The goal of this investigation was to isolate competent polynuclear aromatic hydrocarbons degraders that can utilize polynuclear aromatic hydrocarbons of former industrial sites at McDoel Switchyard in Bloomington, Indiana. Using conventional enrichment method based on soil slurry, we isolated, screened and purified two bacterial species strains PB1 and PB2. Applying the ribotyping technique using the 16S rRNA gene analysis, the strains were assigned to the genus Pseudomonas (Pseudomonas plecoglossicida strain PB1 and Pseudomonas sp. PB2). Both isolates showed promising metabolic capacity on pyrene sprayed MS agar plates during the preliminary investigations. Using time course studies in the liquid cultures at calculated concentrations 123, 64, 97 and 94ppm for naphthalene, chrysene, fluroanthene and pyrene, P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 showed partial utilization of the polynuclear aromatic hydrocarbons. Naphthalene was degraded between 26% and 40%, chrysene 14% and 16%, fluroanthene 5% and 7%; pyrene 8% and 13% by P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 respectively. Based on their growth profile, we developed a model R2=1 to predict the degradation rate of slow polynuclear aromatic hydrocarbon-degraders where all the necessary parameters are constant. From this investigation, we confirm that the former industrial site soil microbial communities may be explored for the biorestoration of the industrial site.
Global industrialization is without consequences, particularly with the deposition of hydrophobic contaminants in sediments, surface soil and dump sites. Polynuclear aromatic hydrocarbons (PAHs), a hydrophobic contaminant have been listed as one of priority/toxic environmental contaminants. They are chemical compounds comprising mainly of carbon (C) and hydrogen (H); arranged in form of two or more aromatic rings with various structural configurations.1 PAHs can be classified as alternant (e.g., benzo[a]pyrene, benz]a]anthracene, chrysene, dibenz[a,h]anthracene) or non-alternant (e.g., fluoranthene, benzo[k]fluoranthene, benzol[j]fluoranthene, indeno[1,2,3-c,d]pyrene). The alternant PAHs comprises of aromatic rings with six carbon atoms while the non-alternant ones contain aromatic rings with less or more than six carbons. This peculiarity is based on the electron density associated with the molecule. Alternant PAHs have an equally distributed electron density, whereas non-alternant PAHs behave almost as if they were two different molecules because of an uneven distribution of electron density from one portion of the molecule to another. PAHs being a derivative of benzene, and thermodynamically stable, have two or more fused aromatic rings arranged in linear, angular, or clustered structures.2,3 PAHs are ubiquitous environmental pollutant, entering the environment from natural and anthropogenic sources such as natural fires, volcanic eruptions, aluminum smelting, coke production, and creosote preservation. The anthropogenic sources typically are as a result of incomplete combustion of the organic substances that make up such substances.4,5
The human body could be exposed to PAHs through inhalation, dermal contact and ingestion. Within the body, PAHs being highly lipid-soluble are quickly absorbed by the fatty tissues such as the kidney, liver and gastro-intestinal tract of mammals. In humans, the highest metabolizing capacity present is the liver, then the lungs, intestinal mucosa, skin and kidneys. Metabolism may also take place in nasal tissues, mammary glands, spleen, brain follicles, erythrocytes, platelets, leukocytes, placenta and uterus. In the liver, PAHs are adapted by cytochrome P450 into epoxides a major intermediates that are reactive and enzymatically metabolized to dihydrodiols and phenols. The dihydrodiols and phenols react against DNA and proteins causing mutagenic damage to cells.6
Naphthalene has been noted to be a hazardous air pollutant.7 When experimental organisms are exposed to naphthalene, it causes decrease in their hemoglobin concentration and inhibits their oxygen consumption. Naphthalene is used as raw chemical for industrial synthases of phthatic anhydride.8 Naphthalene at high concentration may cause hemolytic anemia and some other conditions, whereas the tumorigenic potential is presently considered low.9,10 Naphthalene has been often used as a model PAH due to high speed of utilization by microorganisms compared to other PAH and the relatively simple structure of the intermediates in the catabolic pathways. Thus information on bacterial degradation of naphthalene has been used to understand and predict pathways of most PAHs.
Pyrene is a hydrophobic compounds and its persistence within ecosystems is due to low water solubility, dense clouds of p-electrons on both sides of the ring structures, making them resistant to nucleophilic attack.11
Soil can act as a sink for carbon. It could receive considerable amount of PAHs that may likely remain persistent in the environment due to low solubility and sequestration in soil and sediments; to lack of versatile metabolic capacity of microorganisms to degrade these compound.11 According to Regonne and co-workers, for effective cleanup of PAH- contaminated soils, cheaper and more ecologically friendly options are proposed over chemical and physical processes.12 These options will involve greater understanding of the processes involved and factors that limit the degradation of high molecular and low molecular weight PAHs. Bioremediation is a proficient and safe method to clean up PAH from contaminated sites. It has been applied to both terrestrial and aquatic ecosystems; and may possibly provide a position in biorestoration of contaminated soils. Microorganisms transform the PAHs to CO2 and water through metabolism or co-metabolism. They PAHs serve as carbon and energy sources, thus reducing the associated toxicity and co-metabolic substrates of PAH.11 Since the 1950s efforts have been made to select microorganisms with ability to degrade PAHs from pure cultures.13 Since then, many bacteria strains have been isolated for their ability to transform, degrade and utilize PAHs as a source of energy and carbon.5,14–17 Hitherto, it has been recognized that few bacteria have been isolated that are capable of utilizing PAHs with four or more aromatic rings as sole sources of carbon and energy.18,19 Consequently, it is of paramount importance to isolate and investigate versatile degraders of PAHs. In this study, we report for the first time the isolation and characterization of bacterial strains from a former industrial site in Bloomington in Indiana using conventional enrichment of soil slurry. The bacteria Pseudomonas plecoglossicida strain PB1 and Pseudomonas sp. PB2 were screened for their growth and degradation fluxes in naphthalene, fluoranthene, pyrene and chrysene. We proposed a mathematical model to assist in the simulation of the degradation rates of our bacterial species on the selected PAHs (naphthalene, fluoranthene, pyrene and chrysene).
Materials and methodsChemicalsThe naphthalene, fluoranthene, pyrene and chrysene of analytical grades were purchased from Sigma Aldrich Corp. (St. Louis, MO, USA). Sodium benzoate (99+% purity), 2,2,4,4,6,8,8-heptamethylnonane (HMN), and all other organic solvents were obtained from Fisher Scientific Co. (Springfield, NJ, USA). Hexane, a high purity solvent for GC-chromatograph was obtained from EMD Chemicals Inc. Merck. The PAH analytical standards were procured from Accustandard Inc. (New Haven, CT 06513). All other chemicals and reagents used were of reagent grade or better.
