Different technologies may be used for decolorization of wastewater containing dyes. Among them, biological processes are the most promising because they seem to be environmentally safe. The aim of this study was to determine the efficiency of decolorization of two dyes belonging to different classes (azo and triphenylmethane dyes) by immobilized biomass of strains of fungi (Pleurotus ostreatus – BWPH, Gleophyllum odoratum – DCa and Polyporus picipes – RWP17). Different solid supports were tested for biomass immobilization. The best growth of fungal strains was observed on the washer, brush, grid and sawdust supports. Based on the results of dye adsorption, the brush and the washer were selected for further study. These solid supports adsorbed dyes at a negligible level, while the sawdust adsorbed 82.5% of brilliant green and 19.1% of Evans blue. Immobilization of biomass improved dye removal. Almost complete decolorization of diazo dye Evans blue was reached after 24h in samples of all strains immobilized on the washer. The process was slower when the brush was used for biomass immobilization. Comparable results were reached for brilliant green in samples with biomass of strains BWPH and RWP17. High decolorization effectiveness was reached in samples with dead fungal biomass. Intensive removal of the dyes by biomass immobilized on the washer corresponded to a significant decrease in phytotoxicity and a slight decrease in zootoxicity of the dye solutions. The best decolorization results as well as reduction in toxicity were observed for the strain P. picipes (RWP17).
Synthetic azo-, triphenylmethane, and anthraquinone dyes are commonly used in the textile, food, cosmetics, papermaking and pharmaceutical industries.1,2 They are resistant to light operation, moisture and oxidants because most of them have complex aromatic structures. On the one hand, this is a feature desired by the industry, but on the other hand, it is dangerous for the environment. The presence of synthetic dyes in water causes a reduction in aquatic biodiversity by blocking the passage of sunlight through the water and creates problems for photosynthetic aquatic plants and algae. Many synthetic dyes are toxic, mutagenic and carcinogenic.3–6 In addition, dyes may accumulate in sediments, especially in places of wastewater discharge, and affect the ecological balance of the aquatic system. Leaching of contaminants can affect the groundwater system.7 The most serious problem is associated with effluents from the textile industry, where the dyes used for dyeing and finishing operations vary from day to day and sometimes even several times a day. Imperfection of textile coloration processes may cause losses of applied dyes (even 10–15%).3–5,8 Because of this, the textile finishing wastewater is characterized by a strong color and large number of suspended solids.9
The colored wastewater is mainly cleaned by physical and chemical procedures, such as adsorption, coagulation, flocculation, flotation, precipitation, oxidation and reduction, ozonation and membrane separation. These technologies are very expensive and have drawbacks.4,9,10 That is why bioremediation by microorganisms is still an environmentally friendly and cost-competitive alternative. Treatment of textile effluent requires an efficient system of color removal. There are many publications confirming the high potential of bacterial, fungal and algae species in dye removal.4,11–16 Removal of dyes may be achieved through biodegradation/biotransformation and/or adsorption on biomass. Biotransformation of dye may lead to complete mineralization or formation of less toxic products.4,12,15,16
Fungi may use an extracellular enzymatic system to transform aromatic substances, such as lignin, polycyclic aromatic hydrocarbons (PAH) or pesticides. Currently much attention is focused on fungal decolorization processes. Fungal biomass is used as a sorbent and/or producer of enzymes involved in biodegradation/biotransformation. The process of biosorption is rapid, efficient and adaptable to diverse types of textile effluents. The results of different experiments emphasize that fungal processes are mostly associated with biotransformation but not biosorption.8,12,17,18 Biosorption is observed mostly for non-ligninolytic fungi, such as Aspergillus niger, of which (dead) biomass may be used as an adsorbent.13,19 Among these, the most widely researched are white rot fungi, such as Phanerochaete chrysosporium, Bjerkandera sp., Trametes versicolor, Irpex lacteus, and Pleurotus ostreatus, which produce enzymes, such as lignin peroxidase, manganese peroxidase and laccase. They are able to degrade many aromatic compounds due to their non-specific enzymatic activity.6,13,17,19–21 As described previously, white-rot fungi are capable of decolorizing dyes significantly, and in most cases, this is due to the activities of lignin peroxidase (LiP)22 and Mn-dependent peroxidase (MnP).23 Some studies have demonstrated laccase (Lac)-mediated dye decolorization.12,24 It was also reported that non-ligninolytic enzymes may play a role in the decomposition of dyes, such as triphenylmethane crystal violet.17 Process conditions have a significant influence on biotransformation effectiveness. The best results of dye removal by fungi were obtained in more aerated shaken samples. Living biomass of tested strains removed dyes more effectively than dead biomass.16,17,25,26
Decolorization effectiveness depends mainly on the strain used in the process as well as on the specific structure of the dye and composition of the dye effluents. It was demonstrated that strains isolated from polluted sites had a greater decolorization potential than others. Additionally, the form and composition of the culture medium play an important role in decolorization processes.6,25,27–30 The most important factors are the sources and concentrations of carbon and nitrogen, which have a significant influence on production of ligninolytic enzymes. The influence of different carbon sources on decolorization effectiveness has been extensively studied.19,27,28,31 It should be mentioned that the effectivenes of dye removal depends also on the way biomass is used. Fungal free-cell treatment shows some drawbacks since the mycelium may be more exposed to environmental stresses. Therefore, a good alternative might involve the immobilization of biomass on different supports. Immobilization protects the biomass and improves fungal activity.32 It has been reported that immobilization of fungal cells may stably maintain the production of various enzymes at levels higher than those achieved with suspended or pellet forms.33,34 Moreover, the immobilization of fungal biomass increases fungal resistance to environmental stresses, such as the presence of toxic molecules at high concentrations. Immobilization improves decolorization efficiency of biomass due to less dense fiber packing in comparison with the free fungal biomass. This is because the microorganism has a larger surface area available for dye adsorption. The increase in the surface area of fungal biomass tends to reduce the mass transfer limitations, which in turn increases access to pollutant degradation.32,35–40 Immobilization may allow the use of the system repeatedly, allowing easier liquid–solid separation and avoiding clogging phenomena.32,35
Yesilada et al.5 demonstrated that white rot fungi pellets may be used for effective decolorization of textile dyes. It was also possible to induce dye decolorization activity of Funalia trogii by carefully selecting the optimal culture conditions. Pellets could be used several times and still maintain high decolorization activity. Using pellets would allow treatment of effluents with varying dye compositions and in high concentrations of dyes, which are normally toxic at low concentrations.5 The aim of the present study was to evaluate the influence of the solid support used for biomass immobilization on the decolorization efficacy. Different solid supports were used in the experiment to obtain intense growth of fungal biomass and to assist in the process of dye removal. After the environmental safeties of the solutions after the decolorization processes were assessed, the zoo- and phytotoxicity were evaluated.
