lake, Ceará, Brazil, from March 2011 to February 2012. The obtained yeasts were identified based on morphological and biochemical characteristics. Of the 46 isolates, 37 were Candida spp., 4 were Trichosporon asahii, 3 were Cryptococcus laurentii, 1 Rhodotorula mucilaginosa, and 1 was Kodamaea ohmeri. These isolates were subjected to broth microdilution assay with amphotericin B, itraconazole, and fluconazole, according to the methodology standardized by the Clinical and Laboratory Standards Institute (CLSI). The minimum inhibitory concentrations (MICs) of amphotericin B, itraconazole, and fluconazole were 0.03125–2μg/mL, 0.0625 to ≥16μg/mL, and 0.5 to ≥64μg/mL, respectively, and 13 resistant azole-resistant Candida isolates were detected. A reduction in the azole MICs leading to the phenotypical reversal of the azole resistance was observed upon addition of efflux-pump inhibitors. These findings suggest that the azole resistance among environmental Candida spp. is most likely associated with the overexpression of efflux-pumps.
The quali-quantitative analysis of yeast microbiota is a promising tool to assess the eutrophication status of aquatic systems.1–5 Medeiros et al.,5 for example, studied the biodiversity of yeasts in the lakes and rivers of southeastern Brazil and found that the genus Candida accounted for the largest number of isolates, out of which 50% were resistant to itraconazole and 11% were resistant to fluconazole. Moreover, our group observed, in the freshwater prawn Macrobrachium amazonicum (Amazon River prawn) collected from its natural environment, that 33.3% of the Candida isolates from these prawns were resistant to fluconazole and itraconazole.6
However, none of the mentioned studies investigated the underlying mechanism of the azole resistance present in the Candida strains recovered from aquatic environments. It is well known that one of the main mechanisms of azole resistance among Candida spp. is the increased activity of efflux pumps. This increased activity of efflux pumps is conferred by genes, CDR1 and CDR2, belonging to the superfamily of the ATP binding cassette, and MDR1, belonging to the major facilitator class. The overexpression of these genes and the subsequent increase in the activity of these pumps prevent the accumulation of the drug inside the cell at the site of action impairing its efficacy. The upregulation of CDR1 and CDR2 confers resistance to nearly all azoles, while that of MDR1 provides specific resistance only to fluconazole.7 Thus, the present study aims to investigate the occurrence of antifungal resistance among yeasts obtained from an aquatic environment, and assess the efflux-pump activity in the azole-resistant strains.
