Since 1960s, the organophosphate pesticide chlorpyrifos has been widely used for the purpose of pest control. However, given its persistence and toxicity towards life forms, the elimination of chlorpyrifos from contaminated sites has become an urgent issue. For this process bioremediation is the method of choice.
ResultsTwo bacterial strains, JCp4 and FCp1, exhibiting chlorpyrifos-degradation potential were isolated from pesticide contaminated agricultural fields. These isolates were able to degrade 84.4% and 78.6% of the initial concentration of chlorpyrifos (100mgL−1) within a period of only 10 days. Based on 16S rRNA sequence analysis, these strains were identified as Achromobacter xylosoxidans (JCp4) and Ochrobactrum sp. (FCp1). These strains exhibited the ability to degrade chlorpyrifos in sterilized as well as non-sterilized soils, and were able to degrade 93–100% of the input concentration (200mgkg−1) within 42 days. The rate of degradation in inoculated soils ranged from 4.40 to 4.76mg−1kg−1d−1 with rate constants varying between 0.047 and 0.069d−1. These strains also displayed substantial plant growth promoting traits such as phosphate solubilization, indole acetic acid production and ammonia production both in absence as well as in the presence of chlorpyrifos. However, presence of chlorpyrifos (100 and 200mgL−1) was found to have a negative effect on indole acetic acid production and phosphate solubilization with percentage reduction values ranging between 2.65–10.6% and 4.5–17.6%, respectively. Plant growth experiment demonstrated that chlorpyrifos has a negative effect on plant growth and causes a decrease in parameters such as percentage germination, plant height and biomass. Inoculation of soil with chlorpyrifos-degrading strains was found to enhance plant growth significantly in terms of plant length and weight. Moreover, it was noted that these strains degraded chlorpyrifos at an increased rate (5.69mg−1kg−1d−1) in planted soil.
ConclusionThe results of this study clearly demonstrate that the chlorpyrifos-degrading strains have the potential to develop into promising candidates for raising the productivity of crops in pesticide contaminated soils.
The extensive use of pesticides through field application, crop spraying, handling, rinsing of containers, accidental spills, etc. has a potential to severely contaminate soil.1 Most of the pesticides that are in common usage today are known to adversely affect functional diversity of the soil microbiota leading to loss of soil fertility and plant growth, which in turn put the sustainability of agricultural soil at serious risk.2 To add to the complexity of the situation, pesticide residues and their metabolites often infiltrate through the soil surface into the groundwater and cause widespread contamination of aquatic ecosystems.3
Chlorpyrifos (CP) is a broad spectrum organophosphate insecticide that is classified as moderately toxic. Since 1960s, CP has been extensively used in the agricultural sector for controlling insect infestations of crops such as cotton, cereals, vegetables and fruits.4 Although CP is considered only moderately toxic, it is known to possess neurotoxic and immunotoxic properties and has been shown to be harmful to both animals and humans.5 CP has also been reported to cause a reduction in the bacterial, fungal and actinomycete population of the soil6 and is known to inhibit nitrogen mineralization in soil.7 Detection of CP contamination in surface water bodies and associated sediments has heightened public concern on the topic8,9 and warranted urgent attention and treatment of the problem.
Bioremediation is a method that exploits the potential of microbial degradation for providing a cost-effective and reliable approach to pesticide abatement. Several soil and aquatic environments have been successfully reclaimed from pesticide contamination by using microbes capable of degrading the pollutants.10 Hydrolysis, either chemically or as a result of microbial activity, degrades CP by converting it to diethylthiophosphoric acid (DETP) and 3,5,6-trichloro-2-pyridinol (TCP).11 Pesticide degrading bacteria found in soil are known to have multifarious abilities such as phytohormone production, mineral solubilization, N2-fixation, etc., which are extremely crucial for promotion of plant growth. Presence of the above-mentioned traits underlines and emphasizes the agronomic and environmental significance of such microbes. The potential of microbes to simultaneously detoxify pollutants while enhancing plant growth has been studied previously for pesticides carbofuran and thiamethoxam.12,13
The objective of this study was to isolate and characterize CP-degrading bacteria and to determine the degradation potential of these strains in both sterile as well as non-sterile soil. Moreover, plant growth promoting potential of these bacteria was also assessed; and the strains were tested for their ability to bioremediate soil and enhance plant growth in contaminated soil.
Materials and methodsChemicals and mediaTechnical grade chlorpyrifos (98%) was obtained from the Ali Akbar group, Pakistan. Analytical grade CP and 3,5,6-trichloro-2-pyridinol (TCP) were purchased from Sigma–Aldrich (St Louis, MO, USA). HPLC grade organic solvents were procured from Merck (Pakistan). Microbial isolations were conducted in a mineral salt medium (MSM) (pH 6.8–7.0) containing (gL−1) K2HPO4, 1.5; KH2PO4, 0.5; NaCl, 0.5; (NH4)2SO4, 0.5; MgSO4·7H2O, 0.2, and 10mL of 100× trace element solution. The 100× trace element solution was composed of (mgL−1) Na2EDTA·2H2O, 500; FeCl2·4H2O, 143; ZnCl2, 4.7; MnCl2·4H2O, 3.0; H3BO3, 30; CoCl2·6H2O, 20; CuCl2·2H2O, 1.0; NiCl2·6H2O, 2.0; Na2MoO4·2H2O, 3.0; and CaCl2·2H2O, 100.
