The main objective of this study was to demonstrate the antimicrobial potential of the crude extract and fractions of Chenopodium ambrosioides L., popularly known as Santa-Maria herb, against microorganisms of clinical interest by the microdilution technique, and also to show the chromatographic profile of the phenolic compounds in the species. The Phytochemical screening revealed the presence of cardiotonic, anthraquinone, alkaloids, tannins and flavonoids. The analysis by HPLC–DAD revealed the presence of rutin in the crude extract (12.5±0.20mg/g), ethyl acetate (16.5±0.37mg/g) and n-butanol (8.85±0.11mg/g), whereas quercetin and chrysin were quantified in chloroform fraction (1.95±0.04 and 1.04±0.01mg/g), respectively. The most promising results were obtained with the ethyl acetate fraction, which inhibited a greater number of microorganisms and presented the lowest values of MIC against Staphylococcus aureus and Enterococcus faecalis (MIC=0.42mg/mL), Pseudomonas aeruginosa (MIC=34.37mg/mL), Paenibacillus apiarus (MIC=4.29mg/mL) and Paenibacillus thiaminolyticus (MIC=4.29mg/mL). Considering mycobacterial inhibition, the best results were obtained by chloroform fraction against M. tuberculosis, M. smegmatis, and M. avium (MIC ranging from 156.25 to 625μg/mL). This study proves, in part, that the popular use of C. ambrosioides L. can be an effective and sustainable alternative for the prevention and treatment of diseases caused by various infectious agents.
The World Health Organization (WHO) recommends worldwide development of research on medicinal plants for therapeutic purposes, in order to obtain new possibilities for the treatment of diseases, especially in developing countries,1 considering also, the hope to identify new substances of medicinal character that may serve as raw materials for pharmaceuticals industries. In Brazil the use of plants is very common for the treatment of various diseases, including bacterial and fungal infections, and many studies are conducted to detect secondary metabolites in plants with antimicrobial properties as an attempt to find new antimicrobial or antifungal compounds.2–4 Indeed, because of the increasing bacterial resistance, there is a need to search for new antimicrobial substances from alternative sources, such as plants used in folk medicine. Other important subjects to study are mycobacterial infections, including those caused by Mycobacterium tuberculosis, since tuberculosis caused 8 million new cases and 1.8 million fatalities per annum worldwide.5,6
Chenopodium ambrosioides L. (Dysphania ambrosioides L.) is to current synonymy of the specie belongs to the Amaranthaceae family and is popularly known as “erva-de-santa-maria”. This plant is native from South America and it is widely distributed throughout Brazil, being often used in folk medicine as antirheumatic, anti-inflammatory, antipyretic, antihelmintic, antifungal, anti-ulcer and for the treatment of wounds.7,8 The ointment is prepared with the essential oil of C. ambrosioides inhibited, the growth of dermathophytes and some other fungi.9,10 In 2009, the specie was added to the list of National Medicinal Plants of Interest in Brazil, (SUS RENISUS-BRAZIL) highlighting the need to increase the understanding of the mechanisms behind its medicinal properties.11 Therefore, it is of great importance to conduct experiments in such species, to identify their phytochemical compounds with pharmacological potential and to identify possible alternatives for antimicrobial therapy, since studies using crude extracts and fractions of C. ambrosioides L. are scarce.
Materials and methodsReagentsAll chemicals were of analytical grade. Solvents for the extractions and analytical procedures and, phytochemical screening were purchased from Merck (Darmstadt, Germany). The quercetin, chrysin and, rutin standards were purchased from Sigma–Aldrich (St. Louis, MO, USA). Microbiological assays were performed using Mueller-Hinton Agar and Mueller-Hinton Broth from Sigma–Aldrich.
Plant collectionThe leaves of C. ambrosioides L. were collected in the city of Uruguaiana, from the State of Rio Grande do Sul, Brazil (altitude 57m, 29° latitude, 45′20, 12″S, longitude 57°4′0.28″W) in January of 2013. The exsiccate was archived as specimen in the herbarium of the Departament of Biology at the Federal University of Santa Maria, under the register number of SMDB 137015, for future reference.