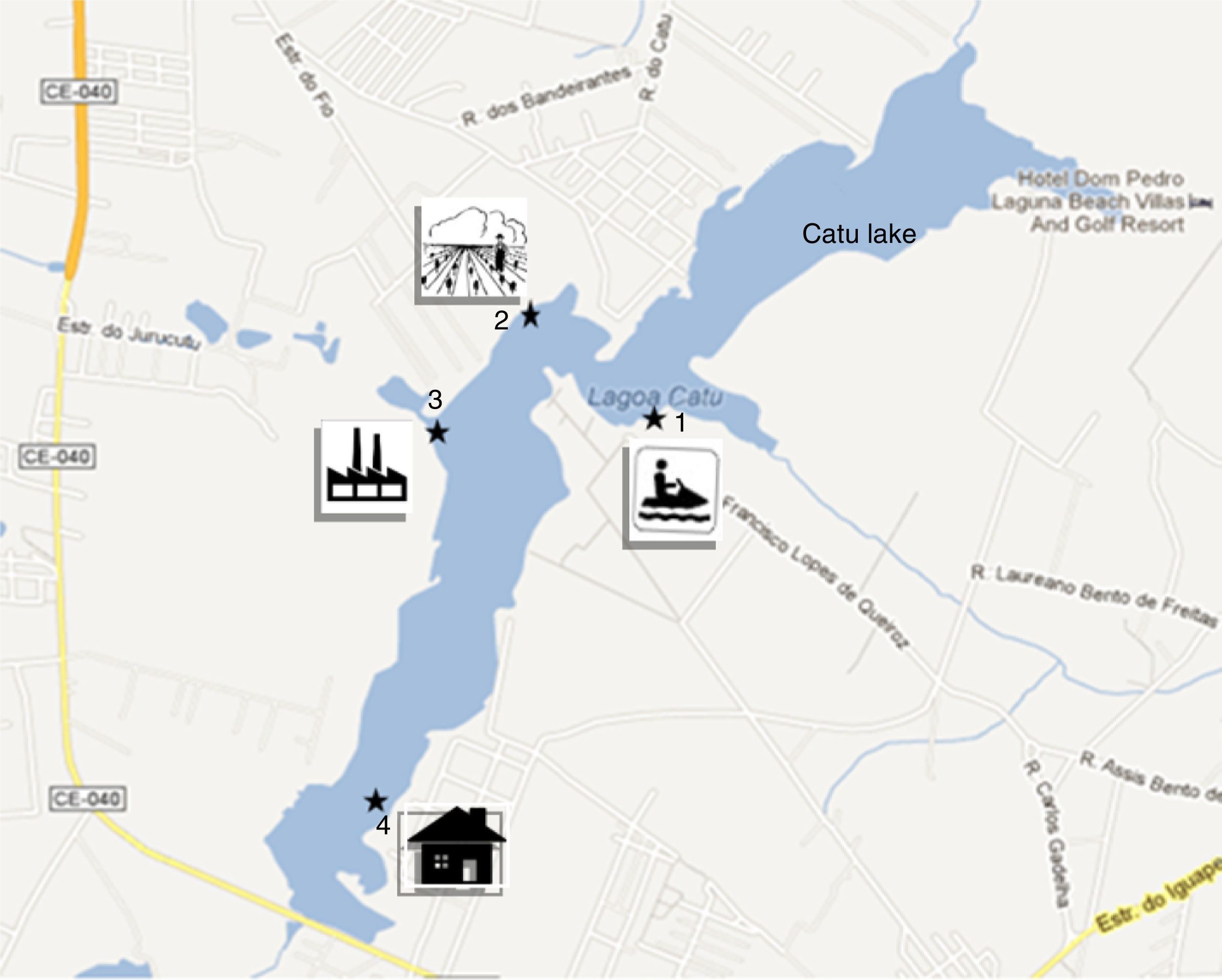
MethodsStudy site and collections of the biological materialThe samples were collected from Catú Lake, located in the municipality of Aquiraz, Ceará State, Brazil (UTM coordinates 0567000E, 9561273N and 0575000E, 9569000N). Catú Lake is a rich freshwater body with mangrove areas sheltering a large number of animal species. However, due to uncontrolled occupation of the surrounding area, water from different zones of this lake is mainly used for human and animal consumption, for industrial, commercial, farming activities, and for leisure activities, such as boat excursions.8
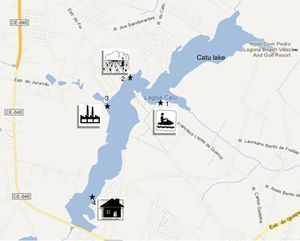
A total of 12 water samples were collected monthly from March 2011 to February 2012, according to the method described by Medeiros et al.,5 with slight modification. The samples were obtained from four collection sites: recreational area point (point 1, 3°55′59.79″S and 38°21′50.10″W); agricultural wastewater point, with possible use of azoles (point 2, 3°55′47.25″S and 38°22′14.16″W); industrial wastewater point (point 3, 3°56′03.70″S and 38°22′25.15″W); Catú River confluence point, residential area with discharge of raw household sewage, (point 4, 3°56′56.72″S and 38°22′31.57″W) (Fig. 1). The water samples were collected in a 1-L Van Dorn bottle, which was rinsed three times with the water from each collection site before the collection. Two samples were collected from each point, one from the surface (SW sample) and the other from the bottom, including sediment (S sample). The study was approved by the Chico Mendes Institute for Conservation of Biodiversity/Biodiversity Authorization and Information System, SISBIO, under the process number 28175-1.
The sample collection points: Catú Lake, Aquiraz, Ceará, Brazil. Point 1: Leisure area: bars, restaurants, boats. The area is used for activities such as boating and jet skiing (3°55′59.79″S and 38°21′50.10″W). Point 2: Agricultural area with potato and bean fields, with possible use of azoles (3°55′47.25″S and 38°22′14.16″W). Point 3: Industrial area, near the state highway (CE-040). (3°56′03.70″S and 38°22′25.15″W). Point 4: Residential area, discharge of raw household sewage, near the confluence with the Catú River. 3°56′56.72″S and 38°22′31.57″W.
