Several strains of lactic acid bacteria (LAB), frequently used in food fermentation and preservation, have been reported to bind different types of toxins in liquid media. This study was carried out to investigate the effect of different concentrations of Lactobacillus rhamnosus GG (ATCC 53103) to bind aflatoxin M1 (AFM1) in liquid media. AFM1 binding was tested following repetitive washes or filtration procedures in combination with additional treatments such as heating, pipetting, and centrifugation. The mixture of L. rhamnosus GG and AFM1 was incubated for 18h at 37°C and the binding efficiency was determined by quantifying the unbound AFM1 using HPLC. The stability of the complexes viable bacteria-AFM1 and heat treated bacteria-AFM1 was tested. Depending on the bacterial concentration and procedure used, the percentages of bound AFM1 by L. rhamnosus GG varied from as low as undetectable to as high as 63%. The highest reduction in the level of unbound AFM1 was recorded for the five washes procedure that involved heating and pipetting. Results also showed that binding was partially reversible and AFM1 was released after repeated washes. These findings highlight the effect of different treatments on the binding of AFM1 to L. rhamnosus GG in liquid matrix.
Aflatoxins (AF) are toxic metabolites that are produced by different kinds of fungi, namely Aspergillus flavus and Aspergillus parasiticus.1 AF production on food, is dependent on mold growth under specific humidity and temperature conditions. It occurs at different stages of food processing, transportation, and storage of crops.2,3
Aflatoxin B1 (AFB1) contained in animal feed is absorbed by the animal's gastrointestinal tract and later converted in the liver into Aflatoxin M1 (AFM1).4 AFM1 could be detected in the blood of the animal within 15min of AFB1-contaminated food ingestion and is then secreted in the milk of lactating animals.4,5 AFM1 is stable during all stages of dairy processing including sterilization and pasteurization.5 To date, some of the best ways to control and prevent AF contamination in food and feed has been performed by improving agricultural practices, in addition to a better control of product storage conditions. AFM1 is not only considered to be carcinogenic and hepatotoxic to humans only but also to other animal species.6 During the early stages of its discovery, the International Agency for Research on Cancer (IARC) classified AFM1 as a group 2B carcinogenic agent.7 Further investigations on its toxicity led to its transfer to the group 1 human carcinogen.8 The maximum acceptable level of AFM1 in milk has been defined by most countries and ranges from as low as 0.05μg/kg, as settled up by the European Union,9 to 0.5μg/kg, as settled up by the Food and Drug Administration.10
Until now, different types of either chemical, physical or biological agents have been tested for their ability to remove AF from feed and food.11,12 It has been reported that the use of lactic acid bacteria (LAB) and other microorganisms reduce mold growth and subsequently AF production in foodstuffs.13,14 Several strains of LAB, including some probiotics, have been reported to effectively bind AF in contaminated milk and media.15–21 Although the stability of the complex formed between AF and bacteria was previously evaluated,16,22,23 still the mechanism of binding between AF and LAB has not been fully clarified yet. It was though that AF bind to cell wall components, including both polysaccharides and peptidoglycans.24 Both hydrogen bonds and Van der Waals interactions have been precisely identified to be involved in AF binding.24,25
Several treatments used in AF binding assays were previously tested such as repetitive washes and/or heat treatment.16,20,22,23 Specifically, in our work we will study the effect of these treatments on AFM1 binding to Lactobacillus rhamnosus GG in liquid medium and on the stability of L. rhamnosus GG/AFM1 complex. In addition, other original treatments of filtration, centrifugation and pipetting were also evaluated and compared. The tested procedures such as pipetting and centrifugation provide different practical applications that could be optimized and used at an industrial scale to reduce AFM1 bioavailability.
