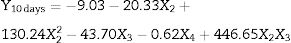An ascomycetes fungus was isolated from brine storage of green olives of the Arauco cultivar imported from Argentina and identified as Monascus ruber. The combined effects of different concentrations of sodium chloride (3.5–5.5%), sodium benzoate (0–0.1%), potassium sorbate (0–0.05%) and temperature (30–40°C) were investigated on the growth of M. ruber in the brine of stored table olives using a response surface methodology. A full 24 factorial design with three central points was first used in order to screen for the important factors (significant and marginally significant factors) and then a Face-Centered Central Composite Design was applied. Both preservatives prevented fungal spoilage, but potassium sorbate was the most efficient to control the fungi growth. The combined use of these preservatives did not show a synergistic effect. The results showed that the use of these salts may not be sufficient to prevent fungal spoilage and the greatest fungal growth was recorded at 30°C.
Table olives are an important fermented fruit product of the western world. The fruit of the olive tree is unsuitable for fresh consumption due to the presence of oleuropein, a glycoside, which is responsible for the bitter taste of raw olives.1 The main reason to process olives is to eliminate oleuropein. There are several different processes, but the most important are: Californian style, Greek style and Spanish style.2
Fungi from genus Monascus are ascomycetes characterized by the production of heat-resistant ascospores, which are able to survive the thermal pasteurization. M. ruber is found in soil and it is related to a post-harvest fruit contamination, and this species was isolated for the first time from pasteurized green olives, in Greece.3
In table olives, M. ruber growth occurs due to its ability to adapt at certain conditions, like growing at very low oxygen tensions, and surviving at low pH and high salt concentrations; in such conditions, M. ruber can produce heat-resistant ascospores.3,4 These conditions allow the microorganism to develop in olives after harvest, which can happen during storage and in canned table olives.3 The M. ruber spoilage results in the fruit softening and a pH increasing in the brine, without necessarily growing any visible fungus mycelium on the brine surface.3,4 Such alterations affect the product quality, causing economic losses. Furthermore, it promotes a risk to the microbiological safety of the product, since the increase of the pH in the brine can allow the growth of several pathogens, such as Escherichia coli O157:H7, Salmonella Enteritidis, Listeria monocytogenes5–7 and Clostridium botulinum.8,9
M. ruber spoilage can be controlled with the combination of intrinsic and extrinsic factors.10 This technique is known as hurdle technology and involves the manipulation of several factors such as: pH, water activity, preservatives and temperature in combination, which may act synergistically to inhibit or retard microbial growth.11,12 However, the antagonistic effect can occur, reducing the effectiveness of the antimicrobials. This fact is more frequent when the ‘hurdles’ have similar mechanisms of action.13,14
The addition of preservatives is a common technique used in food preservation. Food safety is increased and ensured by the use of these additives.12,15,16 The efficiency of preservatives, like benzoate and sorbate is strictly dependent on the pH and the pKa of the acid groups. At low pH, these preservatives are more likely to be in the undissociated form, allowing them to pass freely across the lipid membrane and to inhibit the microbial growth.15,17
Table olives, according to the Trade Standard For Table Olives, can be preserved by heat treatment or by brine with a pH≤4.0, with or without the use of preservatives.18 The preservatives that are allowed include benzoic and sorbic acid, as well as their corresponding salts of sodium and potassium, all within the maximum limits of 1000 and 500ppm, respectively.18 However, information about the combined use of these preservatives on the growth of fungi in the brine of table olives is still scarce.
The aim of this study was to evaluate the effects of the combined use of NaCl, temperature, potassium sorbate and sodium benzoate at different concentrations on the growth of M. ruber on table olive brine after a period of 10, 30 and 50 days.