Enrichment, selection and identification of chlorpyrifos degrading strainsSoil samples from agricultural fields of Jhang and Faisalabad, Punjab, Pakistan, were used for the purpose of enrichment of chlorpyrifos degrading bacteria. Five grams of soil was added to Erlenmeyer flasks (250mL) containing 50mL MSM supplemented with 50mgL−1 CP and the culture was incubated at 150rpm, 30°C. The enrichment process was conducted over a period of four weeks; following an established protocol,14 at regular intervals of one week each of the culture was transferred into fresh growth medium. Subsequent to the last enrichment process, serial dilutions of the culture were spread on MSM plates containing CP (50mgL−1) and the colonies thus isolated were further purified by the streak plate method.
For determining the degradation potential, cultures were grown in triplicate in MSM containing 100mgL−1 CP for a period of 10 days and the residual CP concentration was determined by high performance liquid chromatography (HPLC). CP quantification was analyzed on a SYKAM HPLC (Germany) using an S1122 HPLC Pump, S3210 UV detector, S1122 delivery system and Phenomenex C18 reversed-phase column (150mm). Detector output was processed by the clarity chromatography data system. Samples were eluted using methanol:H2O:acetic acid (80:20:0.5, v/v) at a flow rate of 1.0mLmin−1. Detection was performed at 230nm and the retention times of CP and TCP were determined to be 18.4 and 16.1min, respectively. Two isolates with the highest CP degradation potential, designated as JCp4 and FCp1, were selected for further study.
Bacterial strains were identified by amplification of the 16S rRNA gene using universal primers 27f and 1492R. Amplification was carried out in a 50μL reaction mixture containing 20pmol of primer (F and R) each, 25μL PCR Master Mix (Thermoscientific) and 20ng of template DNA. The PCR thermocycling parameters were as follows: initial denaturation at 94°C for 5min; followed by 25 cycles of 94°C (1min), 50°C (1min); 72°C (1min); and final extension at 72°C for 10min. The amplified PCR products were sequenced and sequence homologies were identified using nBLAST. GenBank accession numbers were assigned for 16S rRNA gene sequences of both the isolates (KJ009240 and KJ009242).
Determination of auxiliary characteristicsIndole acetic acid (IAA) productionLB-broth (50mL), supplemented with 50, 100 and 150μgmL−1 tryptophan, was inoculated with 50μL of cell suspension (OD600=0.5) in triplicate and incubated at 35°C, 120rpm for 72h. At 48 and 72h intervals, the culture was centrifuged and 2mL of the supernatant was mixed with 4mL of Salkowski's reagent. Intensity of colour was taken as OD535 and the amount of IAA produced was quantified using a standard curve. The effect of CP on the IAA production capability of the microbes was evaluated by supplementing the LB-broth with 100 or 200mgL−1 of CP in presence of 100μgmL−1 tryptophan.
Phosphate solubilizationThe ability of the bacteria to solubilize phosphate was determined by plating the bacteria on Pikovskaya agar medium as per the method described previously.15 The presence of a clear zone around bacterial colonies following one week of incubation at 35°C indicated phosphate solubilization. Phosphate solubilization was analyzed by computing the Solubilization Index (SI) which is the ratio of total diameter (colony+halo) to colony diameter. The effect of CP on the phosphate solubilization ability of the bacteria was determined by supplementing Pikovskaya agar plates with 100 and 200mgL−1 chlorpyrifos.
Ammonia productionMcCartney bottles containing 10mL peptone water were inoculated with bacterial strains in triplicate and incubated at 35°C for 96h. The NH3 production was detected by observing the formation of yellow colour in the bottle upon addition of Nessler's reagent and intensity of the colour produced was noted.16 The effect of CP on ammonia production by bacterial strains under study was evaluated by the addition of 100–200mgL−1 CP to peptone water.
Biodegradation studiesInoculum preparationSeed culture for each isolate was grown in a nutrient broth containing 50mgL−1 CP. Following growth, the cultures were centrifuged at 4600×g for 5min, washed and then diluted with MilliQ H2O. Colony forming units (cfumL−1) of these suspensions were determined by the dilution plate counting method. For pesticide biodegradation studies, a cell concentration corresponding to 1.6×107cfumL−1 was used so as to maintain uniformity in cell numbers.