Materials and methodsTested organisms and culture conditionsThe fungal strains P. ostreatus (BWPH), Gleophyllum odoratum (DCa) and Polyporus picipes (RWP17) were isolated by the tissue method (MEA medium (Difco)) with fruiting bodies of fungi collected in the woods near Gliwice (southern Poland, Upper Silesia). Samples were incubated at 26°C. Cultures were maintained in MEA slants and stored at 4°C.
Immobilization experimentWith the aim of improving growth of the fungal biomass, we tested different solid supports: a polyethylene foam, polypropylene washer, polystyrene fitting, tile cross spacers, brush for washing bottles, grid used under plaster and sawdust. The solid supports were added to the flasks containing YEPG medium (glucose 10g/L, peptone 5g/L, yeast extract 2g/L, MgSO4 0.5g/L, KH2PO4 1g/L, pH 5.6) in exact weight and were sterilized by autoclaving (15min, 121°C, 1.5atm). One piece of mycelium (∅5mm) cultured for 7 days on MEA (Fluka Biochemica) was added to each sample. Samples were incubated for 5 days (26°C) on a rotary shaker (110rpm). Observations of biomass growth were performed each day. Adsorption of dyes on all supports was tested. 10mL of water solutions of dye was added (0.1g/L) to samples with supports, and after 1h, absorbance in samples was measured. Percentage removal was calculated according to the formula (1) presented below. For further research, the two supports characterized by a large increase in biomass growth and low adsorption of dyes (polypropylene washer and brush) were selected. It was necessary for evaluating the effectiveness of biological process.
Decolorization experimentThe aim of this study was to determine the effectivenesses of brilliant green (BG – triphenylmethane dye) and Evans blue (EB – azo dye) decolorization by single fungal strains immobilized on solid supports. Based on the previous experiment, two solid supports were chosen: the brush and washer. Samples were prepared by the addition of two pieces of the specified mycelium (∅5mm) cultured for 7 days on MEA (Fluka Biochemica) to Erlenmeyer flasks with 150mL of YEPG medium and the chosen support.
Water solutions of triphenylmethane dye brilliant green (POCh) and diazo dye Evans blue (Sigma-Aldrich) were prepared as described in Przystaś et al.41 Dyes were added to samples to obtain an initial concentration of 0.1g/L. Control samples with dyes were prepared on sterile medium (used for microorganism cultures) and were shaken in the same manner as the inoculated samples. Cultures were incubated at 26°C on a rotary shaker (110rpm). Dead biomass (for estimation of biosorption) was obtained by autoclaving (15min, 121°C, 1.5atm) 5-day-old fungal cultures prepared in the same manner as samples with living biomass. Preparations of all modified and control samples were conducted four times.
Measurement of decolorization effectiveness and sample toxicitySamples were collected after 1, 3, 6, 24, 48, 72 and 96h, and absorbance was measured (UV VIS spectrophotometer Hitachi U1900) at the adequate wavelengths: at −624nm for brilliant green and at 606nm for Evans blue (wavelengths were determined experimentally as the wavelength with maximal absorbance). Percentage dye removal was calculated according to formula (1).
where C – current concentration of dye in a control sample [mg/L], S – current residue concentration of dye in samples with live or dead fungal biomass [mg/L].The toxicities of samples after decolorization processes were determined using two water organisms: Daphnia magna was used for zootoxicity evaluation (OECD Test No. 202: Daphnia sp. Acute Immobilization Test) and Lemna sp. for phytotoxicity evaluation (OECD Lemna sp. growth inhibition test. No. 221). All toxicity tests were conducted four times. Based on these results, the acute toxicity unit (TUa) was calculated (formula (2)), and the toxicity class was established.
EC50 is the Effective Concentration of a wastewater sample that causes inhibition of the test organism by 50%. According to the Final Report of Commissions of the European Communities ACE 89/BE 2/D3, the value TUa<0.4 means the sample is nontoxic (I class), the value 0.4≤TUa<1.0 means the sample is characterized by low toxicity (II class), the value 1.0≤TUa<10 means the sample is toxic (III class), the value 10≤TUa≤100 means the sample is characterized by high toxicity (IV class) and the value TUa>100 means the sample is extremely toxic (V class).