Material and methodsFungal isolates and identificationThe fungus was isolated from plastic drums containing 180kg of olives of the Arauco cultivar in storage brine (pH=3.8 and 10% salt). Isolation was carried out by inoculating 1mL of contaminated brine in Potato Dextrose Agar (PDA) – (Fluka – Sigma–Aldrich®) acidified with 1% (v/v) of sterile tartaric acid (10%) to lower the pH to 3.5, and incubated at 30°C for 7 days. Identification of microorganisms was made from the observation of the micro and macroscopic characteristics, according to the method of key differentiation described by Udagawa and Baba19 and modified by Stchigel et al.20 The microscopic characteristics that were evaluated were the number of ascospores inside the asci, pigmentation of the ascomata, size and form of the ascospores and presence or absence of patches on the ascomata walls. Macroscopic evaluated characteristics were growth or no growth of colonies on the Czapeck Agar Yeast Extract (CYA) – (Himedia®) and Malt Extract Agar (MEA) – (Himedia®), presence or absence of soluble pigment and the color of the pigment formed. The measurements of microscopic structures were taken using lactophenol as the mounting medium. These characteristics were evaluated after fungi growth on CYA or MEA at different temperatures (25 and 30°C for 7 days).
Preparation of ascospores suspensionThe fungus was grown in a Roux bottle containing 200mL of non-acidified Potato Dextrose Agar (PDA) for 1 month at 30°C. Ascospores were harvested after scraping the inoculated Potato Dextrose Agar (PDA) surface with a sterile spatula using 10mL of sterile distilled water containing 0.1% (v/v) Tween 80 followed by filtration through 3 layers of sterile gauze to remove the hyphal fragments. The ascospores suspension was homogenized by vortex for 3min to open the cleistothecia and liberate the ascospores. The procedure was followed by microscopic observation to assure a high proportion (>90%) of free ascospores. Microscopic counting in a Neubauer chamber resulted in a 105ascospores/mL suspension. The ascospores suspension was stored at 4°C and served as the inoculum for all experiments.
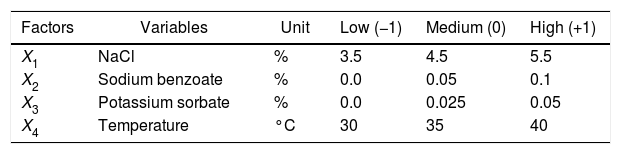