Chlorpyrifos degradation in liquid mediumErlenmeyer flasks (250mL) containing mineral salt medium (100mL) supplemented with 100mgL−1 of CP were inoculated with bacterial cell suspension in triplicates. The flasks were incubated at 30°C with shaking at 150rpm and an uninoculated flask was used as control. For growth study analysis an aliquot of 1mL culture was withdrawn at regular intervals of 2 days each for 10 continuous days and growth was evaluated as OD600. CP residue extraction and estimation by HPLC was done as described previously.14
Biodegradation of chlorpyrifos in soilAnalysis of CP degradation by selected strains isolated from soil was conducted in sterilized (S) and non-sterilized (nS) soil samples. The required soil samples (100g) were spiked with chlorpyrifos to a final concentration of 200mgkg−1 by the addition of an acetone-based CP solution. The solution was initially added to a small portion (10g) of soil, which, following the solvent evaporation was then mixed with the remaining soil quantity. The soil samples were inoculated and incubated at 30°C. The test was performed in triplicate and uninoculated S and nS soils were used as controls. Sample removal, extraction and pesticide residue estimation were carried out as described earlier.14
Plant–microbe interaction and chlorpyrifos degradationPot experiments with Vigna unguiculata (L.) Walp. were conducted in order to examine the effects of bacterial inoculation on plant growth and pesticide degradation. Seeds of V. unguiculata were surface sterilized by treatment with 0.1% HgCl2 solution for 5min followed by washing with sterilized glass-distilled water. Soil samples (1.5kg) were spiked with CP to a concentration of 200mgkg−1 using the same protocol as described above. Samples were then inoculated with microbial suspension to give final concentration of 1.6×107cellsg−1. The test was performed in triplicate and uninoculated spiked and non-spiked soil samples were used as control. Sterilized seeds of V. unguiculata were sown in the sample soils, and then the soil was moistened with water. Care was taken to ensure that the pots were kept at ambient light and temperature. The seed emergence process was observed daily and plants were allowed to grow for 6 weeks. The following parameters of plant growth were recorded: (a) percentage of germination, (b) shoot length (cm), (c) root length (cm), (d) leaf length (cm), (e) shoot fresh weight (g), (f) root fresh weight (g), (g) shoot dry weight (g), and (h) root dry weight (g).
Data analysisThe CP degradation rate constant (k) was determined using the kinetic model Ct=C0×e−kt as described in a previous study.16 Statistical analysis was performed using the IBM SPSS 20 program. Significance (p<0.05) of differences was analyzed by t-test or one-way ANOVA and assessed by post-hoc comparison of means using the Duncan's multiple range test.
ResultsIsolation and characterization of chlorpyrifos degrading strainsFrom the enrichment culture, several promising morphologically different colonies were isolated and purified. Purified isolates were grown in a mineral salt medium supplemented with CP (100mgL−1). Estimation of CP degradation potential by HPLC demonstrated that the isolates were able to degrade 25–84.4% of supplemented CP within a time frame of 10 days. Maximum CP degradation potential was exhibited by isolates JCp4 and FCp1 that degraded 84.4% and 76.8% of the applied CP, respectively. Based on biochemical characterization and 16S rRNA gene analysis, these isolates were identified as Achromobacter sp. and Ochrobactrum sp. The 16S rDNA sequences of strains JCp4 and FCp1 exhibited closest (99%) homology with Achromobacter xylosoxidans (AB547225, FJ639331) and Ochrobactrum sp. (KF479631, KF843719), respectively.
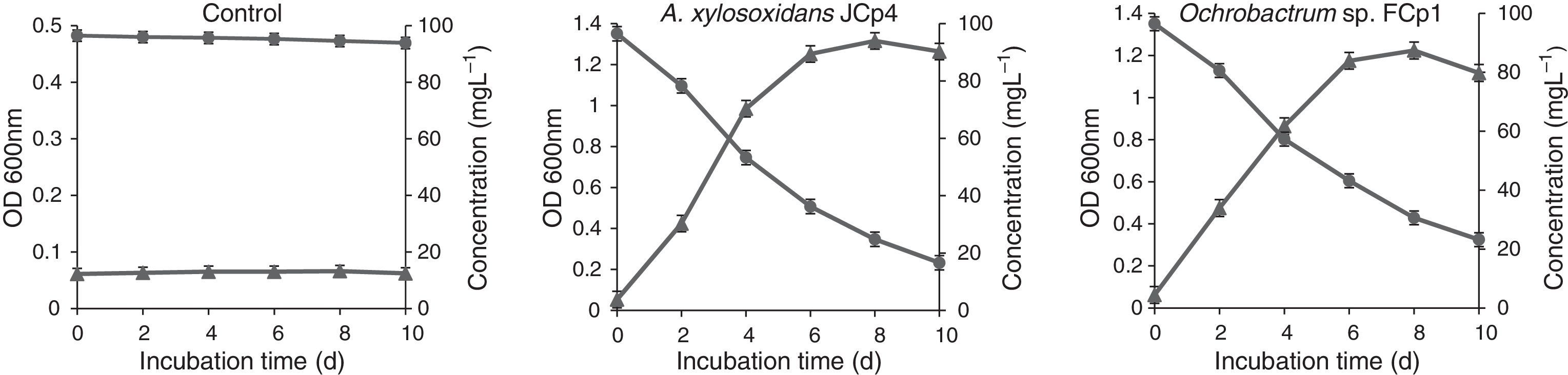
Bacterial growth and biodegradation of CP in liquid mediumThe bacterial strains were able to utilize CP as the sole source of C and exhibited effective growth up to a period 6 days of incubation without undergoing an initial lag phase. Increase in growth was very slow between 6th and 8th day, and thereafter the decline phase was initiated (Fig. 1). CP degradation by bacterial strains was exhibited as a decrease in CP concentration that was proportional to increase in bacterial growth and a time dependent loss of CP was observed in bacterial cultures (Fig. 1). A. xylosoxidans JCp4 degraded 84.4% of CP at the rate of 7.9mgL−1d−1 while Ochrobactrum sp. FCp1 degraded 76.8% of CP at the rate of 7.3mgL−1d−1. In the control flasks, CP degradation, as a byproduct of abiotic losses, was insignificant (6%) at the end of 10 days. HPLC analysis also revealed that A. xylosoxidans JCp4 and Ochrobactrum sp. FCp1 were able to mineralize CP completely such that there was only a transient accumulation of the CP metabolic product TCP (3,5,6-trichloro-2-pyridinol).