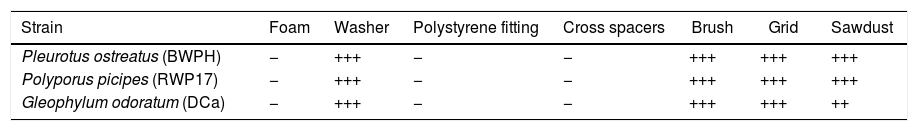
ResultsImmobilization experimentThe results of colonization of tested materials by P. ostreatus (BWPH), P. picipes (RWP17) and G. odoratum (DCa) are presented in Table 1. Growth of the tested strains was observed only on the washer, brush, grid and sawdust. The most intensive growth was observed on the washer and brush. The fungal biomass covered the whole surface of these materials. In samples with sawdust, growth of biomass was only on the top surface of the material.
Growth of fungia on materials used for biomass immobilization after 120h of the experiment.
| Strain | Foam | Washer | Polystyrene fitting | Cross spacers | Brush | Grid | Sawdust |
|---|---|---|---|---|---|---|---|
| Pleurotus ostreatus (BWPH) | − | +++ | − | − | +++ | +++ | +++ |
| Polyporus picipes (RWP17) | − | +++ | − | − | +++ | +++ | +++ |
| Gleophylum odoratum (DCa) | − | +++ | − | − | +++ | +++ | ++ |
Next, we estimated the sorption of used dyes by the chosen solid supports. (Table 2). In general, brilliant green was better adsorbed on tested supports than Evans blue. Sawdust adsorbed 82.49% of the brilliant green and 19.01% of the Evans blue. High adsorption of BG was observed also for the grid (28.44%). The other materials adsorbed less than 10% of both tested dyes. In the case of the brush, an increase in the color of the sample was observed.
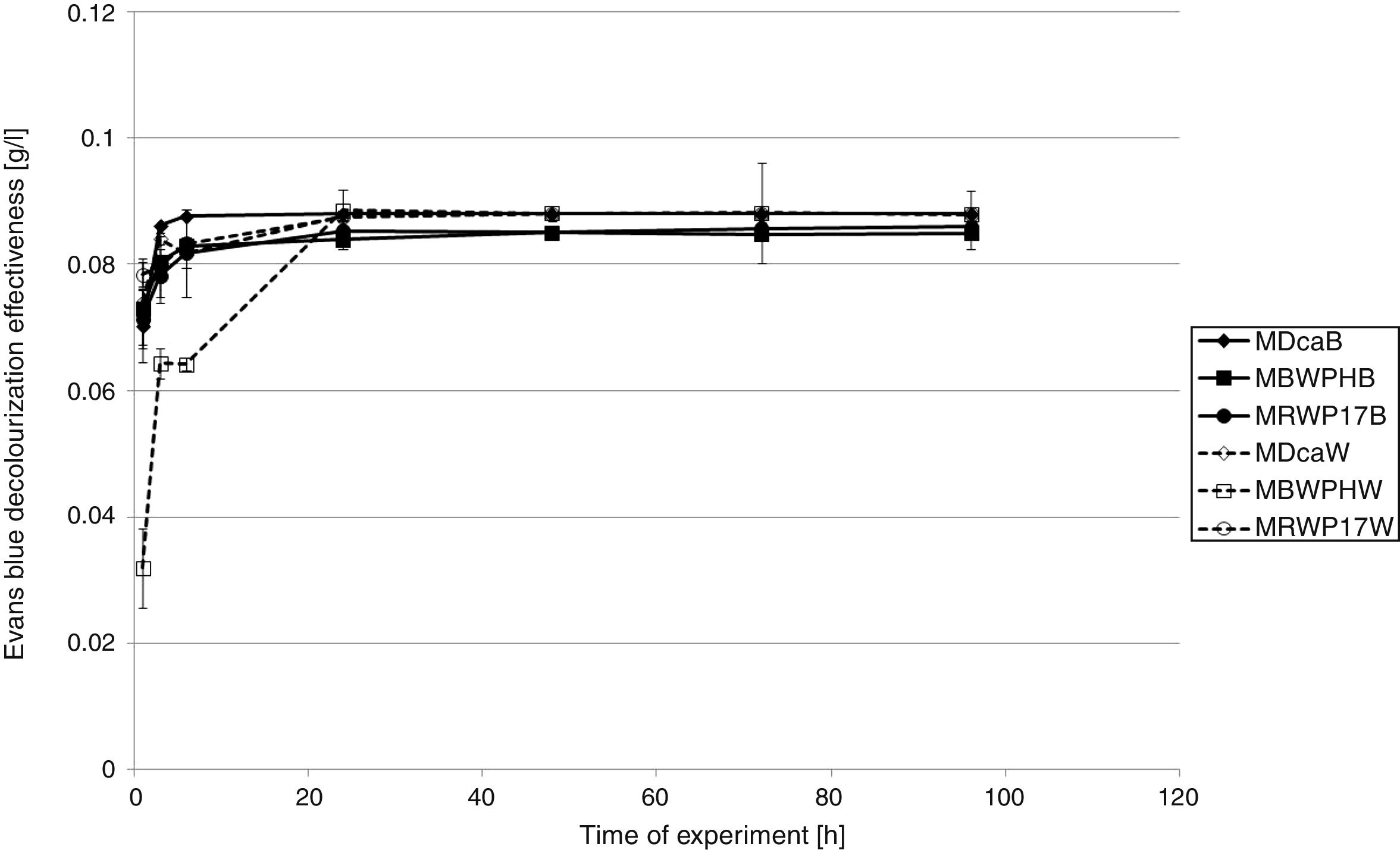
Decolorization experimentThe best result for removal of EB after the 1st hour of the experiment was reached for P. picipes (RWP17) immobilized on the washer (0.059g/L) (Fig. 1). The less effective result was observed for G. odoratum (DCa) immobilized on the brush (no removal). After 24h of the experiment, all strains used immobilized on the washer and P. ostreatus (BWPH) immobilized on the brush removed all color from the samples (0.087g/L). After 48h, P. picipes (RWP17) immobilized on the brush removed all color. All strains completely decolorized samples with EB after 96h, except for P. ostreatus (BWPH) immobilized on the washer. In the sample with P. ostreatus (BWPH) strain immobilized on the washer after 72h, desorption of dye was observed. Dead biomass effectively adsorbed EB from the beginning of the experiment (Fig. 2). After 24h almost all color was removed in samples with biomass of all strains immobilized on washer and brush.