The samples were processed in a biological safety level 2 laminar flow cabinet. Sabouraud agar with chloramphenicol (0.5g/L) was used as the culture medium for primary isolation in Petri dishes. A 100-μL aliquot of the SW samples was spread on the medium after homogenization. The S samples were centrifuged for 20min at 3000rpm and the supernatant was removed and the sediment was resuspended in 2mL of sterile 0.9% NaCl solution. Then, the suspension was agitated in a vortex mixer for 3min and left to rest for 30min at 25°C. Afterwards, 100-μL aliquots of the supernatant of each sample were spread on the culture medium. The inoculated Petri dishes were incubated at 25°C for 10 days, and were with daily observed daily to note any microbiological growth. The colony forming units (CFUs) were counted in all inoculated dishes.
Yeast identificationThe colonies that appeared to be yeast were Gram stained and observed under a light microscope (400×) to check for the presence of blastoconidia, hyphae, or pseudohyphae, and to exclude bacterial contaminations. The yeast colonies were identified through specific macromorphological and micromorphological characteristics, including growth on chromogenic medium for the identification of mixed colonies, and biochemical tests, such as carbohydrate and nitrogen assimilation and urease production. VITEK 2™ microbial identification system (bioMérieux, USA) was used in case of dubious identification to aid the identification procedure.6
In vitro antifungal susceptibility testsThe antifungal minimum inhibitory concentrations (MICs) against these microorganisms were determined through broth microdilution method, as described by the Clinical and Laboratory Standards Institute (CLSI, 2008). Three drugs were tested against the isolates: amphotericin B (0.03125–16μg/mL) (Sigma Chemical Corp.), itraconazole (0.03125–16μg/mL) (Janssen Pharmaceutica, Belgium), and fluconazole (0.125–64μg/mL) (Pfizer, Brazil). Inocula of all tested isolates were prepared from 1-day-old cultures grown on potato dextrose agar at 35°C with RPMI 1640 medium supplemented with l-glutamine (HiMedia Laboratories) and buffered at pH 7 with 0.165M morpholinepropanesulfonic acid. The inocula were adjusted to a final concentration of 0.5–2.5×103cells/mL.6,9 The microdilution plates were incubated at 35°C for 48h and were visually read.9 For each isolate, drug-free and yeast-free controls were included and all the isolates were tested in duplicate. As quality control, for each test performed, Candida parapsilosis ATCC 22019 was included in each test as a quality control measure. The MIC of azole derivatives was defined as the lowest drug concentration capable of inhibiting 50% of growth, when compared with the growth control. For amphotericin B, the MIC was the lowest drug concentration at which no growth was observed. Isolates with MICs >1, ≥1, and ≥64μg/mL were considered resistant to amphotericin B, itraconazole, and fluconazole, respectively.9
Analysis of the efflux-pump activity in the azole-resistant Candida isolatesA phenotypical assay of modulation of efflux-pump activity was carried out based on the method used by Castelo-Branco et al.10 First, the azole-resistant Candida strains (13/37) were tested against two efflux-pump inhibitors, promethazine11 and haloperidol,12 by broth microdilution method,10 and they showed an average MIC value of 98μg/mL and 80μg/mL, respectively. Subsequently, susceptibility test was performed with itraconazole and fluconazole, according to the methodology described above. Sub-inhibitory concentrations of promethazine (MIC/8=12μg/mL) and haloperidol (MIC/8=10μg/mL) were then added to the final fungal inocula. Fluconazole was used in combination with promethazine and haloperidol, while itraconazole was tested only with promethazine, since haloperidol inhibits MDR1 activity, which is mainly involved in fluconazole resistance.12
Statistical analysisDistribution of the different yeast species at the collection sites was analyzed using the Pearson's chi-square test. The exact proportion test, considering a hypothesis of 50%, was used to verify differences in yeast recovery during rainy and dry season. The antifungal MICs obtained for the different yeast species were compared through ANOVA and post hoc Dunnet's test. Correlation between the MICs of each tested drug was measured using the Spearman's correlation coefficient. The Mann–Whitney's nonparametric test was used to compare the antifungal MICs of the strains obtained from different collection sites. p-Values lower than 0.05 indicated statistically significant conclusions.