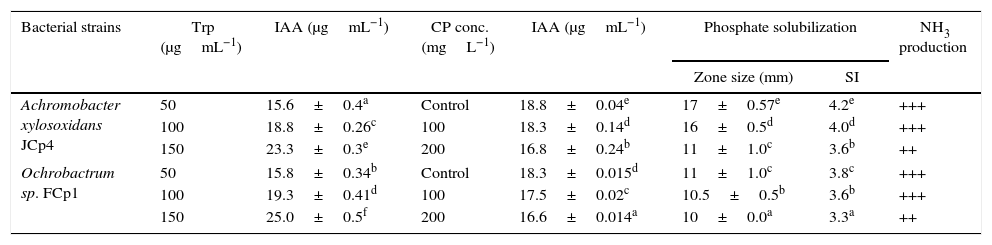
Determination of auxiliary characteristicsThe plant growth-promoting activities of bacterial strains, both in the absence as well as in the presence of CP, were determined (Table 1). The bacterial strains under study exhibited a substantial production of IAA following 24h of incubation. A concentration dependent increase in production of IAA was observed: JCp4 and FCp1 produced 15.6 and 15.8μgmL−1 of IAA at 50μgmL−1, 18.8 and 19.3μgmL−1 at 100μgmL−1, and 23.3 and 25.0μgmL−1 of IAA at 150μgmL−1 of tryptophan, respectively. When the effect of presence or absence of CP on IAA production was compared, it was revealed that there was a reduction in IAA production by 2.65% (JCp4) and 4.3% (FCp1) when CP was present at a concentration of 100mgL−1. A further increase in CP concentration (200mgL−1) suppressed IAA production and resulted in a drop in quantity of IAA produced by 10.6% (JCp4) and 9.2% (FCp1) (Table 1). Both the strains also showed phosphate-solubilizing activity by producing a clear halo around their growth in both conditions. However, phosphate solubilization activity was reduced at higher concentration of CP. Furthermore, bacterial strains were also found positive for ammonia production in both situations (Table 1).
Plant growth promoting activities of cypermethrin degrading strains in presence and absence of chlorpyrifos.
| Bacterial strains | Trp (μgmL−1) | IAA (μgmL−1) | CP conc. (mgL−1) | IAA (μgmL−1) | Phosphate solubilization | NH3 production | |
|---|---|---|---|---|---|---|---|
| Zone size (mm) | SI | ||||||
| Achromobacter xylosoxidans JCp4 | 50 | 15.6±0.4a | Control | 18.8±0.04e | 17±0.57e | 4.2e | +++ |
| 100 | 18.8±0.26c | 100 | 18.3±0.14d | 16±0.5d | 4.0d | +++ | |
| 150 | 23.3±0.3e | 200 | 16.8±0.24b | 11±1.0c | 3.6b | ++ | |
| Ochrobactrum sp. FCp1 | 50 | 15.8±0.34b | Control | 18.3±0.015d | 11±1.0c | 3.8c | +++ |
| 100 | 19.3±0.41d | 100 | 17.5±0.02c | 10.5±0.5b | 3.6b | +++ | |
| 150 | 25.0±0.5f | 200 | 16.6±0.014a | 10±0.0a | 3.3a | ++ | |
Trp=tryptophan; IAA=indole acetic acid; SI=solubilization index.
The values indicate the mean±SD of three replicates.
+++=large amount of ammonia; ++=moderate amount of ammonia.
Means in the same column followed by the different letters are significantly different at p>0.05.