Full 24 factorial designA full 24 factorial design with three central points was carried out to evaluate the effect in the concentration of: NaCl (X1) varying between 3.5 and 5.5% (w/v); sodium benzoate (X2), varying between 0.0 and 0.1% (w/v); potassium sorbate (X3) varying between 0.0 and 0.05% (w/v); temperature (X4), varying between 30 and 40°C, and their interactions on the fungal growth and spoilage. According to full 24 factorial design, 4 negative control (without preservatives) was performed.
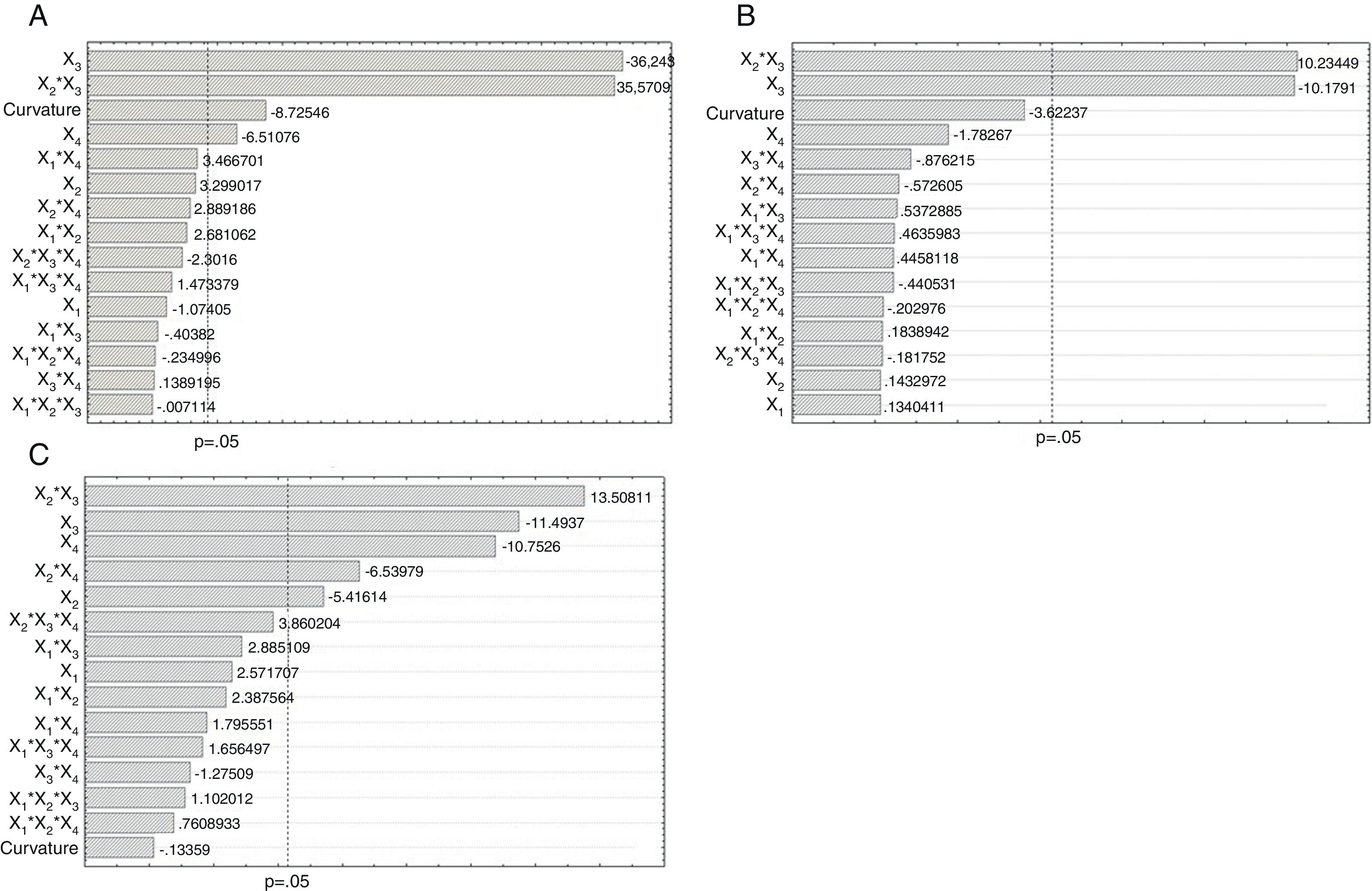
The concentrations of the two independent variables, sodium benzoate (X2) and potassium sorbate (X3) were selected based on the Trade Standards for Table Olives.11 The concentrations of NaCl (X1) were chosen because these are the ones commonly used by the table olive industries in Brazil, while the temperatures (X4) were chosen according to climatic conditions used during storage of table olives in Brazil. Statistical analyses at 95% confidence interval including Analysis of Variance (ANOVA), Pareto Chart of Standardized Effects (Fig. 1) and histogram of normal residual distribution were carried out using the Statistica 7.0 software package (StatSoft Inc, Tulsa, OK, USA).
Pareto chart of absolute standardized values of Monascus ruber growth on table olive brine, at 10 days (A); 30 days (B) and 50 days (C) resulting from the model obtained by a full 24 factorial design with three central points. Monascus ruber growth was analyzed with different concentrations of NaCl (X1) sodium benzoate (X2), potassium sorbate (X3), temperature (X4) and their interactions, performed by full 24 factorial design.
From the results analyzed in the full 24 factorial design, the Pareto chart which display the Standardized Estimate Effect (Absolute Value) was constructed in order to evaluate the significance of each independent factor, their interactions and to check the curvature (Fig. 1). The data from the microbial growth was transformed into mathematical terms like log10, which is standard practise for determining microbial colony counts.21–24
The significance of the curvature suggests that there is a quadratic term in the model, so a polynomial of a higher degree must be used, such as the second-order model,25 so, a FCCCD was used (Table 1). The experimental data were analyzed using response surface methodology (Fig. 2) and fitted to a polynomial model of regression coefficients (Eq. (1)). To evaluate the normality of the residual distribution a Shapiro–Wilk test was performed,21 using the Statistica 7.0 software package (StatSoft Inc, Tulsa, OK, USA).