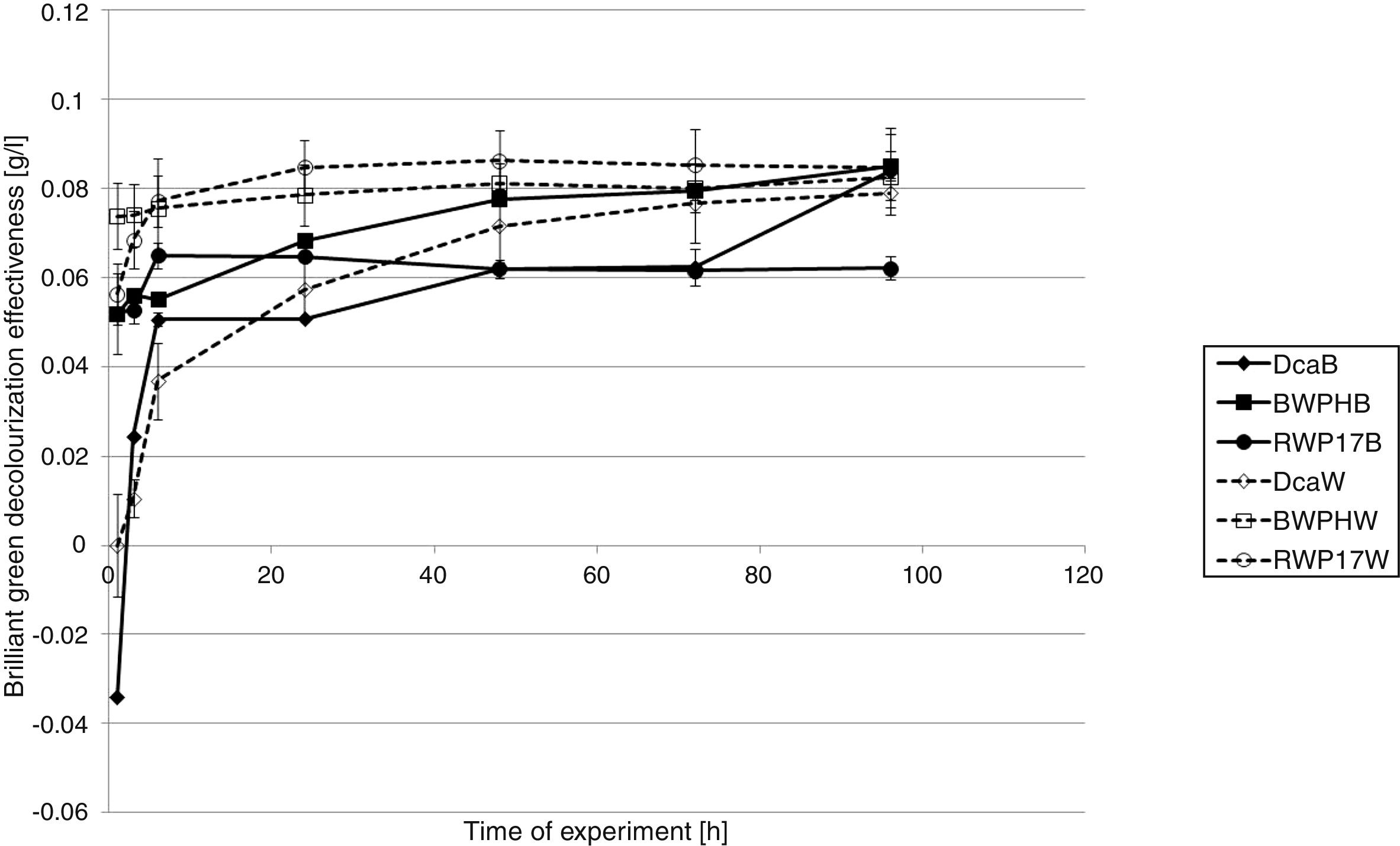
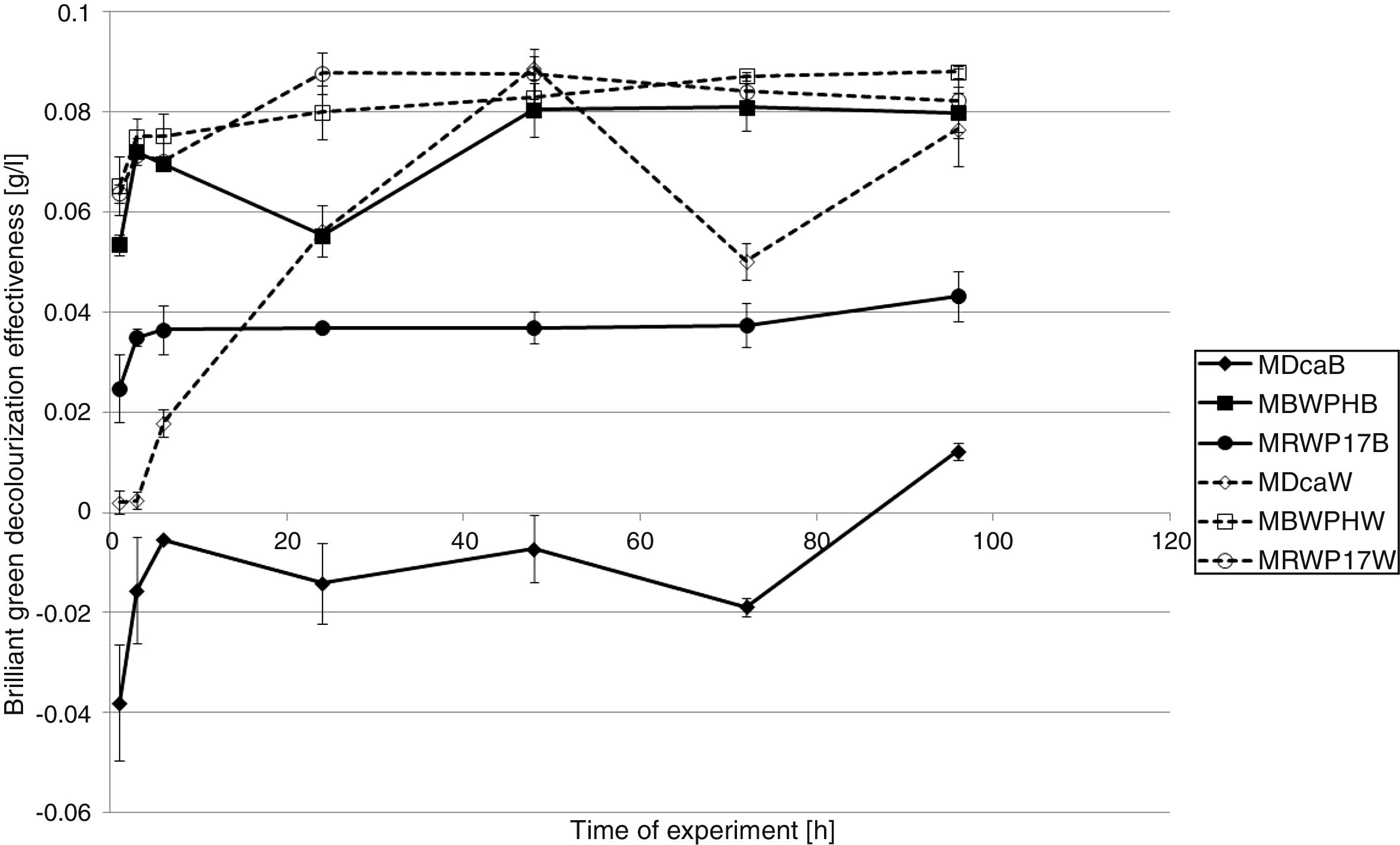
The best results of BG removal were reached in samples with the washer as a support (Fig. 3). Even 0.07g/L of brilliant green was removed after the 1st hour of the experiment (P. ostreatus strain BWPH immobilized on the washer). After 6h, P. ostreatus (strains BWPH) and P. picipes (RWP17) immobilized on the washer removed approximately 0.076g/L of the used dye. After 24h in these samples, almost complete decolorization was reached (0.078 and 0.084g/L, respectively). At the same time, these strains immobilized on the brush removed 0.064 and 0.068g/L, respectively. Finally, all strains immobilized on the washer removed approximately 0.085g/L of BG. P. ostreatus (BWPH) and G. odoratum (DCa) immobilized on the brush also removed approximately 0.085g/L. The worst results were obtained for biomass of P. picipes (RWP17) immobilized on the brush (0.062g/L). Different results were reached in samples with dead biomass (Fig. 4). Only in the case of P. ostreatus (strains BWPH) and P. picipes (RWP17) immobilized on the washer were the results of BG removal comparable for living and dead biomass (approximately 0.08g/L). Slight adsorption was observed for G. odoratum (DCa) immobilized on the brush (only 0.012g/L). Better results for this strain were achieved when biomass was immobilized on the washer. However, in both types of samples, in the 72nd hour of the experiment, we observed desorption. P. picipes (RWP17), which was very effective when biomass was immobilized on the washer, in samples with the brush adsorbed only approximately 0.04g/L of BG.
The results of the toxicity tests are presented in Table 3. Removal of Evans blue by almost all immobilized fungal strains leads to decreases in the zootoxicity and phytotoxicity of the culture solution after the decolorization process. In the case of P. ostreatus (BWPH) immobilized on the brush, samples after decolorization were classified by a zootoxicity test as toxic (III class of toxicity) when the control was extremely toxic (V class of toxicity). Samples with P. ostreatus (BWPH) immobilized on the washer were more toxic (IV/V class of toxicity). Differences between living and dead biomass were observed for modifications with the washer. Samples with living biomass were classified as extremely toxic, like controls, but their TUa value was much lower (169.22 in the control and 108.8 in the sample with BWPH). Samples with dead biomass were very toxic, but the TUa value was 99.1. All samples with G. odoratum (DCa) and P. picipes (RWP17), regardless of the solid support, were classified as very toxic (IV class of toxicity). Slight differences between living biomass and dead biomass were observed for both strains. Similar to that observed in the zootoxicity test, a decrease in phytotoxicity was observed in all samples. Controls with EB were classified as toxic (brush) and as having low toxicity (washer). Samples with living and dead biomass immobilized on the brush were classified as having low toxicity (II class) and samples on the washer were classified as non-toxic (I class).