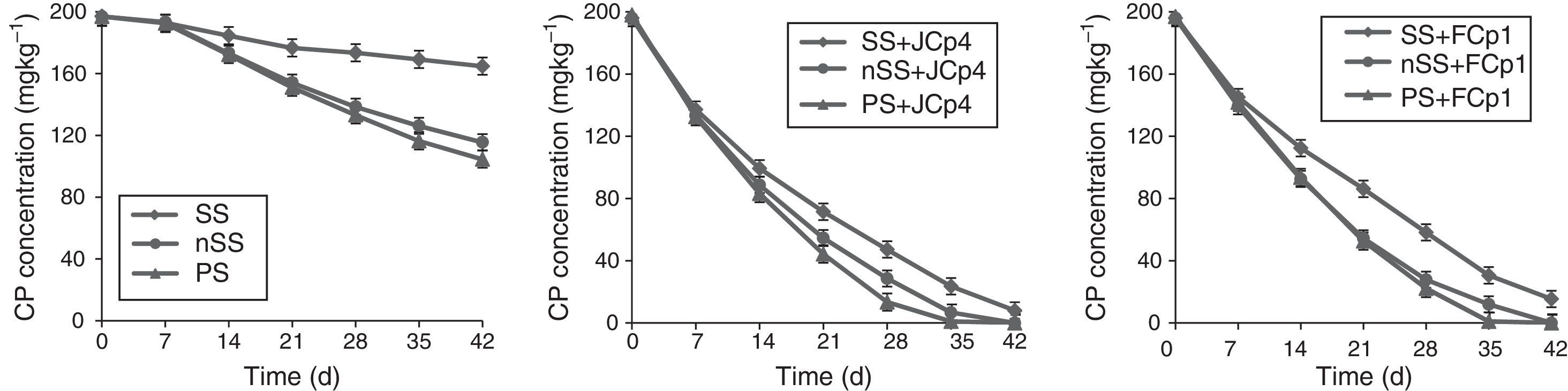
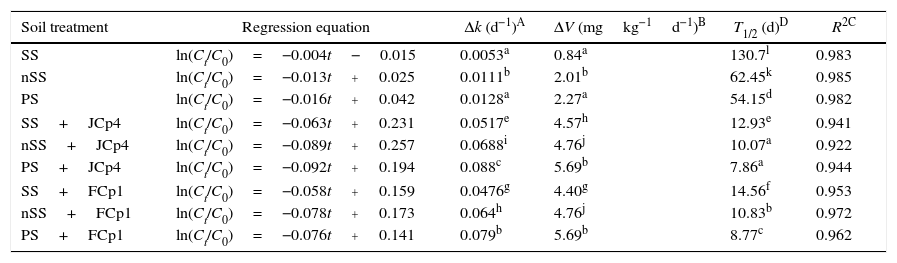
The ability of selected bacterial strains to degrade CP in soil was studied using sterilized (SS) as well as non-sterilized soil (nSS). The CP degradation dynamics revealed significant differences (p<0.05) between different treatments (Fig. 2, Table 2). Moreover, CP degradation in soil suggested a time dependent disappearance that followed first order kinetics. In uninoculated sterilized soil, the decrease in CP concentration was insignificant and it was found that 80% of the applied CP persisted till the end of the incubation period. In non-sterilized soil data clearly showed that indigenous microbes possessed the potential to degrade CP in the amount applied in the experiment and utilized 42.2% of the initial CP amount by the end of the incubation time. However, the initial phase of CP depletion was followed by a lag period of 7 days during which only 3% of initial CP was degraded. Following this phase, CP disappearance was then observed to increase considerably with a rate constant of 0.0111d−1, average rate of 2.01mgkg−1d−1 and T1/2 of 62.45days. Bacterial strains exhibited higher CP degradation potential in non-sterilized soils as compared to sterilized soil. A. xylosoxidans JCp4 degraded 100% of the applied CP within a time frame of 42 days with average rate of 4.57 and 4.76mgkg−1d−1 and rate constant of 0.0517 and 0.069d−1 in sterile and non-sterile soils, respectively. Half-life (T1/2) of CP degradation by JCp4 increased from 10.07 days in non-sterile soil to 12.93 days in sterile soil. Ochrobactrum sp. FCp1 utilized 93% of applied CP with a rate constant and average rate of 0.0477d−1 and 4.40mgkg−1d−1 in sterilized soil and T1/2 of 14.5 days. In contrast, in non-sterilized soil 100% of initially applied CP was degraded by FCp1 with rate constant of 0.064d−1, average rate of 4.76mgkg−1d−1 and T1/2 of 10.6 days (Fig. 2, Table 2).
Degradation dynamics of chlorpyrifos in different soil treatments with initial CP concentration of 200mgkg−1 soil. Symbols: () sterilized soil (SS) or SS inoculated with CP degrading strains, (•) non-sterilized soil (nSS) or nSS inoculated with CP degrading strains, and (▴) planted soil (PS) or PS inoculated with CP degrading strains. Values are the means of three replicates and error bars represent SD.
Kinetic studies of chlorpyrifos degradation in various microbiologically active soils. The initial chlorpyrifos concentration was 200mgkg−1 soil.
| Soil treatment | Regression equation | Δk (d−1)A | ΔV (mgkg−1d−1)B | T1/2 (d)D | R2C |
|---|---|---|---|---|---|
| SS | ln(Ct/C0)=−0.004t−0.015 | 0.0053a | 0.84a | 130.7l | 0.983 |
| nSS | ln(Ct/C0)=−0.013t+0.025 | 0.0111b | 2.01b | 62.45k | 0.985 |
| PS | ln(Ct/C0)=−0.016t+0.042 | 0.0128a | 2.27a | 54.15d | 0.982 |
| SS+JCp4 | ln(Ct/C0)=−0.063t+0.231 | 0.0517e | 4.57h | 12.93e | 0.941 |
| nSS+JCp4 | ln(Ct/C0)=−0.089t+0.257 | 0.0688i | 4.76j | 10.07a | 0.922 |
| PS+JCp4 | ln(Ct/C0)=−0.092t+0.194 | 0.088c | 5.69b | 7.86a | 0.944 |
| SS+FCp1 | ln(Ct/C0)=−0.058t+0.159 | 0.0476g | 4.40g | 14.56f | 0.953 |
| nSS+FCp1 | ln(Ct/C0)=−0.078t+0.173 | 0.064h | 4.76j | 10.83b | 0.972 |
| PS+FCp1 | ln(Ct/C0)=−0.076t+0.141 | 0.079b | 5.69b | 8.77c | 0.962 |
SS, sterilized soil; nSS, non-sterilized soil; PS, planted soil; +, inoculated with.