Materials and methodsBacterial strain and growth conditionsL. rhamnosus GG (ATCC 53103) was purchased, as lyophilized powder, from Microbiologics (St. Cloud, MN, USA). This strain was chosen based on its use in various food products and on its ability to bind different types of food mutagens.23,26L. rhamnosus was incubated for 24h in de Man-Rogosa-Sharpe (MRS) broth (Himedia, Mumbai, India) under aerobic conditions at 37°C. The estimation of bacterial cell concentration in the culture was determined by the turbidimetric method.27 Using the absorbance measured by a spectrophometer (Thermo Fisher Scientific, MA, USA) at 600nm, the bacterial concentration curve over a period of 24h was constructed. After dilution and incubation in MRS agar (Himedia) at 37°C for 48h under aerobic conditions, the logarithmic value of the bacterial concentration was obtained using solid media counting method.28 The equation of the bacterial cell concentration in MRS medium was then generated with a compliant coefficient of determination (R2=0.999).
AFM1 binding assayA standard of AFM1 (10μg/mL), suspended in acetonitrile, was obtained from Sigma (St. Louis, MO, USA). To prepare solutions A and B with a concentration of 100μg/L and 50μg/L, respectively, the AFM1 stock solution was diluted with phosphate-buffered saline (PBS; pH 7.4, 0.05% Tween 20, v/v). Prior to PBS addition, the acetonitrile was evaporated by heating the solutions, in a water bath, at 80°C for 10min. Cell counts of bacterial suspensions were dictated by the generated bacterial cell concentration equation. Following the centrifugation of the suspensions at 3000×g for 10min, bacterial pellets were washed with 5mL of sterile deionized water to avoid the effect of the bacterial concentration on AFM1 removal.15 For the purpose of the experiment, bacterial pellets of 5×108CFU and 1010CFU were prepared. The pellets of 5×108CFU were re-suspended in 1mL of solution A (100μg/L) in 1.5mL microtubes (Eppendorf AG, Hamburg, Germany) while those of 1010CFU were re-suspended in 1mL of solution B (50μg/L) in 14mL tubes (Falcon, Corning Inc., NY, USA). The bacterial pellets used for AFM1 binding were even previously heat treated (1h at 90°C) or not. All suspensions were incubated at 37°C for 18h. A previous pipetting of the suspensions was conducted prior to the incubation for the treatments that included pipetting. Bacterial suspensions of 5×108CFU/mL were then centrifuged at 3000×g for 10min and/or filtered as described below (“Effect of Filtration on AFM1 Quantification” section) while suspensions of 1010CFU/mL were directly centrifuged (3000×g for 10min) after incubation. Supernatants were collected and the concentration of unbound AFM1 was determined by HPLC. Negative (only bacteria suspended in PBS) and positive control (only AFM1 in PBS) were also included.
Effect of filtration on AFM1 quantificationThe effect of the filtration on the separation of AFM1 from L. rhamnosus GG (ATCC 53103) was studied. Due to the volume retention in the filter cake space, it was necessary to increase the volume of the suspension, although the bacterial concentration was kept constant at 5×108CFU/mL and experiments were done with both viable and heat treated bacteria. All heat treated (90°C for 1h) and viable bacterial suspensions were pipetted till homogenization before their incubation at 37°C for 18h in AFM1 solution. In order to study the effect of coupling the steps of centrifugation and filtration on AFM1 adsorption, bacteria were either centrifuged then filtered or filtered directly without a preceding step of centrifugation. Prior to their analysis, all samples were passed through the porous membrane of a syringe filter (Minisart, Sartorius AG, Gottingen, Germany) having a pore diameter of 0.45μm.
Effect of heat treatment and pipetting on AFM1 bindingAfter being suspended in 1mL of either solution A or B, suspensions of both bacterial concentrations (5×108CFU/mL and 1010CFU/mL) were pipetted till complete homogenization for treatments that required pipetting. The used bacterial pellets where previously heat treated in a water bath at 90°C for 1h or kept untreated. After incubation at 37°C for 18h, all samples were centrifuged at 3000×g for 10min. Then, the supernatants of each of the samples were collected and the residual AFM1 was quantified by HPLC.22
Effect of washes on L. rhamnosus GG/AFM1 complexAfter performing the binding assay as described earlier and following the incubation of the samples for 18h at 37°C, the effect of washes on L. rhamnosus GG/AFM1 complex was tested by quantifying the amount of unbound AFM1. Supernatant was collected and the residual AFM1 was quantified by HPLC. Bacterial pellets were washed by suspending each in 1mL of PBS, then kept at room temperature for 10min. Once centrifuged, supernatants were collected and residual AFM1 were quantified by HPLC.22 The washing step was repeated five times.