A FCCCD with three central points: codes, levels, originals variables and results (log10N/N0) of combined factors effects of Monascus ruber growth on table olive brine at 10 days.
| Factors | Variables | Unit | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|---|---|
| X1 | NaCl | % | 3.5 | 4.5 | 5.5 |
| X2 | Sodium benzoate | % | 0.0 | 0.05 | 0.1 |
| X3 | Potassium sorbate | % | 0.0 | 0.025 | 0.05 |
| X4 | Temperature | °C | 30 | 35 | 40 |
| Standard order | X1 | X2 | X3 | X4 | log10N/N0 (10 days) |
|---|---|---|---|---|---|
| 1 | 3.5 | 0.0 | 0.0 | 30 | 1.53 |
| 2 | 3.5 | 0.0 | 0.0 | 40 | 1.09 |
| 3 | 3.5 | 0.0 | 0.05 | 30 | −0.74 |
| 4 | 3.5 | 0.0 | 0.05 | 40 | −1.12 |
| 5 | 3.5 | 0.1 | 0.0 | 30 | 0.26 |
| 6 | 3.5 | 0.1 | 0.0 | 40 | 0.16 |
| 7 | 3.5 | 0.1 | 0.05 | 30 | 0.37 |
| 8 | 3.5 | 0.1 | 0.05 | 40 | 0.04 |
| 9 | 5.5 | 0.0 | 0.0 | 30 | 1.35 |
| 10 | 5.5 | 0.0 | 0.0 | 40 | 1.05 |
| 11 | 5.5 | 0.0 | 0.05 | 30 | −1.03 |
| 12 | 5.5 | 0.0 | 0.05 | 40 | −1.09 |
| 13 | 5.5 | 0.1 | 0.0 | 30 | 0.27 |
| 14 | 5.5 | 0.1 | 0.0 | 40 | 0.28 |
| 15 | 5.5 | 0.1 | 0.05 | 30 | 0.26 |
| 16 | 5.5 | 0.1 | 0.05 | 40 | 0.23 |
| 17 | 3.5 | 0.05 | 0.025 | 35 | 0.25 |
| 18 | 5.5 | 0.05 | 0.025 | 35 | 0.42 |
| 19 | 4.5 | 0.0 | 0.025 | 35 | 0.46 |
| 20 | 4.5 | 0.1 | 0.025 | 35 | 0.67 |
| 21 | 4.5 | 0.05 | 0.0 | 35 | 0.61 |
| 22 | 4.5 | 0.05 | 0.05 | 35 | 0.10 |
| 23 | 4.5 | 0.05 | 0.025 | 30 | 0.00 |
| 24 | 4.5 | 0.05 | 0.025 | 40 | 0.16 |
| 25 (C) | 4.5 | 0.05 | 0.025 | 35 | −0.14 |
| 26 (C) | 4.5 | 0.05 | 0.025 | 35 | −0.11 |
| 27 (C) | 4.5 | 0.05 | 0.025 | 35 | −0.23 |
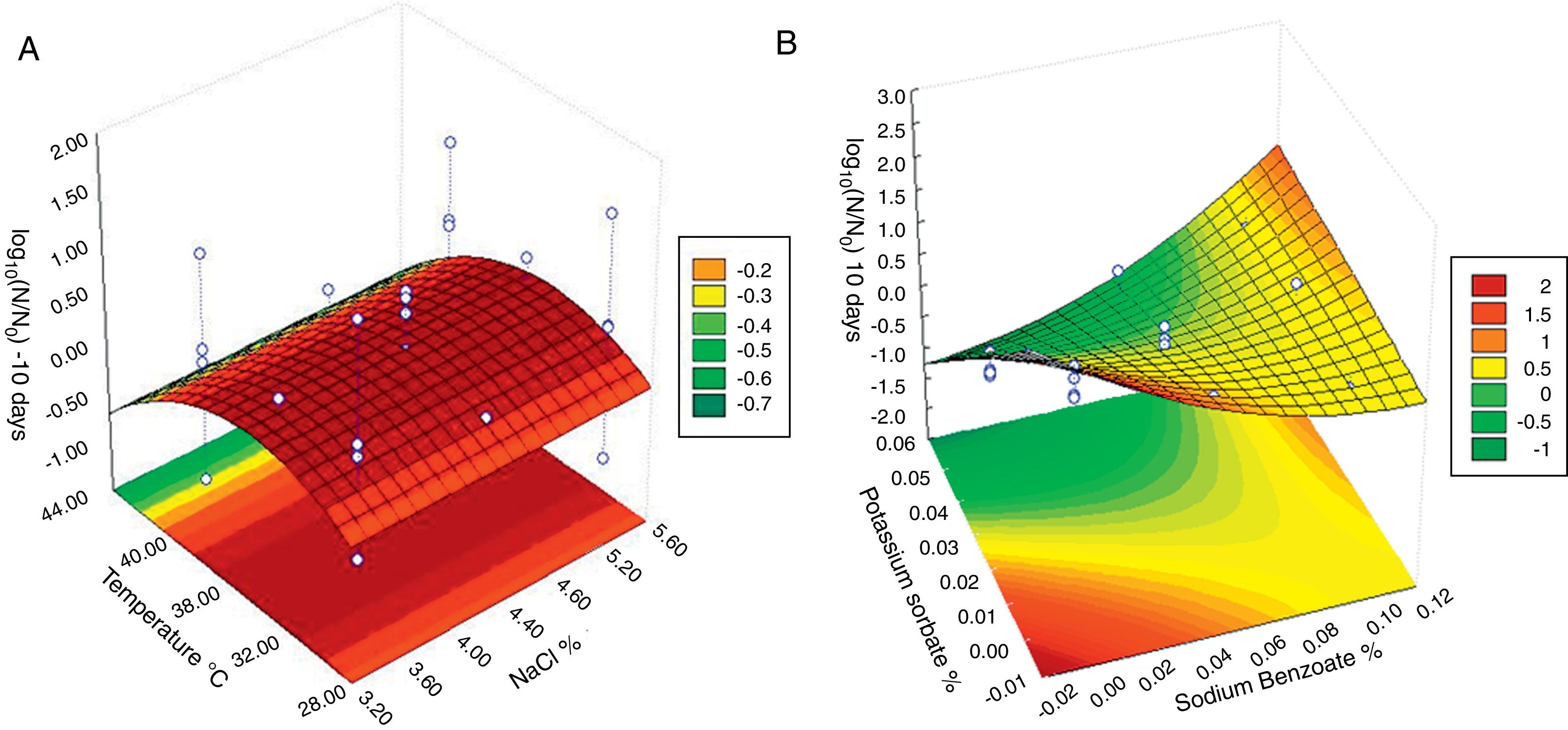
Response surface plots of interaction factors on Monascus ruber growth (log10(N/N0)) on table olive brine after 10 days; (A) interactions of temperature and NaCl; (B) interactions of potassium sorbate and sodium benzoate. Other variables are constant at their center points. The numbers inside the contour plots indicate the Monascus ruber growth.