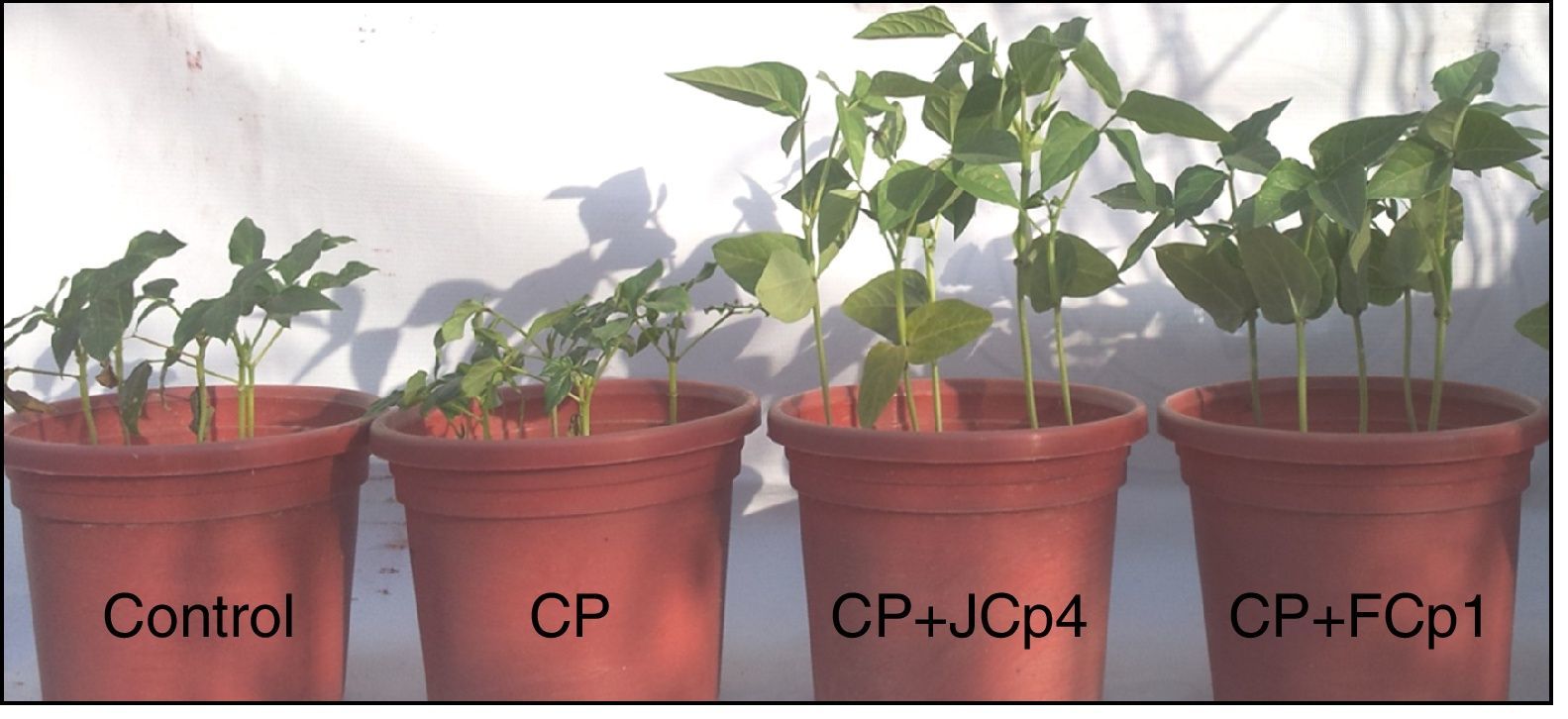
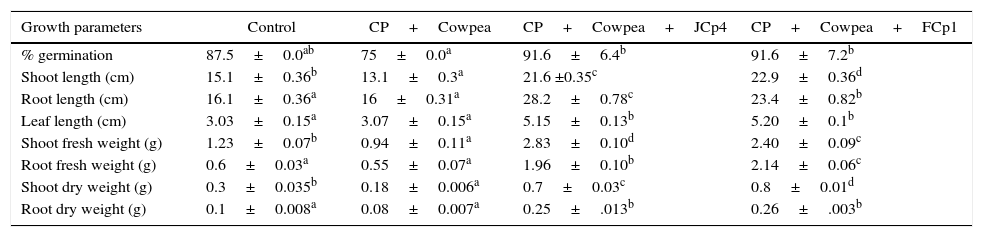
Influence of bacterial presence on plant growth and pesticide degradation was studied using plant growth experiments. CP addition to soil affected a reduction in certain plant parameters such as % germination (14.2%), shoot length (2cm), shoot fresh weight (0.29g), root fresh weight (0.05g), shoot dry weight (0.2g) and root dry weight (0.02g). Plants grown in CP supplemented soils inoculated with CP-degrading bacterial strains exhibited significant enhancement in growth in terms of height and weight. An increase of 6.5 and 7.8cm in shoot length and of 12.1 and 7.3cm in root length was observed in case of JCp4 and FCp1, respectively. Leaf length was enhanced up to 2.1cm. The increase in shoot weight increase was studied in terms of fresh and dry shoot weight; an increase of 1.6 and 1.17g in shoot fresh weight and of 0.4 and 0.5g in shoot dry weight was observed in plants inoculated with JCp4 and FCp1, respectively. Root weight was also enhanced significantly; JCp4 and FCp1 enhanced root fresh weight by 1.36 and 1.54g and root dry weight by 0.15 and 0.16g, respectively (Fig. 3, Table 3).