Residual AFM1 quantitationAfter incubation of bacteria with AFM1, the remaining AFM1 in the supernatant were quantified using reverse-phase HPLC (Waters 2690®, Waters Corp., MA, USA) coupled with a fluorescence detector (Waters 2475®) and a (Supelco Discovery®) C18 column (250mm×4.6mm I.D., 5μm particle diameter) fitted with a C18 guard column (Supelco Supelguard®, Sigma–Aldrich Co., MO, USA). The mobile phase was composed of Milli-Q-water/acetonitrile/methanol (68/24/8, v/v/v) with a flow rate of 1mL/min. The injection volume was 20μL. Detection was done by fluorescence and the wavelengths for excitation and emission were set at 360 and 430nm respectively. The AFM1 retention time was 7.5min. A calibration curve was constructed with AFM1 standard (Sigma–Aldrich, MO, USA) at concentrations ranging from 0.1 to 100μg/L.
Statistical analysisAll assays were carried out in triplicate. Two-way ANOVA was conducted using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA) to identify significant differences between the different procedures. Results with a p<0.05 were considered statistically significant.
Results and discussionComplex stability in filtration procedureWe studied the stability of the complex L. rhamnosus GG/AFM1 at different experimental conditions: filtration, heating, centrifugation and pipetting. The effect of the filtration treatment is highlighted in Table 1. Prior to testing the efficiency of L. rhamnosus GG to bind AFM1, filtration was shown to significantly retain AFM1 where 45.1±0.2% (p<0.05) were retained by this process. The filter may have been involved in AFM1 retention by acting as a barrier to AFM1 passage. This barrier effect may be caused by the adsorption of AFM1 to the filter membrane. In addition, the retention of a certain quantity of AFM1 in the filter cake space and in the cavities of the filter membrane may also explain this high retention rate that will possibly increase the margin of error throughout the filtration procedure. Furthermore, filtration of the supernatant prior to AFM1 quantification (HPLC, ELISA, etc.) may lead to inaccurate results.
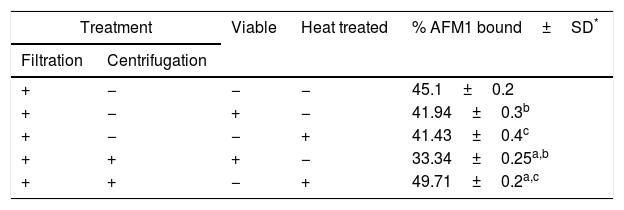
Effect of filtration (coupled or not with centrifugation), heating and pipetting on L. rhamnosus (5×108CFU) binding capacity to AFM1 (100μg/L).
| Treatment | Viable | Heat treated | % AFM1 bound±SD* | |
|---|---|---|---|---|
| Filtration | Centrifugation | |||
| + | − | − | − | 45.1±0.2 |
| + | − | + | − | 41.94±0.3b |
| + | − | − | + | 41.43±0.4c |
| + | + | + | − | 33.34±0.25a,b |
| + | + | − | + | 49.71±0.2a,c |
Indicates a significant binding difference (p<0.05) between:
In the assessment of the binding efficiency using viable bacterial cells, the combination of pipetting and filtration coupled to a centrifugation step seemed to be less efficient in binding AFM1 (33.34%) than that without centrifugation (41.94%). Whereas, an inverse result was obtained when the assessment was done with heat treated bacteria which bind 49.71% of AFM1 when treated by pipetting and filtration coupled to a centrifugation step and only 41.43% when treated by pipetting and filtration without centrifugation. Moreover, the comparison between viable and heat treated bacteria showed a significant difference (p<0.05) when a centrifugation step was introduced. The percentage of bounded AFM1 significantly increased from 33.34% to 49.71% after heat treatment. Thus, centrifugation appears to have a significant impact on AFM1 binding. This effect might be due to the increase of contact between the specific surface of the bacteria and AFM1. Additionally, heat treatment before centrifugation may have further increased the specific surface of the bacteria. This may have evolved from the formation of electrostatic bonds such as hydrogen and Van der Waals bonds.24,25 We hypothesize that centrifugation of viable bacteria leaded to a possible disruption of a number of electrostatic bonds formed with AFM1. This decrease in AFM1 binding was not compensated by any increase in the bacterial specific surface. On the other hand, this increase in AFM1 binding after heat treatment combined with centrifugation step was possibly due to an increase in the number of electrostatic bonds formed with AFM1 following their previous disruption induced by heating.