Stock solutions and mediaFor the enrichment and degradation experiments, chloride free minimal salts (MS) medium as described by20–23,5 were used. The medium consisted of (g) 0.5(NH4)2SO4, 0.1MgSO4·7H2O, 0.076Ca(NO3)2·4H2O and 1.0mL each of trace metal and vitamin solutions per liter of 40mM phosphate buffer (pH 7.25). Naphthalene stock solution were prepared in HMN, a non-degradable carrier to provide an initial concentration of ca. 123ppm. The concentration represents the total mass in both the aqueous and HMN phases, divided by the aqueous volume. The appropriate stock solution was added using a gas-tight syringe in 250-μL aliquots to provide test compound concentration of ca. 100ppm in the final medium. Alongside, chrysene, fluoranthene and pyrene stock solution were prepared differently by dissolving the weighted test compounds in acetone respectively. Fluoranthene, chrysene and pyrene were added from the different stock solution of the test compound into the balch tubes using a Hamilton gas-tight syringe in 250-μL aliquots, to provide test compound concentration of ca. 97ppm for fluoranthene, ca. 64ppm for chrysene, and pyrene ca. 94ppm in the final medium. Solid MS medium was made by the addition of 1.8% Bacto-agar (Difco Laboratories, Detroit, MI, USA).
The naphthalene solution was added with Hamilton gas tight syringe 250μL aliquots into the balch tubes to provide test compound concentration of 100ppm in the final medium. The MS medium was supplemented with the test compound, achieving an experiment dependent concentration. The cultures were incubated at room temperature on a shaker table to aid slow mass transfer of the test compound into the aqueous phase. Initial investigations were carried out using MS medium supplemented with HMN as the sole carbon and energy sources to determine that HMN did not serve as growth substrate.
Soil sample collection and enrichment of PAH degrading bacteriaThe soil samples were collected from former industrial sites at the McDoel switchyard in Bloomington, Indiana. For decades, the site had been contaminated with PAHs, other organic and inorganic pollutants. Soil samples were taken from 6 to 11cm layer of the contaminated soil at three locations, with indications of low to high level of PAH-contamination based on preliminary environmental audit. The soil samples were placed in separate sterile jars and transported back to the lab at ambient temperatures. The samples were dark in appearance. PAH-degrading bacteria were initially isolated by the conventional enrichment methods. For this, 5.0g of the different soil samples were weighed into 160mL serum bottles mixed with 30mL of sterile MS medium. PAH-contaminated soils may have limited bioavailability due to sorption and strong hydrophobicity of PAH. Thus pyrene was added in the enrichment bottles to serve as supplemental carbon and energy source. All the 160mL serum bottle bioreactor was set up in triplicates. The serum bottles were crimp-sealed with teflon-coated, butyl rubber stoppers to prevent losses due to volatilization and/or sorption. These were incubated horizontally on an orbital shaker table (Labline Instruments Inc., Melrose Park, IL, USA) at ambient temperature. Air sparging was done weekly to re-aerate the headspace and biweekly periodic transfers were made using about 15% inoculum into new MS medium supplemented with the test PAHs. The procedure was repeated for seven successive times.
Determination of carbon, hydrogen and nitrogen (CHN) ratio of the soil samplesThe carbon, hydrogen and oxygen ratio of the obtained soil samples from the spatial distribution at McDoel switchyard were determined using the PerkinElmer 2400 series CHN analyzer. The sieved soil samples were thoroughly mixed and oven-dried at 85°C for 24h. Approximately 6mg of the oven-dried soil were weighed into tin capsules. The capsules were then placed into the PerkinElmer 2400 series CHN analyzer for determination of carbon, hydrogen and nitrogen ratio.
Isolation, purification and phylogenetic analysisPure cultures from pyrene-enriched media were isolated by directly plating aliquots (0.2mL) of highly-enriched cultures onto MS agar. Because we wished to prevent loss of catabolic plasmids from capable isolates, we used MS agar medium supplemented with pyrene rather than nutrient agar to maintain selective pressure. The pyrene was added to the medium using the spray plate technique as described by Kiyohara et al.24 Immediately after spread-plating the 0.2mL aliquot of enrichment culture, an ethereal solution of pyrene was uniformly sprayed onto the surface of the agar. The plates were sealed with parafilm film and incubated for 1 week at 30°C in dark. Pyrene-degrading microorganisms were identified by cleared zones around an individual colony. The selected colony was purified by repetitive streaking on MS agar sprayed with pyrene. The most efficient bacterial isolates were selected by comparison of growth rate, generation time, and generation number.
The pure culture were routinely cultured and sustained on solid MS agar plates containing 2.5mM pyrene. For 16S rRNA gene analysis, genomic DNA was isolated from overnight cultures of isolates growing on 2.5mM benzoate using an UltraClean Microbial DNA Isolation kit (Mo Bio Laboratories, Solana Beach, CA, USA). Three eubacterial PCR primers; forward primer 8fm (AGAGTTTGATCMTGGTCAG) and reverse primers 926r (CCGTCAATTCCTTTRAGTTT) and 1387r (GGGCGGWGTGTACAAGGC) were used to amplify the 16S rRNA gene. The reaction mixtures were incubated at 95°C for 2.5min and then cycled 33 times through the following temperature profile: 95°C for 30s, 48°C for 30s, and 72°C for 1.5min, followed by a single 10min incubation at 72°C. About 2μL of each amplification mixture was analyzed by agarose gel electrophoresis 10.0μgmL−1 (w/v) ethidium bromide to establish that amplicons were of the expected length. The PCR amplicons were subsequently cleaned using QIAquick Nucleotide Removal Kit from Qiagen Inc. (Turnberry lane, CA 91355). For the 16S rRNA sequencing, the PCR products were sequenced following an ABI Big Dye Terminator Cycle Sequencing reaction using an Applied Biosystems 3730 automated sequencing system (Applied Biosystems, Inc., Foster City, CA, USA). The following settings were applied: denaturation for 3min at 94°C, 25 cycles at 96°C for 10s, 50°C for 5s and 60°C for 4min. The resultant sequences were edited and aligned using Bio Edit software version 4.8.7 to check for reading errors and when possible, clarify ambiguities.25 Sequences were subsequently compared with deposited sequences in GenBank database using the BLAST algorithm available at URL http://www.ncbi.nlm.nih.gov/BLAST/.26
Growth on the different carbon and energy sourcesInvestigations of the potentials of the pure cultures to grow on naphthalene, fluoranthene, pyrene and chrysene were carried out. The tests were carried out in MS medium supplemented with each PAH test compound as sole carbon source. These investigations were conducted in crimp-sealed tubes (balch tubes) usually utilized for anaerobic studies. For issues of quality control/assurance, tubes utilized for this study were baked in muffle furnace at 500°C to remove organic contaminants. Growth and degradation studies were performed in balch tubes containing 10mL of MS medium, the tested PAH, inoculum, and approximately 15mL air headspace to maintain aerobic conditions. The different tubes were supplemented with different PAHs respectively. Naphthalene was added from an HMN stock solution at a concentration of ca. 123ppm as described above and inoculated with 105cells/mL of phosphate buffer (pH 7.25) washed cells pre-grown in 2.5mM benzoate. All the stock solutions were aseptically prepared before use. Fluoranthene, chrysene and pyrene were added from the stock solution into the balch tubes using a gas-tight syringe in 250-μL aliquots, to provide test compound concentration ca. 97ppm for fluoranthene, ca. 64ppm for chrysene, and pyrene ca. 94ppm in the final medium. Balch tubes were crimp-sealed with teflon-coated, butyl rubber stoppers to prevent losses due to volatilization or sorption. The tubes were incubated horizontally on a shaker table at (120rev/min) at ambient temperature. Epifluorescence microscopic examination was utilized to monitor growth by counting the cells numbers using replicate tubes. The cells were stained with acridine orange stain after fixation with 50μL of glutraldehyde. The acridine orange stain bind to the DNA of the cells and is usually used to determine the total bacteria cells present. Visual examinations in agreement with periodic GC analyses to measure the test compound disappearance was also done. In this study, growth was positive when there is an increase in turbidity greater than the killed or abiotic control that was used. For statistical estimate, at least 10 microscopic fields were randomly chosen and a minimum of 1000 cells were counted. Data are presented as the mean cell numbers±the SEM.