All the samples of brined table olives were prepared using fifteen green olives of the Arauco cultivar (graded-size 320/360olives/kg), with 200mL of acidified brine, to analyze the fungal growth under real conditions. The formulation was prepared according to the experimental design. The final pH of 3.5 was reached by the addition of lactic acid 85%.
Initially, the samples were pasteurized at 100°C for 10min, in order to eliminate the natural microbiota and thus be able to evaluate exclusively the deterioration resulting from contamination by M. ruber. Preliminary experiments showed that the time×temperature used were the most appropriate thermal treatment conditions. The experiments were carried out in quintuplicate where, it was not observed any growth after plating with 1mL of samples in Potato Dextrose Agar (PDA) – (Fluka – Sigma–Aldrich®) and Plate count agar (PCA) – (Himedia®) after 3–5 days of incubation at 30–35°C.
Pasteurized samples (100°C/10min) were inoculated with 0.2mL of the ascospores suspension (“Preparation of ascospores suspension” section), to reach a concentration of around 102ascospores/mL of brine. These were the samples incubated at temperatures set in the experimental design (30, 35, 40°C). Inoculum concentrations between 102 and 103CFU/mL are commonly used and recommended in studies involving microbial behavior.26
Microbiological analyses and pH measurementsFor enumeration of microbial growth, 1mL of the brine sample aliquot was added to dilution tubes containing 9mL of sterile distilled water. After subsequent decimal dilutions, the inoculation was carried out using the spread plate technique with non-acidified PDA (Fluka – Sigma–Aldrich) in duplicate. All plates were incubated at 30°C for 48–72h. For each sample, the results were the average of two plates and expressed as log10 values. The pH was measured in samples incubated according to the FCCCD design, to analyze the spoilage fungi.
ResultsFungus identificationThe fungus was isolated from brine storage of green olives of the Arauco cultivar imported from Argentina. This may be due to the few studies involving deterioration of olives in Brazil. The fungus was identified as M. ruber (IOC 4667) and registered at the Culture Collection of Filamentous Fungi of Oswaldo Cruz Foundation (CCFF/IOC).