Complex stability in five washes procedureThe effects of heating, pipetting and five washes treatments in addition to their coupling effects are highlighted in Tables 2 and 3 when using different bacterial concentrations (5×108CFU/mL and 1010CFU/mL). At a bacterial concentration of 5×108CFU/mL and AFM1 concentration of 100μg/L, not all the treatments showed significant differences in the binding of AFM1 (Table 2).
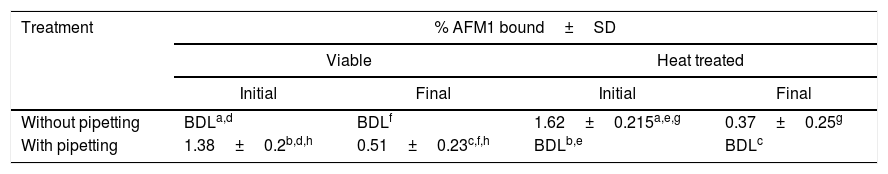
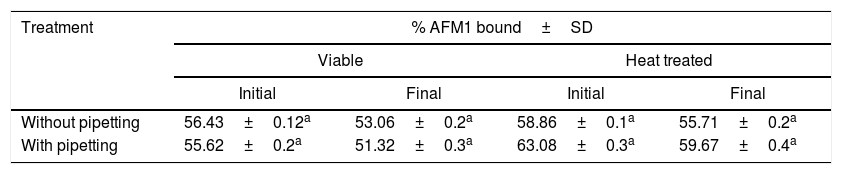
Effect of pipetting, heating and five washes treatments on L. rhamnosus (5×108CFU) binding capacity to AFM1 (100μg/L) before (initial) and after (final) up to five washes with PBS.
| Treatment | % AFM1 bound±SD | |||
|---|---|---|---|---|
| Viable | Heat treated | |||
| Initial | Final | Initial | Final | |
| Without pipetting | BDLa,d | BDLf | 1.62±0.215a,e,g | 0.37±0.25g |
| With pipetting | 1.38±0.2b,d,h | 0.51±0.23c,f,h | BDLb,e | BDLc |
Results are the average±SD for triplicate samples. BDL: below HPLC detection limit (0.03μg/L).
Indicates a significant binding difference (p<0.05) between:
Effect of pipetting, heating and five washes treatments of L. rhamnosus (1010CFU) on AFM1 (50μg/L) binding before (initial) and after (final) up to five washes with PBS.
| Treatment | % AFM1 bound±SD | |||
|---|---|---|---|---|
| Viable | Heat treated | |||
| Initial | Final | Initial | Final | |
| Without pipetting | 56.43±0.12a | 53.06±0.2a | 58.86±0.1a | 55.71±0.2a |
| With pipetting | 55.62±0.2a | 51.32±0.3a | 63.08±0.3a | 59.67±0.4a |
Results are the average±SD for triplicate samples.
Heat treatment and heating coupled with pipetting showed a significant effect on the amount of bounded AFM1 that was respectively revealed by an increase of 1.62% (from BDL: below detection limit to 1.62%; p<0.05) and a decrease of 1.38% (from 1.38% to BDL; p<0.05). Moreover, when heating was followed by the five washes treatment no significant effect on the amount of bounded AFM1 was observed (from BDL to 0.37%; p>0.05). When heating was coupled with five washes and pipetting treatments a significant decrease in bounded AFM1 was shown (from 0.51% to BDL; p<0.05). Actually, the treatments in combination with heating (pipetting and pipetting combined with five washes) showed a decrease in the binding of AFM1. This decrease in AFM1 binding may be caused by a disruption of a number of electrostatic bonds due to the used treatments.