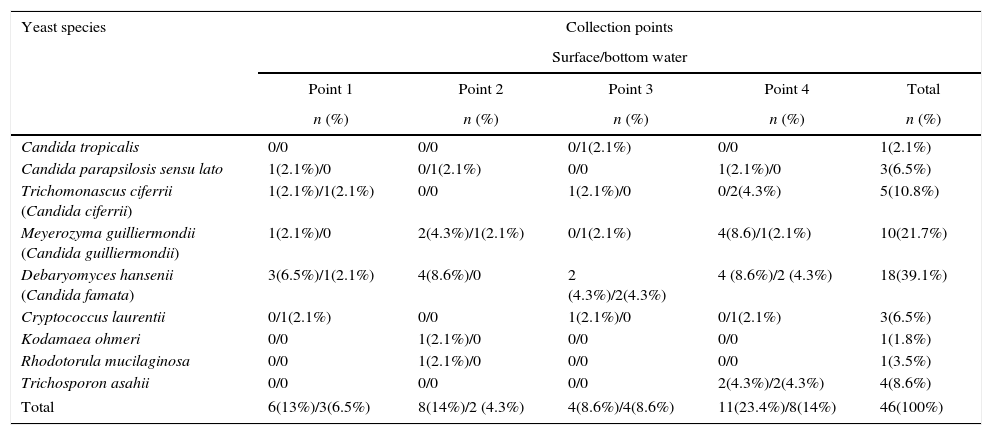
Results and discussionA total of 46 isolates were obtained, belonging to eight genera and nine species. Of this, 30 (65.2%) were from surface water samples, 2 C. parapsilosis sensu lato, 2 Trichomonascus ciferrii (Candida ciferrii), 7 Meyerozyma guilliermondii (Candida guilliermondii), 13 Debaryomyces hansenii (Candida famata), 2 Cryptococcus laurentii, 1 Kodamaea ohmeri, 1 Rhodotorula mucilaginosa, and 2 Trichosporon asahii; and 16 (34.8%) were from sediment samples, 1 Candida tropicalis, 1 C. parapsilosis sensu lato, 3 T. ciferrii, 3 M. guilliermondii, 5 D. hansenii, 1 C. laurentii, and 2 T. asahii (Table 1). When considering the collection sites, point 4 was the one with the highest amount of isolates (n=18, p<0.01), followed by point 2 (n=11), point 1 (n=10), and point 3 (n=7). There were no predominant species among the collection sites. With respect to seasonal variation, 84.8% of the yeasts (39/46) were isolated in the dry season (July to December, p<0.01) and 15.2% (7/46) were isolated in the rainy season, including the three strains of the Cryptococcus genus.
Yeast species isolated from different collection points at Catú Lake.