Effect of individual hurdles on microbial growthThe Pareto chart (Fig. 1) shows that the interaction effect between benzoate and sorbate was significant (p<0.05) and showed the same response trend obtained at 10, 30 and 50 days of growth; thus the results for 10 days can be used to describe the trend of growth and spoilage for 30 and 50 days. The results of the temperature and saline concentration interactions did not show any significant effect, consequently these parameters were not considered important to control fungus growth.
Furthermore, as the Pareto chart (Fig. 1) showed that the curvature only had a significant influence for 10 days, a second-order model was applied. Based on the results of growth and the influence of the curvature, a surface model design was used to find the optimal conditions to reduce or eliminate the fungal growth and the resulting deterioration of the table olives.
Response surface methodology of FCCCDAfter evaluating the importance of the factors on the fungal growth at 10, 30 and 50 days using the full 24 factorial design, a FCCCD with three central points was used to find the best set of values, which would give the optimal condition to control the fungal growth and the resulting deterioration. The FCCCD was carried out only considering the result from the 10 days of growth of the preliminary analysis from the full 24 factorial design, which presented a significant curvature, as shown in Fig. 1A. The factors that showed a strong and weak effect on the fungal growth, in other words the significant (p<0.05) and the marginally significant (0.05>p<0.1) factors, were chosen to be analyzed in FCCCD although the NaCl concentration was not significant, this factor was analyzed by FCCCD, because the interaction with temperature (X4) was marginally significant (Fig. 1A).
Table 1 lists the codes, concentrations and original values of the variables used, as well as the results obtained after 10 days of fungal growth resulting from the FCCCD. From ANOVA, a polynomial model was obtained based on the regression analysis of the original significant and marginally significant values. The model resulting from the regression analysis is shown in Eq. (1):
Based on ANOVA of the data from the FCCCD, the quality of fit of the response model was evaluated. Thus in order to determine the accuracy of the quadratic response surface to be able to predict the fungal growth in table olives the R2 (0.9223), the adjusted-R2 (0.8937) and the distribution of the residuals were observed.
The normal distribution of residuals was analyzed using the normality method of Shapiro–Wilk, because numerical approaches are the best way to test for the normality of data.21,27 The Shapiro–Wilk test proved to be the most powerful and efficient in comparison to other tests, like: Anderson–Darling, Kolmogorov–Smirnov, and Lilliefors.27
Fig. 2 presents the response surface plot of significant and marginally significant interaction factors and reveals that the use of preservatives has an important influence on fungal growth. In Fig. 2A the interaction of temperature and concentration of NaCl for all the conditions tested was not sufficient to prevent the fungal spoilage. Fig. 2B shows that sorbate was more efficient than benzoate to inhibit fungal growth. An increase in the concentration of sorbate has a greater effect on the prevention of microbial growth compared to benzoate. The initial concentration of the fungus could only be reduced with sorbate, demonstrating its fungicidal effectiveness. In Fig. 2B the region that provided satisfactory (log10(N/N0)<0.5)26,28 results in the fungal growth, involved concentrations of potassium sorbate higher than 0.025%.
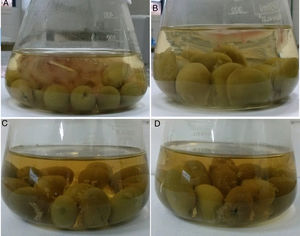
Fig. 3 shows the development of a mycelial mat on the brine surface of the table olives. In addition to visual growth, the pH was measured to evaluate deterioration. The results showed that in the samples without preservatives there was a greater pH increase when compared to samples with preservatives. The largest development of a mycelial mat and the greatest increase in pH (pH=4.0) occurred in the sample incubated at 30°C with 3.5% salt (Fig. 3A).
The development of Monascus ruber mycelial mat on the brine surface of green table olives (Arauco cultivar). Letters (A) and (B) refer to samples without preservatives (sodium benzoate and potassium sorbate), incubated at 30°C and with 3.5% and 5.5% of NaCl, respectively. Letters (C) and (D) correspond to samples without preservatives (sodium benzoate and potassium sorbate), incubated at 40°C and with 3.5% and 5.5% of NaCl, respectively.