Pipetting treatment and pipetting coupled with five washes treatment showed a significant binding difference with an increase of 1.38% (from BDL to 1.38%; p<0.05) and 0.51% (from BDL to 0.51%; p<0.05) of the amount of bounded AFM1 respectively. Thus, due to the absence of detectable AFM1 binding before pipetting or washing, these treatments may have initiated this binding by increasing the contact between bacteria and AFM1. In addition, when pipetting was coupled with heat treatment a significant decrease of 1.62% (from 1.62% to BDL; p<0.05) of the amount of bounded AFM1 was shown. This decrease in binding is possibly due to the increase of the applied forces on the bacteria. On the other hand, no significant difference in AFM1 binding was observed after coupling pipetting with heating and washing treatments (from 0.37% to BDL; p>0.05).
Five washes coupled with heating and five washes coupled with pipetting treatment showed a significant binding difference with a decrease of 1.25% (from 1.62% to 0.37%; p<0.05) and 0.87% (from 1.38% to 0.51%; p<0.05) of the amount of bounded AFM1 respectively. Furthermore, the percentage of bounded AFM1 after five washes treatment and five washes combined with pipetting and heat treatments were all BDL. Therefore, the five washes effect is not clearly shown at a bacterial concentration of 5×108CFU/mL.
Stability at a bacterial concentration of 1010CFU/mLIn the five washes procedure, after increasing the number of L. rhamnosus GG to 1010CFU as previously used in other AF binding assays16,23 and decreasing the concentration of AFM1 to 50μg/L, results showed a significant difference in AFM1 binding in all treatments (Table 3). The binding of AFM1 to L. rhamnosus GG increased from less than 2% to more than 50%, which is a normal increase between bacterial concentration and the bounded amount of AFM1.
Heat treatment and heating coupled with five washes treatment showed a significant difference in the amount of bounded AFM1 with an increase respectively of 2.43% (from 56.43% to 58.86%; p<0.05) and 2.65% (from 53.06% to 55.71%; p<0.05). In addition, when heating was combined with pipetting treatment, higher significant binding increase of 7.46% (from 55.62% to 63.08%; p<0.05) was observed. Similarly, when heating was coupled with pipetting and washing treatments a significant increase in AFM1 binding of 8.35% (from 51.32% to 59.67%; p<0.05) was shown. Thus, we hypothesize that in the absence of pipetting, bacterial aggregate and heat treatment promotes formation of inter and intra bacterial bonding, leaving possibly the superior layer of this mass mostly exposed to AFM1. These results emphasize the implication of bacterial surface in AFM1 binding, this is in compliance with others who reported the implication of the bacterial cell wall polysaccharide and peptidoglycan as the two main elements in mutagens binding to LAB.29–32 Furthermore, the positive effect observed after heat treatment on AFM1 binding was in accordance with other previous studies. Elsanhoty et al. while studying the detoxification of AFM1 in yoghurt using probiotics and LAB found that heat treatment of L. rhamnosus can significantly increase its binding to AFM1.22 Similarly, Haskard et al. showed that a significant increase in the binding of AFB1 by heat treated L. rhamnosus GG was observed.23 Therefore, we speculate that heat treatment may increase the disruption of the inter and intra-bacterial electrostatic bonds, and consequently the surface of the bacteria becomes free to form additional bonds with AFM1.
Pipetting treatment and pipetting coupled with five washes treatment showed a significant binding difference with a decrease of 0.8% (from 56.43% to 55.62%; p<0.05) and of 1.74% (from 53.06% to 51.32%; p<0.05) respectively in the amount of bounded AFM1 to viable bacteria. These decreases may possibly be due to the increase in the forces exerted on bacteria leading to the disruption of a number of electrostatic bonds formed with AFM1. In contrast, when pipetting was combined with heat treatment a significant increase in the amount of bounded AFM1 of 4.22% (from 58.86% to 63.08%; p<0.05) was observed. This increase may be explained by the exposition of the totality of the bacterial specific surface to AFM1. The released quantity after pipetting was probably compensated by an increase in the bacterial specific surface caused by the possible disruption of electrostatic bonds after heat treatment. Similarly, when pipetting was coupled with heating and five washes treatments the percentage of bounded AFM1 significantly increased by 3.96% (from 55.71% to 59.67%; p<0.05).