Transformation of PAH compounds – naphthalene, fluoranthene, pyrene and chrysene experimentsThe degradation studies of the PAHs – naphthalene, fluoranthene, pyrene and chrysene were likewise conducted in the balch tubes. The tubes were inoculated with the different bacterial cultures, crimp sealed and incubated horizontally on the shaker table at ambient temperature. After 14 days, the degradation reactions were stopped for naphthalene while experiments with fluoranthene, pyrene and chrysene were stopped after 21 days. 5mL of hexane was added, vortexing for 1–2min and subsequently, mixed continuously on a tube rotator for 12h to stop the degradation study. Beckman GS-6 series centrifuge at 2190rpm for 20min was used to separate the hexane fraction and the aqueous phase. The hexane extracts were collected for further analysis. The extracts were stored in target vials with a headspace of 1mL and crimp sealed using an 11mm Teflon rubber stopper from National scientific and preserved at 4°C prior to analysis.
Analytical methodsGas chromatography coupled flame ionization detector and statistical analysisThe hexane extracts were analyzed on an HP 5890 Series II gas chromatography GC (Hewlett Packard Co., Palo Alto, CA, USA) fitted with an HP 3396 series II integrator and equipped with a flame ionization detector (FID). Hexane extracts (5μL injection volume) were injected using a 10-μL Hamilton gas-tight syringe through a 30m HP-5 megabore fused-silica capillary column (J & W Scientific, Folsom, CA, USA; 0.32mm id, 0.25μm film thickness). The GC utilized helium (He) as the carrier gas with a linear velocity He flow rate=∼3.33mL/min; H2=∼30mL/min; air=∼400mL/min. The injector and detector temperatures ranged between 180 and 300°C with two different rates. The program cycle for naphthalene was at an initial temperature of 50°C; this was held for 5min then ramped at 30°C/min to 180°C for 2min, then ramped to 300°C at 40°C/min for 4min. Analytical standards of PAHs were prepared in hexane. Typical coefficients of correlation for standard curves were 0.98–0.99. Statistical tests was performed using the Prism 4.0 computer software program (Graph Pad Software, San Diego, CA, USA) and statistical package for social scientist (SPSS) 15.0.
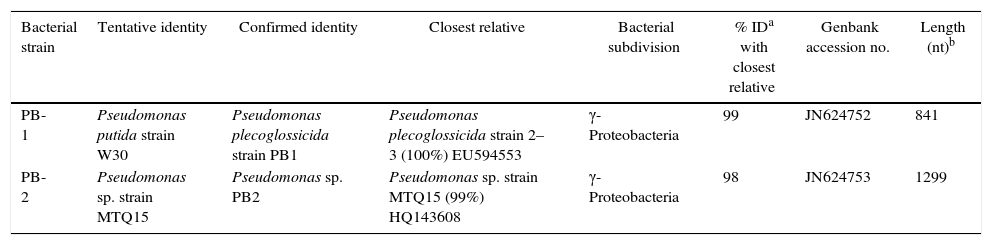
ResultsIsolation and phylogenetic characterization of the PAH degrading strainsTen morphological different microbial colonies were selected from the MS agar plates following initial enrichment on pyrene. Upon screening individual isolates for growth on MS salicyclic acid, MS benzoate and we selected two isolates for further study. The colony morphology of some isolates observed under the fluorescent microscope showed uniform bacillary rods, producing non-fluorescent diffusible blue pigment. 16S rRNA phylogenetic analyses placed our strains PB-1 and PB-2 within the genus Pseudomonas (Table 1). The closest relative of strain PB-1 had 99% similarity as P. plecoglossicida strain 2–3 (EU594553),27 a root organism isolated from root of sugar beet. Strain PB-2 had 98% homology as Pseudomonas sp. strain MTQ15 (HQ143608),28 isolated from the soil around the rhizosphere surrounding tobacco plant in China. We have classified our isolates as P. plecoglossicida strain PB1, and Pseudomonas sp. strain PB2 (GenBank database accession numbers JN624752 and JN624753 issued).
Cloned fragments of 16S rRNA genomic DNA of PAH degrading bacterial species.
| Bacterial strain | Tentative identity | Confirmed identity | Closest relative | Bacterial subdivision | % IDa with closest relative | Genbank accession no. | Length (nt)b |
|---|---|---|---|---|---|---|---|
| PB-1 | Pseudomonas putida strain W30 | Pseudomonas plecoglossicida strain PB1 | Pseudomonas plecoglossicida strain 2–3 (100%) EU594553 | γ-Proteobacteria | 99 | JN624752 | 841 |
| PB-2 | Pseudomonas sp. strain MTQ15 | Pseudomonas sp. PB2 | Pseudomonas sp. strain MTQ15 (99%) HQ143608 | γ-Proteobacteria | 98 | JN624753 | 1299 |
The obtained quantity of carbon, nitrogen and hydrogen from the soil samples from McDoel switchyard from different locations within the site is as shown in Table 2. It was apparent that each of the soil sample examined had similar trend where nitrogen being the lowest values and carbon the highest values.