Zoo- and phytotoxicity of samples with Pleurotus ostreatus (BWPH), Gleophyllum odoratum (DCa) and Polyporus picipes (RWP17) immobilized on different solid supports after 96h of decolorization.
| Strain | Type of biomass | Material used for immobilization | Zootoxicity (Daphnia magna) of samples with Evans blue – toxicity class (TUa) | Zootoxicity (Daphnia magna) of samples with brilliant green – toxicity class (TUa) | phytotoxicity (Lemna sp.) of samples with Evans blue – toxicity class (TUa) | phytotoxicity (Lemna sp.) of samples with brilliant green – toxicity class (TUa) |
|---|---|---|---|---|---|---|
| BWPH | Living | Brush | III (4.6) | IV (13.0) | II (0.67) | II (0.88) |
| Dead | Brush | III (9.9) | V (100.0) | II (0.65) | II (0.98) | |
| DCa | Living | Brush | IV (23.8) | IV (58.8) | II (0.62) | II (0.64) |
| Dead | Brush | IV (32.3) | IV (83.8) | II (0.54) | II (0.59) | |
| RWP17 | Living | Brush | IV (37.0) | IV (52.4) | II (0.42) | II (0.51) |
| Dead | Brush | IV (58.8) | IV (59.6) | II (0.64) | II (0.88) | |
| BWPH | Living | Washer | V (108.8) | V (120.7) | I (0.12) | I (0.14) |
| Dead | Washer | IV (99.1) | V (160.9) | I (0.32) | I (0.34) | |
| DCa | Living | Washer | IV (16.0) | V (144.1) | I (0.27) | I (0.36) |
| Dead | Washer | IV (29.6) | V (190.8) | I (0.28) | I (0.39) | |
| RWP17 | Living | Washer | IV (29.7) | IV (16.0) | I (0.08) | I (0.10) |
| Dead | Washer | IV (31.3) | IV (16.2) | I (0.22) | I (0.22) | |
| Controls with dyes | Brush | V (169.22) | V (189.31) | III (7.8) | III (9.9) | |
| Washer | V (114.48) | V (150.4) | II (0.86) | II (0.98) | ||
Controls with brilliant green were also extremely toxic to D. magna. Similar to that observed for EB toxicity, controls with the brush were more toxic than controls with the washer. A decrease in brilliant green zootoxicity was observed for P. picipes (RWP17) regardless of solid support (IV class of toxicity) in G. odoratum (DCa) in samples with the brush, and the same was observed for P. ostreatus strain BWPH (but only in samples with living biomass). The test with Lemna sp. classified controls with BG and the washer as having low toxicity (II class) and with the brush as toxic (III class). A decrease in phytotoxicity was observed for all modifications. Slight differences in the TUa value were observed that were higher in samples with dead biomass than in samples with living biomass. Samples with strains immobilized on the brush, as observed in the case of EB, showed low toxicity (II class), and samples with biomass immobilized on the washer were not toxic (I class).
DiscussionImmobilization experimentThe positive influence of microbial immobilization on the effectiveness of biological decolorization processes has been emphasized by Kim et al.,33 Nakamura et al.,34 Yesilada et al.,5 Rodriguez-Couto,32 Gao et al.35 and Castillo-Carvajal et al.36 Not all synthetic or natural materials may be colonized by microorganisms. The best growths of the tested fungal strains were observed on the washer, brush and grid used under plaster in buildings (Table 1). Another support that was also very well colonized by the strains was the straw, but in the case of this carrier, high adsorption of dyes was observed (Table 2).
High capacities of dye adsorption on different natural materials were widely presented. Different wastes from agriculture were used by Gao et al.42 and Kurniawan et al.,43 wastes from the fishing industry by Piccin et al.,44 chitosan by Dotto and Pinto45 and McKay et al.,46 and sawdust by Asfour et al.47 and Garg et al.48 In the study conducted by Namasivayam et al.,49 it was demonstrated that different acid dyes (acid violet and acid brillant blue) as well as rhodamine-B and methyl blue may be effectively adsorbed by coconut mesocarp (even 95%). Efficiency of adsorption was strictly associated with the pH of solution and type of dye.49 A similar kind of waste was also used by Vieira et al.50 High removal efficacy was reached in the cases of Blue Remazol R160 (BR 160), Rubi S2G (R S2G), Red Remazol 5R (RR 5), Violet Remazol 5R (VR 5) and Indanthrene Olive Green (IOG). It was shown that even 10.0g of coconut mesocarp as a waste material was enough to absorb dyes from one liter of wastewater. Namasivayam et al. demonstrated that orange peel may effectively absorb Kongo red, procion orange and rhodamine-B. The first dye was removed at 76.6% in pH 5, rhodamine-B at 67% and Procion Orange at 49% in pH 3.51 Orange peel was also used by Sivaraj et al.,52 and as suggested by the authors, it may be reused.
The present results showed better adsorption of brilliant green than Evans blue, which was associated with the different structures of the tested dyes. They are representatives of different classes of dyes. As mentioned above, the sawdust adsorbed 82.49% of BG, and EB was adsorbed at almost 20%. Such high adsorption on this material prevented sawdust from being considered for further studies. High adsorption of BG was also observed for the grid (∼28%), and therefore, we decided to use two other materials that adsorbed less than 10% of both dyes in further studies. In the case of the brush, an increase in the color of liquid samples was observed. We suppose that this may be associated with an interaction between metals released from the brush and an interaction with used dyes. The confirmation of such an interaction may be observed in the results of the toxicity tests, where the EC50 values in the phytotoxicity test were always higher in the samples with brush (Table 3). The aim of the test on different materials was to find solid supports which allowed for intensive growth of fungal biomass and were characterized by low adsorption of dyes. Materials that met the established criteria were the polypropylene washer and brush.