Five washes and its coupling treatments showed a significant negative effect on the amount of bounded AFM1. The decreased amount of bounded AFM1 in five washes steps and five washes coupled with heat treatment was respectively 3.37% (from 56.43% to 53.06%; p<0.05) and 3.15% (from 58.86% to 55.71%; p<0.05). Furthermore, when the five washes treatment was coupled with pipetting or with heating and pipetting treatments the amount of bounded AFM1 significantly decreased by 4.3% (from 55.62% to 51.32%; p<0.05) and 3.41% (from 63.08% to 59.67%; p<0.05) respectively. This partial reversibility of binding between bacteria and AFM1 presents an evidence of the presence of a non-covalent type of bonds as hydrogen and Van der Waals interactions as it was previously reported. Yiannikouris et al. found that beta-d-glucans, a cell wall component of many microorganisms was implicated via these interactions in the binding of AF and other types of mycotoxins.25
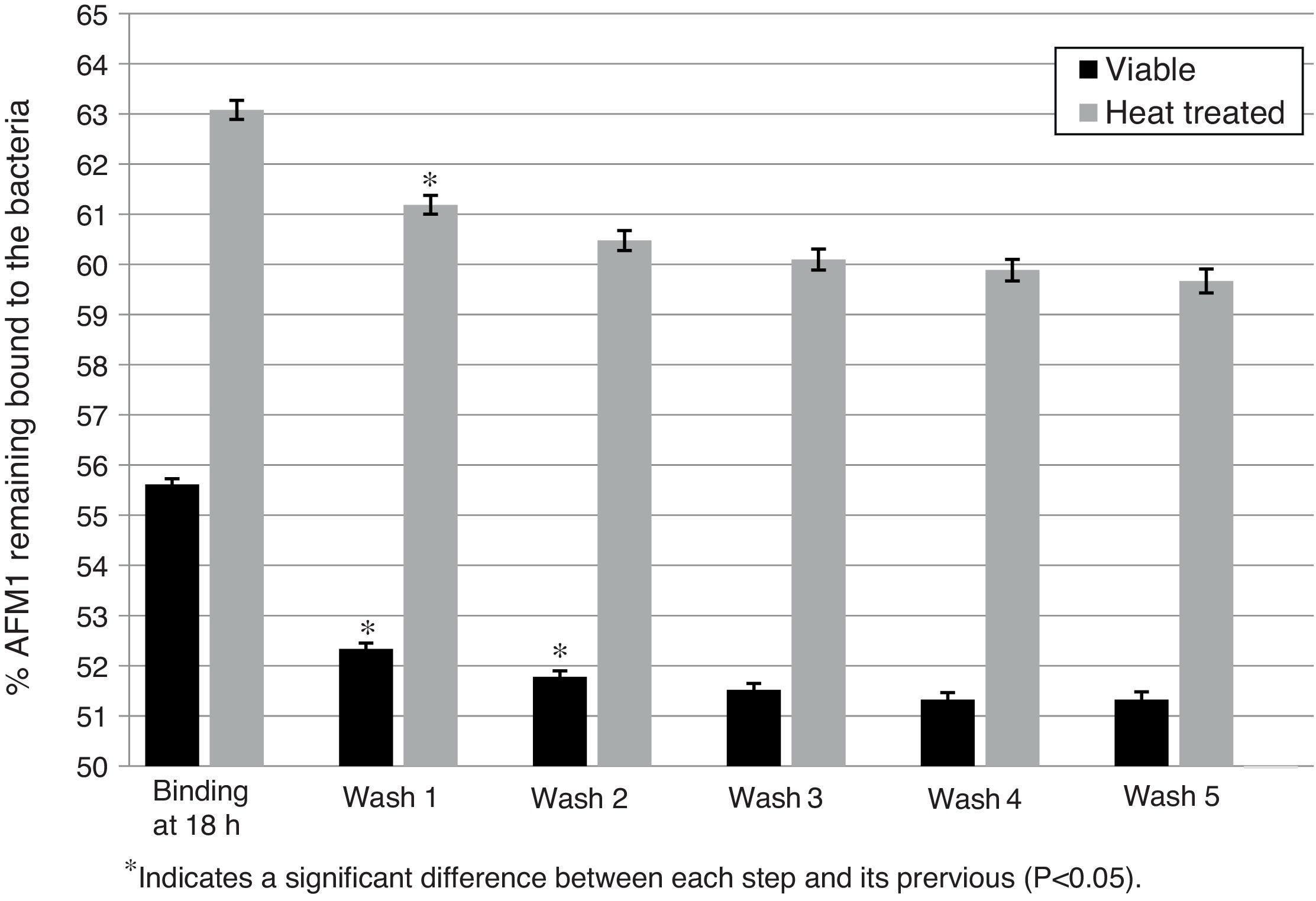
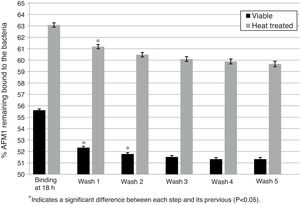
Stability after each washing stepThe effect of successive washing steps on the AFM1 binding by viable and heat treated L. rhamnosus GG is presented in Figs. 1 and 2. Previously pipetted viable and heat treated bacteria showed a significant decrease in the percentage of bounded AFM1 (p<0.05) of 3.84% after the first two washes of viable bacteria and of 1.89% after the first wash of heat treated bacteria with a trend to a decrease in the following washes (Fig. 1). After five washes, the total residual amount of AFM1 with viable and heat treated bacteria was respectively 4.3% and 3.41%.