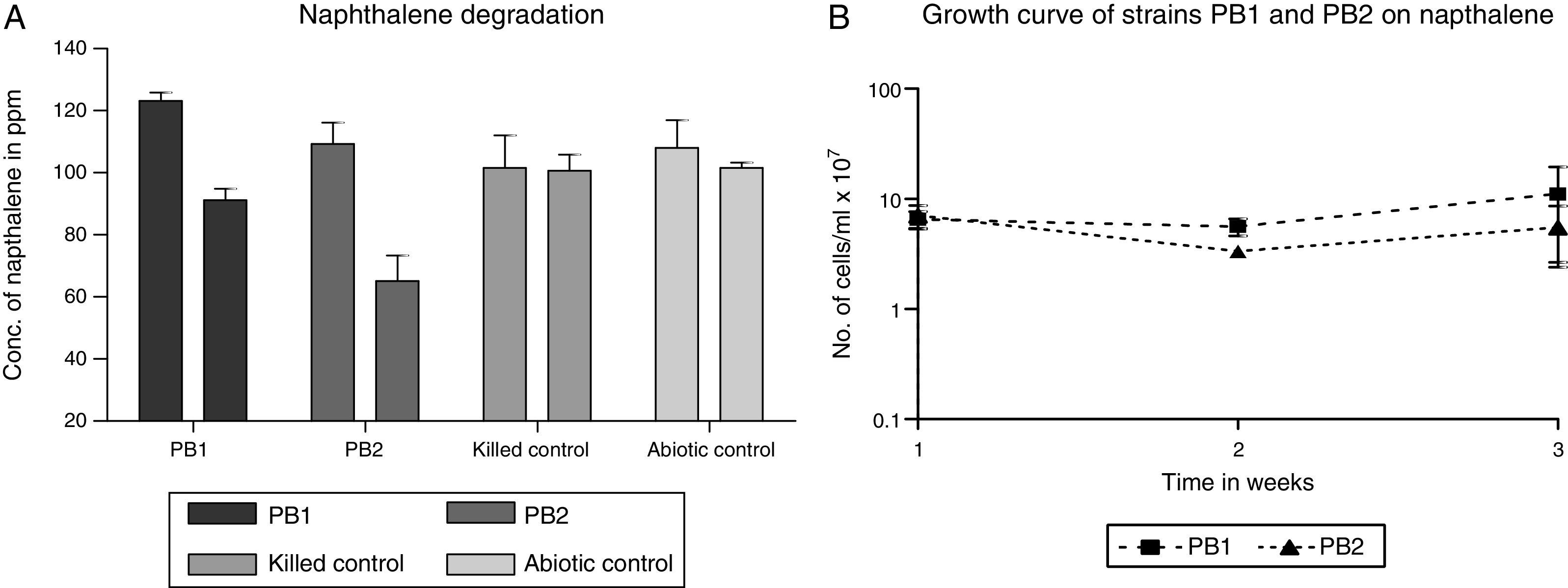
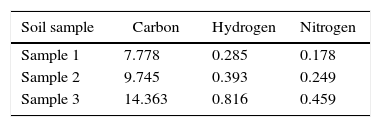
Degradation of naphthaleneIn this study, we observed that the initial and final values (data not shown) obtained for the abiotic and biotic controls had no significant difference with the values obtained at the initial stage for the test samples. The isolated strains PB-1 and PB-2 were evaluated on naphthalene to determine its degradative potential on the test compound. The evaluation was done with strains PB-1 and PB-2 washed cells grown on MS-benzoate. We employed no other carbon source other than the provided naphthalene. Following 14 days of incubation, strains PB-1 and PB-2 ability to utilize naphthalene was appraised by comparing the GC peak areas of the initial day time (0) and the final time (t). We established the growth of our strains by the intense increase in the turbidity of the test sample and considerable reduction in the concentration of naphthalene. (Fig. 1A) shows the values of the net reduction (percent reduction in total naphthalene content) in naphthalene concentration. These were 26% and 40% respectively for strain PB-1 and PB-2. The initial concentration of naphthalene at time zero was ca. 123ppm while the final concentration ranged between 65 and 91ppm. The mean biodegradation rate of naphthalene by strain PB-1 was 0.095±0.004mgL−1h−1. The mean PB-2 biodegradation rate for naphthalene was 0.131±0.005mgL−1h−1.
(A) Degradation of naphthalene by MS-benzoate grown cells of PB-1, PB-2, incubated for 14 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Naphthlane-dependent growth and cell numbers distribution of strains PB-1, PB-2, in naphthalene incubated for 14 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (14 days) represented as (3) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The large error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
In this study, we defined growth as increase in the cell number of at least one-order-of-magnitude and concomitant disappearance of the parent compound when compared to the biotic and abiotic control. We determined that growth observed was not from HMN by supplementing it as the only carbon source. It was observed that no appreciable growth occurred for strains PB-1 and PB-2 in HMN. Consequently in all cases, cell numbers increased by a significant orders-of-magnitude than the balch tubes of the abiotic and biotic control. This evidently demonstrated growth on naphthalene. (Fig. 1B) shows the results of growth profiles of strains PB-1,2. Cell numbers were counted after 7 days. Strains PB-1 and PB-2, had decline in the cell number when compared at time (0) after one week. Over the course of this investigation, strain PB-2, exhibited an increase in the cell numbers that occurred until the end of 14 days incubation period. Possibly the observed decline in cell numbers may be due to stress experienced by the cells that were active at log phase having been pre-grown in fresh MS-benzoate.
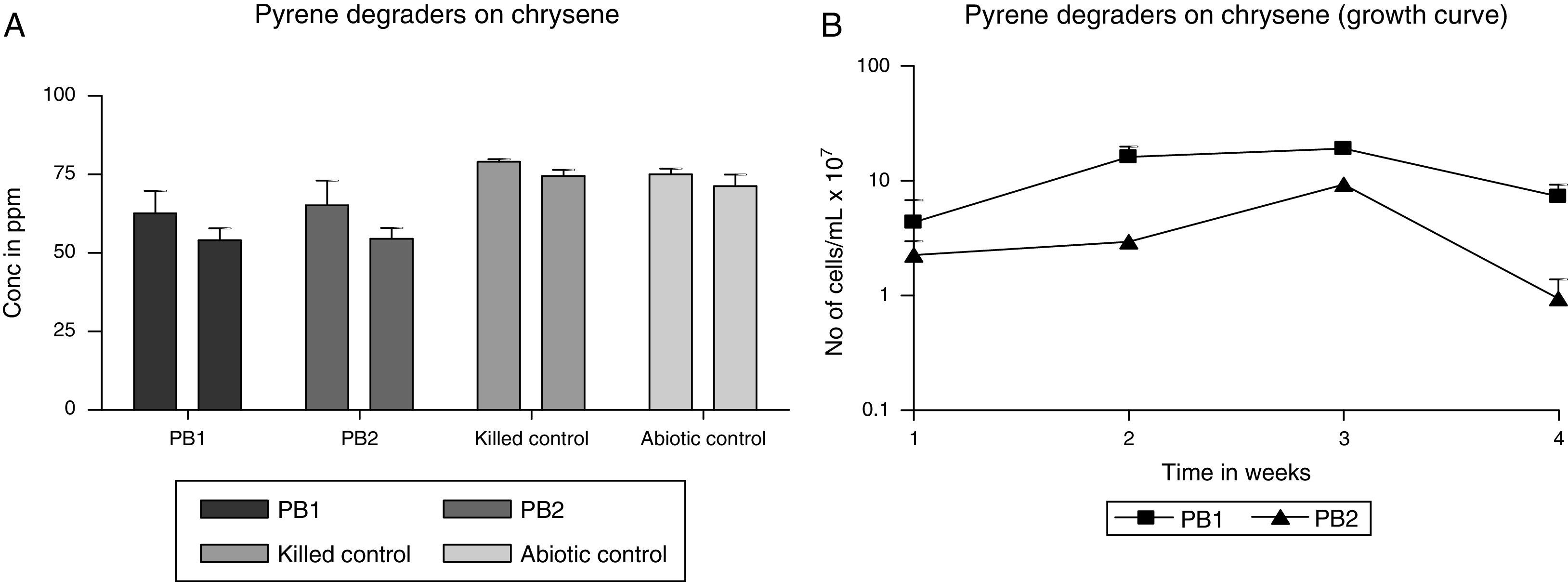
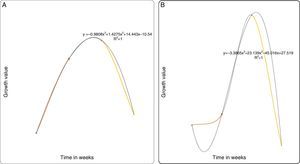
Degradation of chryseneThe strains PB-1 and PB-2 consumed chrysene as a source of its energy and carbon sources. Evidently, the strains exhibited increases in their cell numbers. However, each of the strains had a different growth pattern as shown by the growth profile graph (Fig. 2B). The strain PB-2 was able to degrade more of the chrysene than strain PB-1. The (mean and standard deviation values) of chrysene used in this investigation was ca. 64ppm. At the end of the 21 days incubation period, the strains degraded about 14–16% of the chrysene. The final concentration (mean and standard deviation) assessed was 54ppm. Strain PB-1 consumed the chrysene at 14% at volume biodegradation rate of 0.017±0.011mgL−1h−1. Strain PB-2 utilized 16% of chrysene at the volume biodegradation rate of 0.021±0.009mgL−1h−1. It was evident from the growth profile study in Fig. 2B that after the second week, there was a decline in the cell numbers of PB-1 and PB-2, this may perhaps be due to dead end products or intolerance to the produced intermediate products.