| Yeast species | Collection points | ||||
|---|---|---|---|---|---|
| Surface/bottom water | |||||
| Point 1 | Point 2 | Point 3 | Point 4 | Total | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Candida tropicalis | 0/0 | 0/0 | 0/1(2.1%) | 0/0 | 1(2.1%) |
| Candida parapsilosis sensu lato | 1(2.1%)/0 | 0/1(2.1%) | 0/0 | 1(2.1%)/0 | 3(6.5%) |
| Trichomonascus ciferrii (Candida ciferrii) | 1(2.1%)/1(2.1%) | 0/0 | 1(2.1%)/0 | 0/2(4.3%) | 5(10.8%) |
| Meyerozyma guilliermondii (Candida guilliermondii) | 1(2.1%)/0 | 2(4.3%)/1(2.1%) | 0/1(2.1%) | 4(8.6)/1(2.1%) | 10(21.7%) |
| Debaryomyces hansenii (Candida famata) | 3(6.5%)/1(2.1%) | 4(8.6%)/0 | 2 (4.3%)/2(4.3%) | 4 (8.6%)/2 (4.3%) | 18(39.1%) |
| Cryptococcus laurentii | 0/1(2.1%) | 0/0 | 1(2.1%)/0 | 0/1(2.1%) | 3(6.5%) |
| Kodamaea ohmeri | 0/0 | 1(2.1%)/0 | 0/0 | 0/0 | 1(1.8%) |
| Rhodotorula mucilaginosa | 0/0 | 1(2.1%)/0 | 0/0 | 0/0 | 1(3.5%) |
| Trichosporon asahii | 0/0 | 0/0 | 0/0 | 2(4.3%)/2(4.3%) | 4(8.6%) |
| Total | 6(13%)/3(6.5%) | 8(14%)/2 (4.3%) | 4(8.6%)/4(8.6%) | 11(23.4%)/8(14%) | 46(100%) |
In the present study, the genus Candida showed highest number of species, similar to what was observed by Medeiros et al.,5 with the presence of opportunistic pathogens such as C. tropicalis, M. guilliermondii, D. hansenii, T. ciferrii, and C. parapsilosis sensu lato. Of these, D. hansenii was the most isolated species from both the surface water and sediment (27.7% and 10.6%, respectively), followed by M. guilliermondii (14.9% and 6.4%, respectively), together accounting for 59.6% of the isolates found in this study. These two species are isolated from aquatic environments, eutrophized or contaminated with domestic sewage and industrial wastewater.5,6,13,14D. hansenii, in particular, besides indicating eutrophication, appears to be closely associated with the removal of these pollutants from contaminated water.14 Moreover, most of the isolates were obtained during the dry period, which may be related to the decrease in water volume and consequent concentration of nutrients that eventually may have favored the growth of yeasts. The nutritional concentration may also explain the larger number of strains found at point 4, which comprises an area where there is the discharge of domestic sewage.15
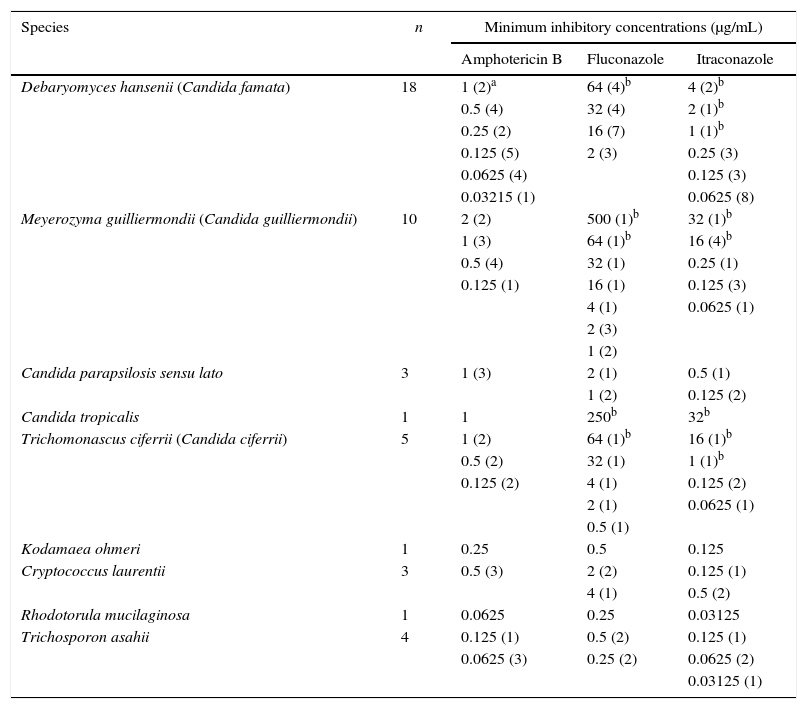
The MIC values obtained from the in vitro susceptibility tests of all the isolates are shown in Table 2. In relation to Candida (Debaryomyces, Meyerozyma, Trichomonascus) species, the MICs for amphotericin B varied from 0.03125 to 2μg/mL, with two resistant M. guilliermondii isolates (MIC=2μg/mL), which were also resistant to itraconazole. The MICs for fluconazole ranged from 0.5 to 500μg/mL and those for itraconazole varied from 0.0625 to 32μg/mL. A positive correlation was observed between amphotericin B and itraconazole (rho=0.19; p<0.01) and fluconazole and itraconazole (p<0.01). Of the 37 Candida isolates, 7 (18.9%) were resistant to both azole derivatives, 5 (13.5%) were resistant to itraconazole, and 1 (2.7%) was resistant to fluconazole. Among the azole-resistant isolates, three were obtained from point 1 (D. hansenii, M. guilliermondii, and T. ciferrii), two from point 2 (D. hansenii and M. guilliermondii), four from point 3 (2 D. hansenii, C. tropicalis, and T. ciferrii), and four from point 4 (3 M. guilliermondii and T. ciferrii). No statistically significant differences were observed in the antifungal MICs against strains from different collection sites. The MICs for amphotericin B, fluconazole, and itraconazole against the other yeast genera varied from 0.0625 to 0.5μg/mL, 0.25 to 4μg/mL and 0.03125 to 0.5, respectively (Table 2). The antifungal MICs of the T. asahii isolates were statistically lower than those of the other yeast genus (p<0.05) (Table 3).