Effect of washes on L. rhamnosus GG/AFM1 complex stability. Binding was determined after viable and heat treated bacteria (1010CFU) were incubated with AFM1(1mL, 50μg/L) at 37°C for 18h with previous pipetting. The complexes formed were exposed to five washes with 1mL of PBS and the residual AFM1 was quantified. Error bars represent the SD (standard deviation).
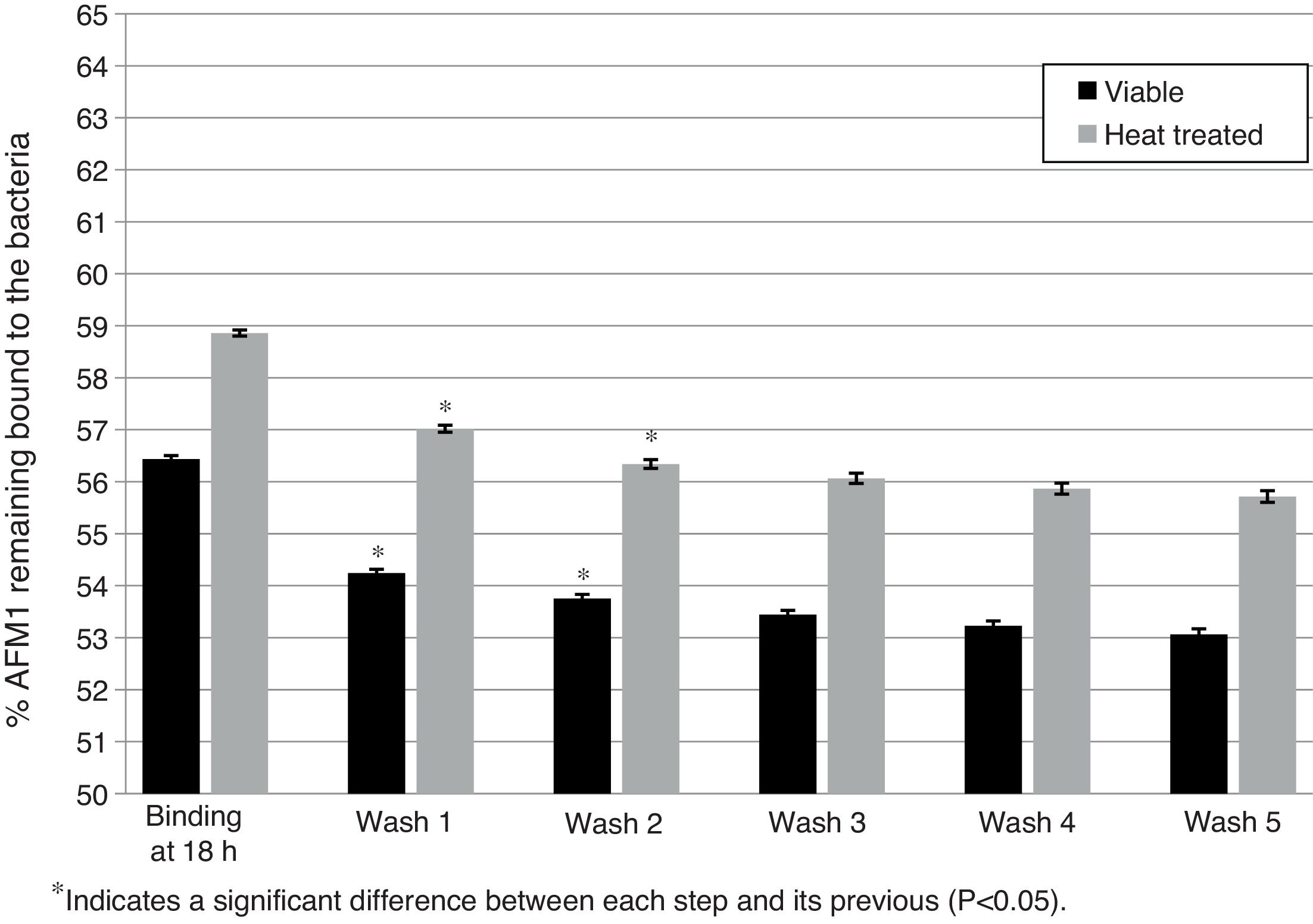
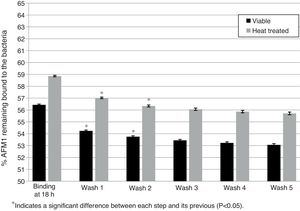
Effect of washes on L. rhamnosus GG/AFM1 complex stability. Binding was determined after viable and heat treated bacteria (1010CFU) were incubated with AFM1 (1mL, 50μg/L) at 37°C for 18h without previous pipetting. The complexes formed were exposed to five washes with 1mL of PBS and the residual AFM1 was quantified. Error bars represent the SD.
However, non-pipetted viable and heat treated bacteria showed a significant decrease in the percentage of bounded AFM1 (p<0.05) of 2.68% and 2.52% respectively after the first two washes with a trend to a decrease in the following washes (Fig. 2). After five washes, the total residual amount of AFM1 with viable and heat treated bacteria was respectively 3.37% and 3.15%.
Therefore, after washing the L. rhamnosus GG/AFM1 complexes with PBS, significant amounts of unbound AFM1 were released back into solution. This reversible binding may be due to unbound AFM1 stacked among the bacterial cells. Additionally, due to the washing steps the disruption of the weak bonds established with AFM1 might increase the amount of residual AFM1.