(A) Degradation of chrysene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Chrysene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
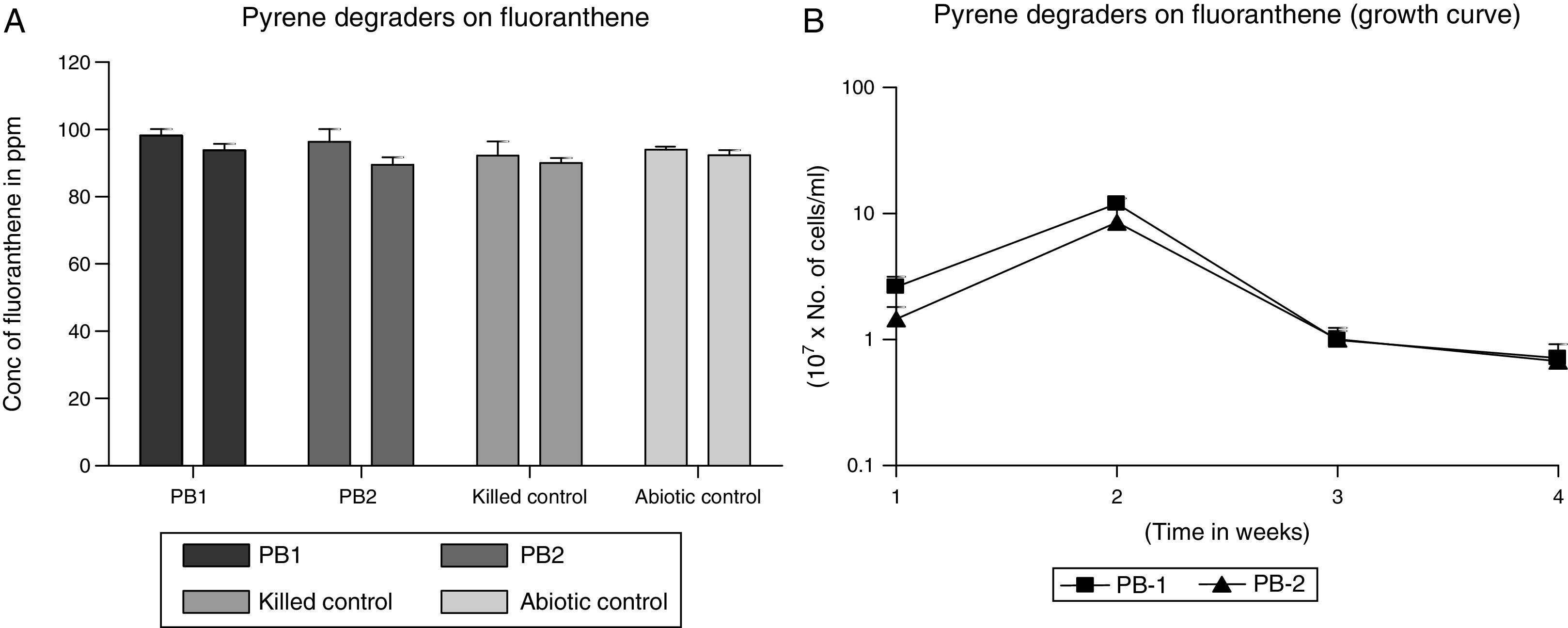
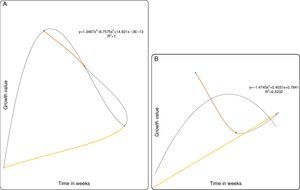
The abilities of strains PB-1 and PB-2 to degrade fluoranthene was assessed using washed benzoate-grown cells. The percentage net reductions for fluoranthene are 5% and 7% for strains PB-1 and PB-2 respectively. Representing the concentration in ppm the initial concentration ca. 97ppm and the final ca. 91ppm thus this signifies minimal utilization of fluoranthene (Fig. 3A). The mean biodegradation rate used by strain PB-1 was 0.009±0.0001mgL−1h−1. Strain PB-2 consumed fluoranthene at the rate of 0.013±0.005mgL−1h−1. From the growth profile in Fig. 3B, the strains did not show any lag phase possibly due to endogenous utilization of the substrates. This lasted for about a week period. Thereafter, there was sharp decline in the cell numbers that continued to the end of the investigation. This may suggest that the organisms could not tolerate high concentration of fluoranthene. In addition, the rate of decline for strain PB-1 and PB-2 were similar.