Minimum inhibitory concentration (MIC) of amphotericin B, itraconazole and fluconazole against 46 yeast isolates from Catú Lake.
| Species | n | Minimum inhibitory concentrations (μg/mL) | ||
|---|---|---|---|---|
| Amphotericin B | Fluconazole | Itraconazole | ||
| Debaryomyces hansenii (Candida famata) | 18 | 1 (2)a | 64 (4)b | 4 (2)b |
| 0.5 (4) | 32 (4) | 2 (1)b | ||
| 0.25 (2) | 16 (7) | 1 (1)b | ||
| 0.125 (5) | 2 (3) | 0.25 (3) | ||
| 0.0625 (4) | 0.125 (3) | |||
| 0.03215 (1) | 0.0625 (8) | |||
| Meyerozyma guilliermondii (Candida guilliermondii) | 10 | 2 (2) | 500 (1)b | 32 (1)b |
| 1 (3) | 64 (1)b | 16 (4)b | ||
| 0.5 (4) | 32 (1) | 0.25 (1) | ||
| 0.125 (1) | 16 (1) | 0.125 (3) | ||
| 4 (1) | 0.0625 (1) | |||
| 2 (3) | ||||
| 1 (2) | ||||
| Candida parapsilosis sensu lato | 3 | 1 (3) | 2 (1) | 0.5 (1) |
| 1 (2) | 0.125 (2) | |||
| Candida tropicalis | 1 | 1 | 250b | 32b |
| Trichomonascus ciferrii (Candida ciferrii) | 5 | 1 (2) | 64 (1)b | 16 (1)b |
| 0.5 (2) | 32 (1) | 1 (1)b | ||
| 0.125 (2) | 4 (1) | 0.125 (2) | ||
| 2 (1) | 0.0625 (1) | |||
| 0.5 (1) | ||||
| Kodamaea ohmeri | 1 | 0.25 | 0.5 | 0.125 |
| Cryptococcus laurentii | 3 | 0.5 (3) | 2 (2) | 0.125 (1) |
| 4 (1) | 0.5 (2) | |||
| Rhodotorula mucilaginosa | 1 | 0.0625 | 0.25 | 0.03125 |
| Trichosporon asahii | 4 | 0.125 (1) | 0.5 (2) | 0.125 (1) |
| 0.0625 (3) | 0.25 (2) | 0.0625 (2) | ||
| 0.03125 (1) | ||||
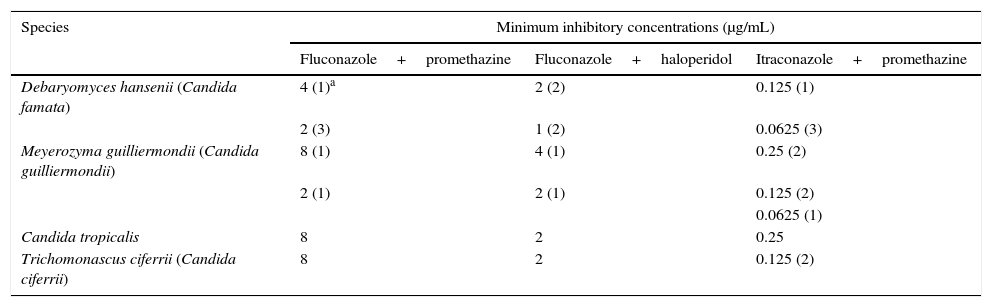
Minimum inhibitory concentration (MIC) of fluconazole and itraconazole after a combination with efflux pump inhibitors against the resistant yeast isolates from Catú Lake.