Accordingly, the use of L. rhamnosus may provide a potential element to detoxify liquid products such as milk and needs further studies to elucidate its application. In addition, it may be important to optimize the binding of L. rhamnosus GG to AFM1 by assessing the effect of several experimental parameters for example: incubation time, temperature and centrifugal force. Furthermore, the binding of AFM1 to L. rhamnosus GG using different treatments such as pipetting and centrifugation can be additionally studied for possible industrial application during milk processing in order to reduce AFM1 bioavailability. Thus, using a customized industrial agitator that could increase the contact between L. rhamnosus GG and AFM1 by combining the effects of pipetting and centrifugation may lead to increase the binding of AFM1 in milk and other liquid foods.
ConclusionsIn this study, different procedures for binding of AFM1 by L. rhamnosus GG were used. Heat treatment of L. rhamnosus GG significantly increased AFM1 binding in a five washes procedure. In addition, pipetting and centrifugation of bacteria after a heat treatment increased the percentage of bound AFM1. The reversibility of binding was shown with the bacterial washing steps, suggesting the implication of non-covalent electrostatic bounds, such as hydrogen bonds and Van der Waals interactions. In addition, filtration appears to be inefficient compared to the five washes procedure. This work highlights the ability of L. rhamnosus GG as a potential binder of AFM1 in liquid foods such as milk.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the research council and the research and analysis center (CAR) at Saint-Joseph University (USJ).