(A) Degradation of fluoranthene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Fluoranthene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
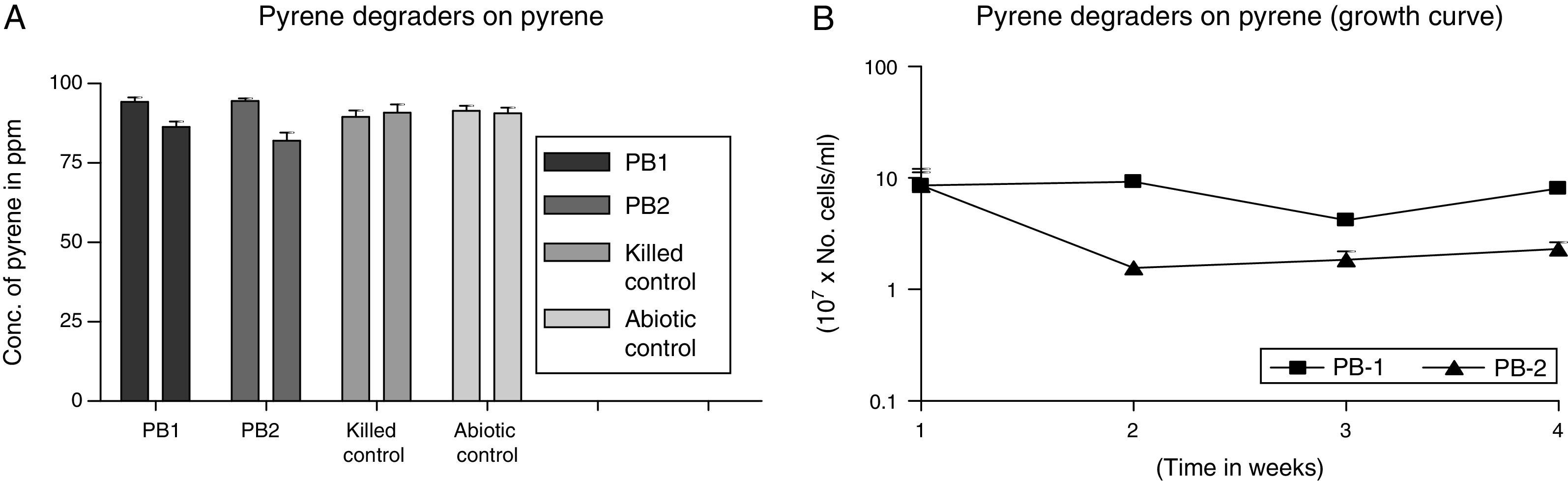
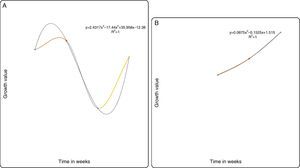
The degradation potential of the MS-benzoate washed cells of strains PB-1 and PB-2 were evaluated on pyrene, having exhibited optimum potential in degrading pyrene on MS-agar sprayed pyrene. Nonetheless, the organisms exhibited low consumption of pyrene in balch tubes supplemented with initial concentration of pyrene ca. 94ppm and the final concentration ca. 83ppm After 21 days of incubation, strain PB-1 were able to consume about 8% of pyrene at the biodegradation rate of 0.016±0.001mgL−1h−1 while strain PB-2 utilized 13% of pyrene at the biodegradation rate of 0.024±0.005mgL−1h−1. In the growth profile in Fig. 4B), it showed that the organisms had a lag period, that resulted in the decline of cell numbers following the assay carried out the first week. After the third week, there was an increase in the cell numbers. Nevertheless, the multiplication in cell numbers was not a sharp logarithmic growth. It showed a gradual increase. There are possibilities, that the organisms might be synthesizing different metabolic pathway having been grown in MS-benzoate before this evaluation. The slow rate of the biodegradation suggests that these strains could utilize pyrene minimally in a liquid medium when compared with growth on the sprayed solid MS-agar. In addition, it is evident that these organisms may require long adaptation period for effective utilization of pyrene.
(A) Degradation of pyrene by MS-benzoate grown cells of PB-1 and PB-2, incubated for 21 days. Data represent the mean and standard deviation of triplicate determination of initial and final concentration respectively. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes. (B) Pyrene dependent growth and cell numbers distribution of strains PB-1 and PB-2, in chrysene incubated for 21 days. Data represent the mean of replicates tubes for initial time (0) cell density represented as (1) and final time (21 days) represented as (4) respectively. The x-axis value range was chosen as such to allow for even spread of the growth curve. The error bars (Standard Deviation) were due to differential response of cells in triplicate tubes.
Many PAHs have been noted to be toxic and as such interest in understanding the physicochemical processes and microbial degradation activities that influences these PAHs in soil are important. Soils subjected to a constant contamination can yield a natural selection of autochthonous pollutant – biodegrading microbial population.29 Microorganisms obtained from such polluted soil are efficient in using hydrocarbons as carbon and energy sources. Thus the art of application of microorganisms for the bioremediation of PAH-contaminated environment is an attractive technology for the reclamation and sustainable development of polluted sites. In this study, we have isolated and characterized P. plecoglossicida strain PB1, and Pseudomonas sp. strain PB2 with capacity to grow and partially degrade the selected PAHs – naphthalene, fluoranthene, pyrene and chrysene.
Often times, sites contaminated with hydrocarbon could be difficult reclaiming, partly due to the imbalance in the carbon:nitrogen:phosphorus (C:N:P) ratio, caused by high carbon and low nitrogen levels might slow down or even prevent biodegradation. The percentage of C:H:N ratio obtained from the soil analyses (samples 1, 2, 3) from the McDoel switchyard is as shown in Table 2. An imbalance in the C:N:P ratio has been found to reduce the capacity of microbes to form viable biomass in using PAHs as carbon/energy source. Van Hamme and co-workers30 revealed that nitrogen and phosphorus contents greatly affect microbial degradation of hydrocarbon. Besides organic compounds as carbon source (C), productive PAH-metabolizing microorganisms require inorganic macronutrients such as nitrogen and phosphorus for biomass production. Thus the macronutrients requirements for C, N and P for bacterial growth are not constant but vary with type, carbon source utilized and the environmental habitat. In addition, Verde and co-workers,31 reported that the amount of N and P required for metabolism of a certain amount of PAHs depends on the bacterial specific growth rate, the elemental composition of the bacterial cells formed and the maximum carbon conversion efficiency. By itself, the PAH conversion rate and growth of PAH-degrading species in a soil environment very much relies on the available concentrations of N and P. Thus from the obtained results of C:H:N ratio, these variations in these essential nutrients might have accounted for the high levels of PAH recorded during the environmental audit.
The evolution of degradative abilities among bacterial strains may be as a result of the plasmids. Plasmids allow bacterial populations to access the horizontal gene pool for adaptive traits that may be useful for their survival under local selective pressures imposed by organic pollutants.
On the assessment of our strains PB-1 and PB-2 for their degradation and growth studies on naphthalene, we believed that the HMN a highly branched alkane did not induce aromatic dioxygenase, thus functioned as intended, i.e., reduction in volatility and facilitated mass transfer of naphthalene into the medium. For naphthalene, the amount utilized by strains PB-1 and PB-2 were 26% and 40% respectively from initial concentration ca. 123ppm. These values seemed low when compared to other bacterial strains known to degrade naphthalene. This may suggest that previous exposure of our strains to pyrene during enrichment may influence the type of plasmid/catabolic enzymes expressed by the bacterial species. From the growth profile in Fig. 1B, there was a decline in the cell number which suggests that the organisms may had to adapt to the new substrate and synthesize new pathway for utilization of naphthalene. It may also be that naphthalene caused a physiological stress on strain PB-1 and PB-2. From studies of Foght and Westlake,32 a plasmid may encode a complete degradative pathway or partial degradative step. Some other plasmids may code for enzymes that have specificity for several substrates. In addition, Foght and Westlake in their investigation reported of genes encoding the upper and lower pathways of naphthalene within the NAH plasmids of several pseudomonads. The noted of broad specificities that allowed the host to grow on several two and three-ring PAHs, as sole carbon and energy sources. In view of this, we assessed the degradative capacity of strain PB-1 and PB-2 on chrysene. From the obtained values, it was evident that they could utilize chrysene partially at 13% and 16% from the original concentration of about 79ppm. In fluoranthene, the bacterial strains (PB-1 and PB-2) utilized 4% and 7% from the initial concentration of 98ppm respectively. On pyrene, 8% and 13% were utilized from the initial concentration of 94ppm. It is obvious from the growth profile, that chrysene and pyrene were partially degraded by these bacterial strains. However, a decline in cell numbers was evident in the fluoranthene study. It can be deduced from our obtained results, that the strains utilized naphthalene, pyrene and chrysene at slow rate. The slow rate of utilization of pyrene may be that the organisms were not able synthesize enough biosurfactants that will aid mass transfer of the substrate.