Measurement of growth parameters of V. unguiculata in different microbiologically active soils supplemented with CP (200mgkg−1).
| Growth parameters | Control | CP+Cowpea | CP+Cowpea+JCp4 | CP+Cowpea+FCp1 |
|---|---|---|---|---|
| % germination | 87.5±0.0ab | 75±0.0a | 91.6±6.4b | 91.6±7.2b |
| Shoot length (cm) | 15.1±0.36b | 13.1±0.3a | 21.6 ±0.35c | 22.9±0.36d |
| Root length (cm) | 16.1±0.36a | 16±0.31a | 28.2±0.78c | 23.4±0.82b |
| Leaf length (cm) | 3.03±0.15a | 3.07±0.15a | 5.15±0.13b | 5.20±0.1b |
| Shoot fresh weight (g) | 1.23±0.07b | 0.94±0.11a | 2.83±0.10d | 2.40±0.09c |
| Root fresh weight (g) | 0.6±0.03a | 0.55±0.07a | 1.96±0.10b | 2.14±0.06c |
| Shoot dry weight (g) | 0.3±0.035b | 0.18±0.006a | 0.7±0.03c | 0.8±0.01d |
| Root dry weight (g) | 0.1±0.008a | 0.08±0.007a | 0.25±.013b | 0.26±.003b |
The values indicate the mean±SD of three replicates.
Different letters in same rows indicate significantly different values.
Within six weeks 48% of CP was degraded in un-inoculated soils at the rate of 2.27mgkg−1d−1 and rate constant of 0.0128d−1. The half-life of CP in un-inoculated planted soils was calculated to be 54.15days. In inoculated soils 100% of applied CP was degraded within 35d with average rate of 5.7mgkg−1d−1. A. xylosoxidans JCp4 degraded 93.3% CP in planted soil with rate constant of 0.088d−1 and T1/2 was observed to be 7.86 days. Ochrobactrum sp. FCp1 utilized 89% CP within 28 days with rate constant of 0.079d−1 and T1/2 of 8.77days (Fig. 2, Table 2).
DiscussionChlorpyrifos is a very popular pesticide that is used extensively for the purpose of pest control in vegetable and cotton fields. However, it is well known that CP is toxic for mammals and can lead to contamination of soil and water resources which makes its removal from the environment an extremely urgent issue. Bioremediation is a process that utilizes the degradation potential of microbes to provide a cost-effective and reliable approach for pesticide abatement. To this purpose, CP degrading bacterial strains were isolated from contaminated agricultural soil samples. Two promising CP-degrading isolates that were identified were A. xylosoxidans JCp4 and Ochrobactrum sp. FCp1 which were found to degrade 84.4% and 78.6% of CP (100mgL−1), respectively, within a time period of 10days. In a few previously published reports, Achromobacter17 and Ochrobactrum18,19 have been studied for their role in pesticide biodegradation but, to the best of our knowledge, this is the first study that aimed to analyze simultaneously CP degradation and plant growth promotion by these bacterial strains.
For analyzing CP degradation, an inoculum size of 1.6×107cfug−1 was used. Incidentally, it was noted that the inoculum density used was capable of removing CP proficiently without undergoing a lag phase. As reported in previous studies inoculum density is an essential factor influencing the efficient degradation of applied pesticides. In a previous report it was found that an inoculum density greater than 106cfug−1 was capable of degrading chlorpyrifos without lag phase.20 One possible explanation for requiring a high initial inoculum density for pesticide degradation could be that a higher number of bacteria are required to start and sustain fast degradation during incubation. Soil degradation studies revealed that the selected CP-degrading strains were capable of degrading CP both in liquid media as well as in soil. Previous studies on pesticide degradation have reported that microbes that are capable of degrading these compounds in culture media are also capable of degradation in soil.3,21,22 The degradation kinetics were determined using first order rate equation Ct=C0×e−kt because the pesticide disappearance was noted to be time-dependent. The rate constants for CP degradation by bacterial strains in soil ranged from 0.047 to 0.069d−1 with T1/2 of 10–14.5days. Similar first order rate equations have been used to study degradation kinetics of pesticides in soil in a few previously published reports.23,24 Comparable degradation kinetic values have been reported in earlier studies that analyzed degradation of CP. For example, Cycon et al. noted that Serratia marcescens was capable of degrading CP at rate constant ranging from 0.017 to 0.052d−1 with T1/2 of 13.6–37days in different types of soils.25
CP degradation was observed to be higher in uninoculated non-sterilized soils (42.2%) as compared to sterilized soils (17.2%). This finding demonstrates that CP degradation is mediated by soil microflora and that indigenous microbes are capable of degrading CP. In earlier studies, authors observed an 80% increase in diazinon degradation in non-sterilized soils as compared to sterilized soils.24 Degradation of CP was high in inoculated non-sterile soils as compared to inoculated sterile soils indicating the bioremediation potential of CP degrading strains. An increase in CP-degradation was observed in non-sterile soils supplemented with CP-degrading strains A. xylosoxidans JCp4 (9.6%) and Ochrobactrum sp. FCp1 (7.75%). The observed enhancement in CP utilization can be attributed to the fact that inoculation of soil with degrading bacteria leads to an increase in its catabolic potential. Also, the ability of the indigenous microbes to utilize the applied pesticide compound most likely played a synergistic role in their biodegradation. Increased rate of depletion of pesticides in contaminated soils by augmentation of potential degrading strains has been observed in earlier studies. For instance, Burkholderia sp. FDS-1 enhanced the fenitrothion utilization rate when inoculated in FT contaminated soil containing native microflora.26 In a similar vein, S. marcescens degraded deltamethrin more efficiently in non-sterile soils as compared to sterile soils.27