| Species | Minimum inhibitory concentrations (μg/mL) | ||
|---|---|---|---|
| Fluconazole+promethazine | Fluconazole+haloperidol | Itraconazole+promethazine | |
| Debaryomyces hansenii (Candida famata) | 4 (1)a | 2 (2) | 0.125 (1) |
| 2 (3) | 1 (2) | 0.0625 (3) | |
| Meyerozyma guilliermondii (Candida guilliermondii) | 8 (1) | 4 (1) | 0.25 (2) |
| 2 (1) | 2 (1) | 0.125 (2) | |
| 0.0625 (1) | |||
| Candida tropicalis | 8 | 2 | 0.25 |
| Trichomonascus ciferrii (Candida ciferrii) | 8 | 2 | 0.125 (2) |
Savini et al.16 reported that M. guilliermondii is less susceptible to amphotericin B, in contrast to other Candida species. In this study, the susceptibility of Candida isolates to the azole derivatives corroborates our earlier study on yeasts isolated from wild-harvested freshwater prawns (M. amazonicum), wherein, azole-resistant isolates were obtained in a high number.6 In the present study, of the 37 Candida isolates, 13 (35.14%) were resistant to azoles, 4 among them were D. hansenii and 5 were M. guilliermondii. Unlike clinical isolates of D. hansenii and M. guilliermondii, antifungal resistance is more often seen in the environmental isolates of these Candida species.5,6,17,
Several studies focusing on the isolation of antifungal-resistant environmental yeasts responsible for the deterioration of aquatic systems have been published.5,6,17 An insight on the mechanisms of antifungal resistance can help better understand the relationship between the development of antifungal resistance and environmental pollution. In order to investigate the mechanisms involved in the azole resistance, the efflux-pump inhibition assay was performed with promethazine and haloperidol, which resulted in the reversal of resistance to itraconazole and fluconazole in all tested Candida isolates. The addition of promethazine led to an MIC reduction of 8-256 fold and of 8-62.5 fold, for fluconazole and itraconazole, respectively, while the addition of haloperidol led to a 32–125 fold reduction of the MICs for fluconazole (Table 2).
Promethazine is a phenothiazine derivative that acts on MDR and CDR efflux pumps,11 while haloperidol acts only on MDR pumps.12 Thus, the inhibition of the efflux-pump activity by promethazine and haloperidol, resulting in the reversal of the azole resistance, suggests that the azole resistance among the Candida spp. is related to the enhanced activity of these pumps. This increased activity is a direct result of the upregulation of the CDR and MDR genes, possibly, as a consequence of the presence of different chemical compounds in Catú Lake, secondary to human activities. It is believed that this resistance phenomenon is related to the discharge of industrial wastewater and other pollutants into the aquatic environment, which may lead to alterations in gene expression or gene sequence in the microorganisms.18–20 These alterations have been found as the main genetic variations associated with the development of antifungal resistance.21
The present study reports a high rate of azole-resistant Candida spp. (Debaryomyces, Meyerozyma, and Trichomonascus) obtained from an aquatic environment, which may represent a risk for environmental and human health. This article is the first report of the involvement of efflux pumps in the azole resistance among Candida spp. from environmental sources. In this study D. hansenii and M. guilliermondii were the most commonly isolated species and presented the highest rate of azole resistance. Considering that these species are associated with environmental deterioration, monitoring their phenotypical features might serve as an indicator of the environmental health of water bodies.
Conflict of interestThe authors declare no conflicts of interest.
This work was supported by grants from the National Council for Scientific and Technological Development (CNPq; Brazil; Processes 562296/2010-7, 504189/2012-3, 443167/2014-1) and the Coordination Office for the Improvement of Higher Education Personnel (CAPES/PNPD 2103/2009, AE1-0052-000650100/11).