Pyrene a HMW PAH of ubiquitous distribution, environmental persistence, and potentially with deleterious effect on human health has prompted the development of clean up of pyrene contaminated soils. Conversely, most bacterial species show limited ability to degrade these hydrophobic compounds.33,34 From this study, the strains PB-1 and PB-2 may possess broad spectrum catabolic plasmids having shown potentials of utilizing the HMW PAHs – pyrene and chrysene. In addition, the limited degradation of chrysene and pyrene may be explained based on the stereochemistry of their structures. Chrysene and pyrene have four of six membered rings. The only difference is in their arrangement, where pyrene is fused together to form a crystalline ball structure while chrysene is almost linear combination of all rings. In this study, strains PB-1 and PB-2 were able to exhibit similar behavior to chrysene and pyrene but not to fluoranthene with three six membered ring and one five membered ring. It goes to show that the stereochemistry influences the recalcitrance of most aromatic hydrocarbons. Boldrin and other workers35 isolated Mycobacterium strain BB1 from a former coal gasification site. The organism had an exponential growth on solid pyrene (0.04h−1) as sole carbon and energy source. Kastner and co-workers29 isolated Gordona sp BP9 capable of using pyrene as a sole carbon and energy source from PAH contaminated site using the plate screening technique.
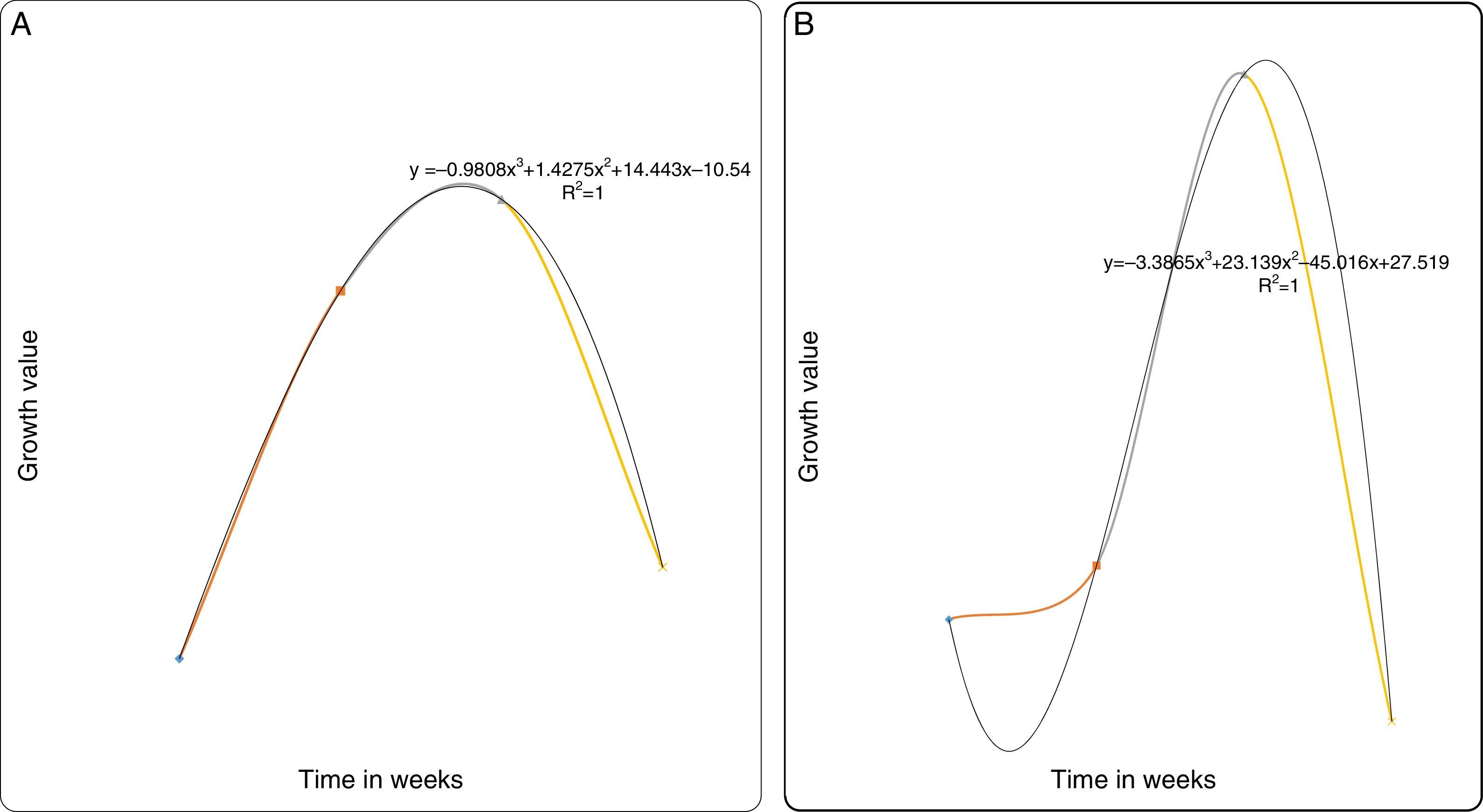
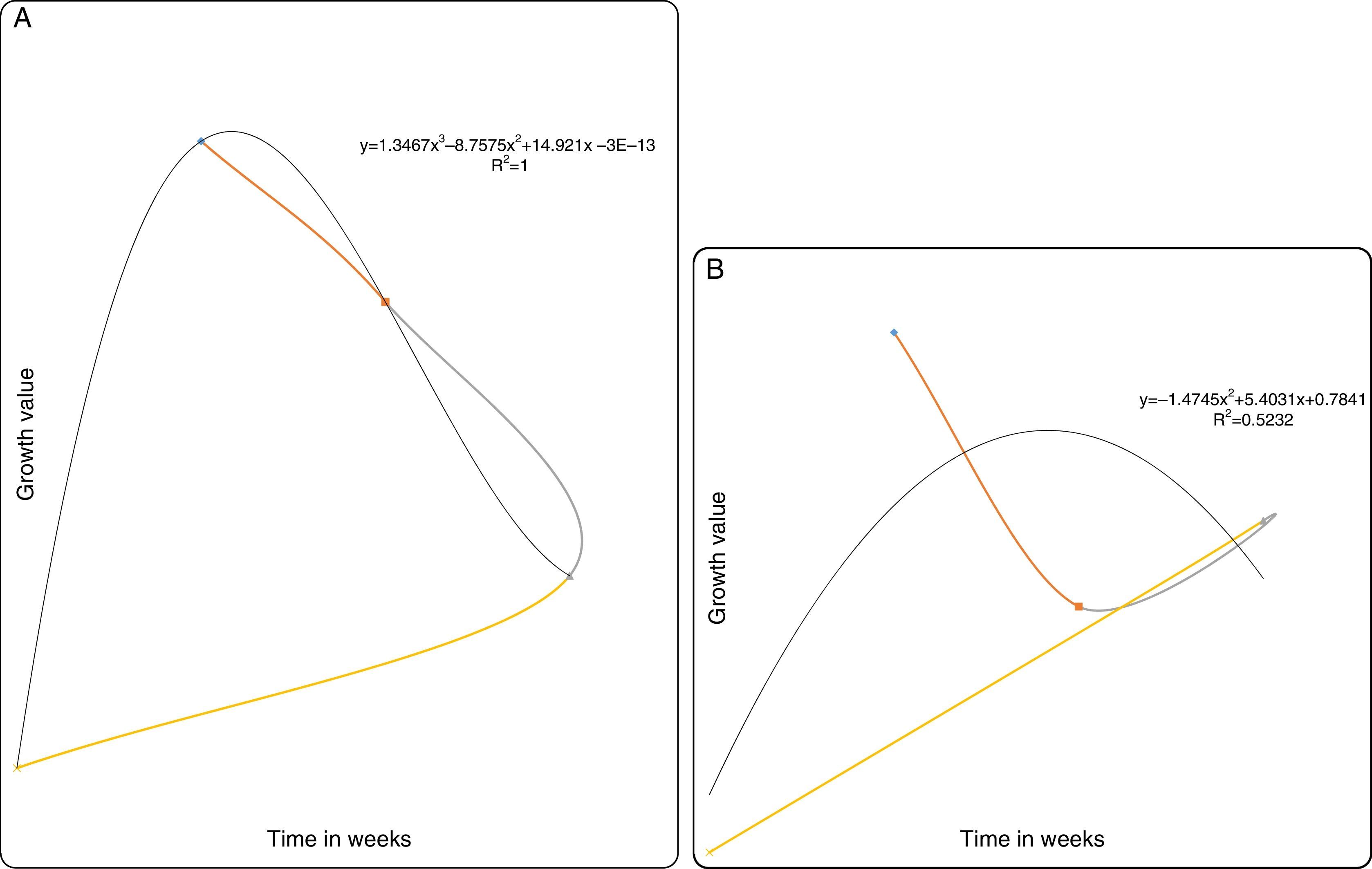
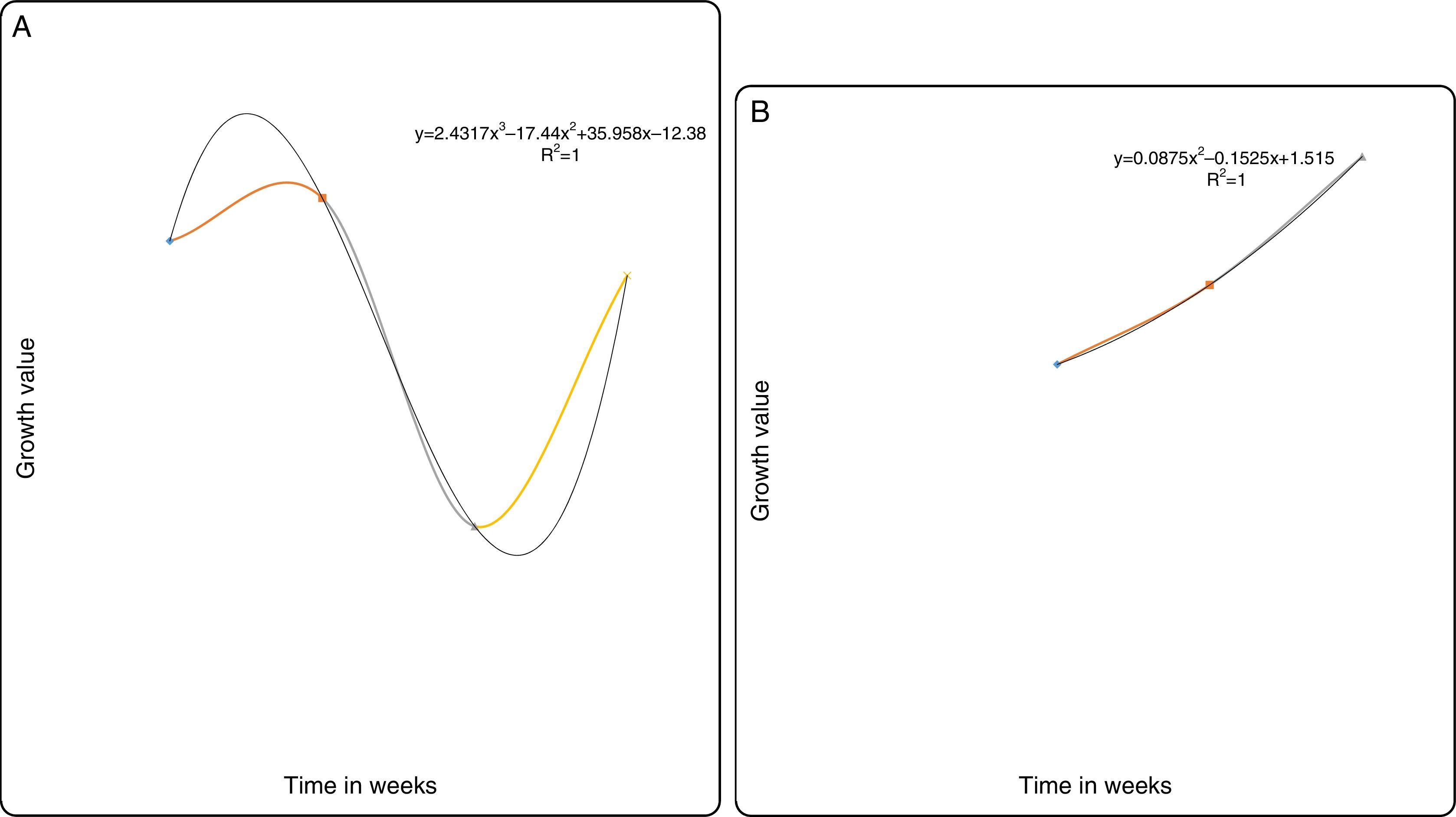
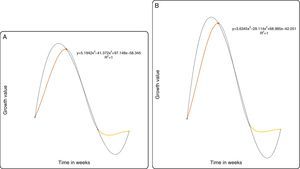