The first research involving M. ruber contamination in table olives was carried out by Panagou et al.3 who isolated this fungus from thermally processed green olives of the Conservolea cultivar; where it had shown relative resistance to heat. The authors reported that the fungal spoilage resulted in fruit softening and increased pH of the brine, and they reported that M. ruber was an important spoilage fungus in table olives.
In the present work, M. ruber was isolated from the spoiled brine of green olives of the Arauco cultivar, and the effects of salt (NaCl), potassium sorbate, sodium benzoate and temperature on the fungus growth were studied. Based on the results, one can conclude that the fungus is salt tolerant and the deterioration (visible growth and pH increased) occurred in samples without preservatives. These results showed that the use of salt may not be sufficient to control the fungal spoilage. This conclusion is consistent with Panagou et al.29 who reported that this fungus could even grow in table olive brine containing 9% NaCl, proving its tolerance to salt.
Influence of temperature on fungal growth was foremost when incubated at 30°C, but the growth occurs at all temperatures evaluated. This result is also in agreement with the previously mentioned study by Panagou et al.29 who evaluated the fungal growth by the gradient plate technique at 20, 30 and 35°C and observed that the optimal conditions were also obtained when incubated at 30°C.
Preservatives are used in various products to control fungal growth and spoilage. Organic acids such as sorbic and benzoic acids are the most common preservatives in foods.30 The effectiveness of these compounds depends on many environment factors, like: pH, temperature, aw, the physical matrix of the food and their concentrations.31 The action of the preservatives is extremely pH-dependent because the microbial inhibition is due to their undissociated acidic form, and is influenced by the acid pKa. This form allows the preservatives to penetrate into the cell and to promote the acidification of the cytoplasm, thus inhibiting the fungal growth.32,33
In general, sorbic acid (pKa=4.76) tends to be more effective than benzoic acid (pKa=4.20) due to the higher pKa. Consequently, there will always be a higher concentration of the undissociated form in sorbic acid compared to benzoic acid.16 In our work, the results showed that potassium sorbate had greater efficiency in inhibiting fungal growth compared to sodium benzoate. The maximum efficient was when sorbate was used at only 0.05%, which allows the destruction of the initial fungus concentration. Similar results were obtained when evaluating the effect of these preservatives in inhibiting a cocktail of native yeasts isolated from table olives.34
López et al.35 evaluated the influence of potassium sorbate (0.05%) on the resistance of Issatchenkia occidentalis isolated from table olive brine. The results showed that this fungus was very resistant to preservatives, and was inhibited by 0.03% potassium sorbate. These results are somewhat similar to the ones found for M. ruber in our work that showed inhibition of the fungus at potassium sorbate concentrations higher than 0.025%. Turantaş et al.24 reported that in addition to the antifungal effect of potassium sorbate, the use of this preservative at 0.05% enhanced the production of lactic acid during the fermentation of black olives.
The combined use of two antimicrobial agents can produce different effects on the microbial population such as synergism, antagonism, and additive effects.36 The results of this research showed that the combined use of preservatives in table olives did not result in synergistic effect, as expected. However, when used in combination, benzoate inhibited the action of sorbate on the fungus. This effect can be clearly seen in Fig. 2. These results were similar to those found by Arroyo-López et al.34 who evaluated the effect of using sorbate and benzoate on a native yeast cocktail in table olives using the FIC methodology.
Other research suggests that this phenomenon is due to the fact that these preservatives have the same mechanism of action on the microbial cell. The synergistic effect generally occurs when several antimicrobial factors that have different mechanisms of action are used in combination, thus promoting a greater associated impact than the additive or individual effect.14,37
ConclusionsThe results of this research showed that the combined use of potassium sorbate and sodium benzoate did not present a synergic effect, as expected. Potassium sorbate was the most efficient preservative to prevent table olive deterioration by M. ruber. Based on the response surface, the minimum inhibitory concentration was around 0.03% of potassium sorbate. These results could be used in the table olive industry to assist in preventing M. ruber spoilage. Its survival and growth can lead to olive deterioration including an increase in pH up to the dangerous levels favorable for pathogen growth. A knowledge of the combined technologies with the appropriate concentrations of the ‘hurdles’ in combination can contribute to the design and control of the process in order to assure the safety and quality of the products demanded by the consumer market.
Conflicts of interestThe authors declare no conflicts of interest.