Decolorization experimentThe differences in decolorization efficiencies of both dyes were observed for all tested strains (Figs. 1–4). In the case of Evans blue (Figs. 1 and 2) after the 1st hour of the experiment, the best results of removal of EB were obtained in the sample with living biomass of P. picipes (RWP17) immobilized on the washer (0.059g/L), and the worst was observed in the case of G. odoratum (DCa) immobilized on the brush. Such results suggest that P. picipes (RWP17) has a high adsorption capacity for these dyes. During the study, in the case of this strain, we did not observe desorption of dye and coloration of biomass, suggesting that biological transformation also takes part in the process and was the next step after adsorption. Confirmation of such a statement may be found in the results of decolorization reached after 24h, when in all samples with biomass immobilized on the washer, removal of EB was at the level 0.087g/L. Complete decolorization of samples with this dye was reached finally after 96h. The exception was samples with P. ostreatus (BWPH) immobilized on the washer. In these samples, desorption of dye was observed from 72h of the experiment.
Adsorption as the main process in the decolorization of EB is consistent with the results of the test with dead biomass (Fig. 2), which effectively adsorbed EB from the beginning of experiment. After 24h, almost all color was removed in all modifications for all used strains. No desorption was observed in any sample. However, the results of this test did not provide complete information about the process. The results in samples with dead biomass are even better than those in samples with living biomass. It is important to acknowledge that preparation of dead biomass is associated with thermal processes that may change the biomass properties due to changes in chemical composition of the cell wall and cell membrane as well as their physical properties. It has been demonstrated previously that in some cases, thermal processes may have an influence on the rate of dye adsorption and may improve the sorption properties of biomass.26,41
As in the case of EB, the best results of BG removal were reached also in samples with the washer as a support for the biomass (Fig. 3). P. ostreatus (BWPH) immobilized on the washer removed 0.07g/L of brilliant green after the 1st hour of the experiment, which suggests that adsorption may be a main mechanism through which this strain removes BG. In the case of other strains, the results were worse. After 24h, almost complete decolorization was reached in samples with biomass of strains P. ostreatus (BWPH) and P. picipes (RWP17) immobilized on the washer (0.078 and 0.084g/L, respectively). At the same time, the results in samples with mycelium immobilized on the brush were about ∼0.01g/L worse than those in samples with the washer. Finally, all strains immobilized on the washer removed approximately 0.085g/L of BG, the same as P. ostreatus (BWPH) and G. odoratum (DCa) immobilized on the brush. Different results were observed in samples with dead biomass (Fig. 4). The comparable efficiency of dye removal was reached only in the case of dead biomass of strains P. ostreatus (BWPH) and P. picipes (RWP17) immobilized on the washer. When the brush was used as a support for P. picipes (RWP17), removal in samples with dead biomass was much lower than in samples with living biomass. No apparent desorption process or discoloration of the biomass during the process suggests that an enzymatic mechanism plays a major role in the removal of this dye. Slight adsorption observed for G. odoratum (DCa) immobilized on the brush suggests that the living biomass of this strain may also transform BG.
A positive influence of immobilization of fungal biomass on dye removal effectiveness was demonstrated. Iqbal and Saeed53 used the biomass of Phanerochaete sp. immobilized on a natural sponge loofa and obtained better results for the removal of used dyes than in the case of using biomass suspended in media.53 Nilsson et al.54 showed better removal of reactive azo dye 4 and reactive azo dye 2 with biomass of T. versicolor immobilized on a natural sponge. They reached a 70% reduction in color after 3 days of the experiment.54 Neelamegan et al.55 removed 90–95% of dyes when biomass was immobilized on rice straw. Dominguez et al.56 reported removals of 95% of indigotine and 69% of phenol red after 24h of contact of dyes with biomass of Trametes hirsuta immobilized on alginate.56
Karimi et al.57 indicated that P. chrysosporium immobilized on Kissiris may remove all methyl blue. They showed that immobilization was associated with an increase in manganese peroxidase activity. An increase in enzymatic activity during immobilization was also observed by Rodriquez et al.24 and Casieri et al.58 Both teams used fungi from genera Pleurotus. Immobilization has the most critical influence on the effectiveness of dye removal by non-ligninolytic fungi. Tišma et al.59 used Aspergillus ochraceus immobilized on wastes from the food industry. They observed production of different exo-enzymes that transformed different dyes.59 Decolorization of Evans blue and Brilliant green by G. odoratum (DCa) was analyzed previously in the case of suspended biomass. The biomass of this strain was not very effective in the removal of both dyes. The adsorption properties were also low.41 Immobilization of biomass improved the decolorization properties of this strain. Similar results may be observed in the case of P. picipes (RWP17). In previous studies, almost complete removal of the dye mixture was reached after 96h by suspended biomass,60 where in this study it was observed after 24h for Evans blue when biomass of P. picipes (RWP17) was immobilized.