For the predictive models based on the growth curve plots Figs. 5–8(A and B), it showed that a nonlinear relationship of the polynomial type describes adequately the relationship between both the PB-1 and PB-2 with time. Thus, based on the results of the statistical nonlinear regression modeling presented by the predictive growth plots, the values of the measure of explanatory power of the model (R2) is indicative that the models predicts accurately changes in the growth pattern as the time in weeks changes. Hence, variability in the growth patterns of the PBs is associated with the length of time of action.
In conclusion, P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 could partially degrade pyrene, chrysene, naphthalene and fluoranthene at low concentrations. The predictive model (R2) revalidates the growth profile of our bacterial strains PB1 and PB2. However, this model may be applied to determine degradation rates of slow-growing PAH degraders. As a whole, the results suggested that P. plecoglossicida strain PB1 and Pseudomonas sp. PB2 would be an excellent candidates with a potential for future application in the bioremediation of PAH-contaminated sites.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the financial support of Institute of International Education, administrators of the Fulbright Scholarship which supported O. Nwinyi and the School of Public and Environmental Affairs Indiana University, Bloomington, IN, USA, for additional support. The authors appreciate the inputs of Associate Professor Flynn W. Picardal and An Thuy of the School of Public and Environmental Affairs Indiana University, Bloomington, IN, USA.