Determination of plant growth promoting characteristics revealed presence of substantial phosphate solubilization, IAA and ammonia production by CP degrading strains. Bacterial stains belonging to genera Achromobacter28 and Ochrobactrum15 have been isolated and studied for their plant growth promoting traits. Usually bacteria from plant rhizosphere and bulk agricultural soil possess plant growth promoting traits; some of these microbes also contain pesticide degrading capacities as a result of continuous exposure to these compounds. In earlier studies, diverse bacterial strains isolated from a soil enrichment culture responsible for thiamethoxam degradation were also found to possess IAA production and N2-fixation abilities.13 Chlorpyrifos degrading Acinetobacter calcoaceticus also showed PGP traits.29 Significant decrease in PGP activities of CP-resistant bacteria was observed in presence of chlorpyrifos. CP has been previously reported to exert an inhibitory effect on soil microbial activities.2 Similarly, another study has also reported a decrease in plant-growth promoting traits of bacteria in presence of pesticides above the recommended dose.30 Pesticides affect the soil microbiological activities by altering the population of cellulolytic and phosphate solubilizing microorganisms and thereby changing the nitrogen balance and ammonification of soil.31
The growth of V. unguiculata was inhibited when soil was supplemented with 200mgkg−1 of CP. CP addition to soil also reduced certain plant parameters such as % germination (14.2%), shoot length (13.2%), shoot fresh weight (23.5%), root fresh weight (8.3%), shoot dry weight (40%) and root dry weight (20%). Bidlan et al. has reported that use of lindane at a concentration of 100μgL−1 inhibited the germination process in green gram and radish.32 Different pesticides such as aldrin, carbofuran, fenamiphos and phorate have been found to have adverse effects on plant growth parameters such as plant height, weight and root nodulation.33 The negative effect of pesticides on plant growth can be attributed to inhibition of electron flow in the photosynthetic chain. OPs and DDT have been found to affect photosynthesis by uncoupling the photosynthetic electron flow such that ATP synthesis is blocked.34 Enhanced plant (V. unguiculata) growth was observed when soils were inoculated with CP bacterial strains. Following inoculation with A. xylosoxidans JCp4 and Ochrobactrum sp. FCp1, increase in plant growth was monitored by tracking parameters such as % germination (4.68%), plant height (59.6% and 48.6%), plant fresh weight (193.8% and 176.6%) and plant dry weight (137.5% and 165%). The enhanced rate of seed germination and plant growth can be attributed to IAA production, phosphate solubilization and decrease in toxicity of pesticides owing to its increased degradation.
Degradation of CP was also studied in planted soils inoculated with bacterial strains. In CP supplemented soils, A. xylosoxidans JCp4 and Ochrobactrum sp. FCp1 utilized 93.3% and 88.9% of applied CP in 28 days and were found to degrade CP completely after 35 days. The increase of CP degradation in planted soil could be attributed to the greater pesticide degrading activity of the inoculated microbes. Plant roots secrete exudates that can serve as a source of carbon for microbial growth which in turn degrades pesticide at an accelerated rate. In several studies, the plant seedlings have been shielded from the toxic effects of various pesticides by bio-augmenting the soil with pesticide degrading bacteria.32,35
In conclusion, the CP-degrading bacterial isolates obtained as a result of this study exhibited strong CP-degradation potential and were able to bioremediate soil with CP concentration as high as 200mgkg−1. These bacterial strains were found to possess not only pesticide degrading capacities that lower the toxic effects of these pesticides on plants but also various other traits that helped in plant growth promotion. These traits included production of phytohormones such as IAA that enhance cell growth, solubilization of phosphate for root uptake and N2-fixation for plant uptake. Collating efficient biodegradation potential along with multiple biological properties, these isolates have the potential to develop into valuable candidates for development of bioremediation strategies.
Conflict of interestThe authors declare that no conflict of interest.
The financial support for the PhD work was provided by Higher Education Commission (HEC), Islamabad, Pakistan through Indigenous scholarship for work in Pakistan and International Research Support Initiative Program (IRSIP) funds to visit the Faculty of Agriculture and Environment, University of Sydney.