Ecotoxicity testsThere is still limited information about the influence of decolorization processes’ end products on water ecosystems. The few studies about this topic are mostly concentrated on terrestrial plants.60–63 Only in a few studies water plants were used. Casieri et al.58 tested phytotoxicity of decolorization products with Lemna minor, as we presented above. Zootoxicity is more frequently evaluated. The most frequently used organism in this case is that used in the present study, i.e. D. magna. The results of zootoxicity tests with D. magna were presented by Elisangela et al.64 and Porri et al.65 A decrease in the toxicity of dye solutions after fungal decolorization processes has been observed by many authors.58,60,66 Zhuo et al.67 showed that decolorization of textile dyes by Ganoderma sp. En3 reduced the negative influence of dyes on plant germination as well as on root and stem growth. T. pubescens was used by Anastasi et al.68 A bioreactor prepared by these scientists effectively decolorized dyes and significantly reduced toxicity of textile effluents. Similar results were achieved by Shedbalkar et al.69 when Penicillium ochrochloron was used for removal of Cotton blue. A significant reduction in toxicity by Triticum aestivum and Ervum lens was presented by these authors. Another filamentous fungus (Fusarium oxysporum) was used by Porri et al.65 A reduction in GAD-4 toxicity in D. magna after the decolorization process was indicated. Casieri et al.58 found a reduction in dye phytotoxicity for Lemna sp. when Trametes pubescens and P. ostreatus were used in the decolorization processes. We observed the same phenomenon as Casieri et al.,58 in that there was no correlation between the effectiveness of dye removal and a toxic effect, which may be associated with production of different toxic metabolites of dye biotransformation.
The results of the toxicity tests are presented in Table 3. Both dyes used in the experiment were extremely toxic to D. magna and toxic for Lemna sp. Removal of Evans blue by almost all immobilized strains was associated with a decrease in zootoxicity as well as phytotoxicity. In the case of the test with D. magna, extreme toxicity indicated for control samples was reduced by P. ostreatus (strain BWPH) immobilized on the brush to III class toxicity. Samples with P. ostreatus (BWPH) immobilized on the washer were more toxic. We observed differences between living and dead biomass immobilized on the washer. Extreme toxicity (V class of toxicity) was observed in samples with living biomass of this strain, where samples with dead biomass were very toxic (IV class of toxicity). This may suggest that the process of biological transformation may be associated with the production of toxic metabolites, but it should be noted that in spite of the classification of samples to different toxicity classes, the differences in TUa value in both types of samples were not significant (108.8 in sample with living biomass and 99.1 in samples with dead biomass). A decrease in zootoxicity was observed in all samples with G. odoratum (DCa) and P. picipes (RWP17), regardless of the solid support used. In these cases, the observed differences in toxicity between living biomass and dead biomass were slight.
A decrease in brilliant green zootoxicity was observed only in some samples, mostly when the biomass was immobilized on the brush. In these samples, removal was lower than that in samples with the washer. The same tendency was observed in the case of the results of the phytotoxicity test. Differences in TUa value observed between samples with living and dead biomass suggest that the process of BR removal was mostly biological and that the metabolites are less toxic to the tested organisms than the dye.
Both the living biomass as well as dead biomass of all strains used for decolorization of EB reduced phytotoxicity of solution of this dye. The class of toxicity was reduced from III to II or I. The results of the phytotoxicity test for both tested dyes are very important. Controls with EB and the brush were classified as toxic, and those on the washer as having low toxicity, which suggests that the dye reacts with that brush or that some substances from brush were released into solution, e.g., during thermal preparation of the samples. After decolorization, we observed the same tendency. Samples with living and dead biomass immobilized on the brush were classified as having low toxicity, and samples on the washer were classified as non-toxic. Such results confirm the suggestion above that the brush releases some substances, probably metals, that enhance toxicity. Confirmation of such a phenomenon may be observed in the TUa values of the control samples in the zootoxicity test, which are higher in the control with the brush. Similar to the case of EB for controls with the brush and BG, control samples with the brush and the dye were more toxic than controls with the washer and the dye. Such results confirm the release of some substances from the brush and their possible interaction with dyes.
It should be mentioned that there was no correlation among the effectiveness of decolorization by dead biomass and zoo- and phytotoxicity. Complete removal of Evans blue by dead biomass was associated with a decrease in toxicity to IV class in tests with D. magna, and in the case of P. ostreatus (BWPH) immobilized on the brush to III class. The results are similar to those reached in the sample with living biomass. There is only a difference in the case of P. ostreatus (BWPH) immobilized on the washer.
ConclusionsDifferent natural and synthetic solid supports may be used for immobilization of fungal biomass. Among the different tested supports the best growth of P. picipes (RWP17), P. ostreatus (BWPH) and G. odoratum (DCa) was observed on the grid, sawdust, brush and polypropylene washer. Because the supports may take part in the decolorization process, the level of adsorption on them was estimated for both tested dyes. The sawdust and grid intensively adsorbed brilliant green (>82% and >19%, respectively) and were eliminated from further study. Adsorptions of both dyes on the washer were negligible, and the brush changed the color of the sample to a more intense color. These two materials, as materials with low adsorption capacity, were used for immobilization of fungal biomass. The result after 24h of the experiment was almost complete removal of Evans blue observed in samples with biomass immobilized on the washer. Samples with biomass immobilized on the brush needed more time for complete dye removal. Also in samples with brilliant green, in the first hours, decolorization was faster in samples with biomass immobilized on the washer. Finally, after 96h, approximately 0.85g/L of both dyes was eliminated from the samples. The type of solid support had a significant influence on the results of the toxicity tests. Reductions in zoo- and phytotoxicity were observed in all samples with fungal biomass. Phytotoxicity was completely eliminated in all samples with fungal biomass immobilized on the washer. Zootoxicity was also reduced from a V to III/IV class according to the modifications used in the experiment.
According to the results of the phytotoxicity test, where samples with the brush were classified into a higher toxicity class, we suggest that the washer was the best solid support for biomass immobilization and for quick decolorization. Among all tested strains, the strain P. picipes (RWP17) is the most promising in the dye removal processes. The decolorization process by this strain may reduce the color and toxicity of different dyes.
Conflicts of interestThe authors declare no conflicts of interest.
This research was supported by the Ministry of Science and Higher Education grant (2007–2010) research project number N523 17 85 33 and co-financed by BK-217/RIE-8/2016.