Extraction of the plant leavesPlant material was dried at room temperature and powered in a knife mill. The leaves of the plant (500g) were macerated at room temperature with ethanol 70% and subjected to a daily shake-up for a week. The extract was collected and filtered and the remaining plant material was subjected to more extraction. After filtration, the hydroalcoholic extract was evaporated under reduced pressure in a rotary evaporator to remove the ethanol. A share of this hydroalcoholic extract was dried in a stove (temperature below 40°C) yielding the dried crude extract. The remaining of the hydroalcoholic extract was partitioned with solvents of increasing polarity: chloroform, ethyl acetate and n-butanol. Lastly, the fractions obtained were fully dried in a rotary evaporator.
Phytochemical screeningThe phytochemical screening followed a series of characterization reactions which used hydroalcoholic extract, according to.12,13 The identification of alkaloids was performed using Dragendorff reagent, the technique is based on the formation of precipitates after the addition of the reagent, which demonstrates the presence these metabolites. The flavonoids were identified using the reaction with aluminum chloride, filter paper strips were moistened with hydroalcoholic extract, after was added one drop of 5% AlCl3 solution, the presence of flavonoids was revealed by intensification of the yellowish green color on fluorescence. The tannins were identified through of the reaction that used ferric chloride, in which drops of 1% FeCl3 solution in methanol were added to 2mL of hydroalcoholic extract, the blue color indicated the presence of tannins. The anthraquinone compounds were identified by Bornträger reaction. Briefly, was added small fragment of the drug (about 0.2g) in a test tube and added 5mL of diluted NH4OH solution, the reaction was characterized as positive by pink or reddish coloration. The cardiotonic heterosides were characterized through of the Liebermann-Buchard reaction where acetic anhydride and sulfuric acid were added together to a part of the hydroalcoholic extract. The green coloration characterized the presence of steroidal nucleus, found in cardiotonics compounds.
HPLC–DAD analysis of polyphenols compoundsThe analysis were performed with a Prominence Auto-Sampler (SIL-20A) equipped with Shimadzu LC-20AT (Shimadzu, Kyoto, Japan) reciprocating pumps connected to a DGU-20A5 degasser and CBM-20A integrator. UV-VIS detector DAD SPD-M20A and Software LC solution 1.22 SP1 were used. Reverse phase chromatography analyses were carried out with a Phenomenex C-18 column (4.6mm×250mm) packed with 5μm diameter particles, volume injection was 40μL, and the gradient elution was conducted with slight modifications in the method.14 The mobile phase consists of water containing 2.0% acetic acid (phase A) and methanol (phase B), and the elution gradient was 2min to achieve 5% of B, and changed to obtain 25%, 40%, 50%, 60%, 70% and 100% B at 10, 20, 30, 40, 50 and 70min, respectively. The UV absorption spectra of the standards as well as the samples were recorded in the range of 230–400nm. Stock solutions of standards were prepared in methanol in the range of 0.0025–0.060mg/mL. Samples and standards solutions as well as the mobile phase were degassed and filtered through 0.45μm membrane filter (Millipore). Chromatographic operations were carried out at ambient temperature (22°C) and in triplicate. The identification of the compounds was done by comparing its retention time and UV absorption spectrum with the standards.
Antimicrobial potentialSample preparationThe sample for the microbiological tests was prepared from the raw extract of C. ambrosioides L. at the concentration of 275mg/mL mixed in a vortex mixer and totally dissolved in DMSO with the aid of ultrasound.
Microorganisms and preparation of inoculumsThe following microorganisms used in the evaluation were obtained from the American Type Culture Colletion (ATCC): Bacillus cereus (ATCC 9634), Listeria monocytogenes (ATCC 7644), Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 35218), Pseudomonas aeruginosa (ATCC 340), Salmonella choleraesuis (ATCC 10708), Candida albicans (ATCC 90028). Clinical and environmental isolates provided by the Department of Microbiology, from the Universidade Franciscana (UNIFRA), were also used to test the antimicrobial activity of C. ambrosioides L. extract and fractions: Paenibacillus thiaminolyticus, Paenibacillus pabuli, Paenibacillus azotofixans, Paenibacillus allginolyticus, Paenibacillus validus, Saccharomyces sp., Staphylococcus saprophyticus, Salmonella enteritidis, Citrobacter freundii, Klebsiella pneumoniae, Streptococcus sp. All isolates were maintained on nutrient agar slants at 4°C. The inoculum of the tested microorganisms was prepared according to the guidelines of the Clinical and Laboratory Standards Institute,15 adjusted to 0.5 Mc Farland standard.
Disk diffusion test and determination of minimal inhibitory concentrations (MICs)The disk diffusion test was chosen as the screening test, following CLSI parameters, aiming the selection of sensitive microorganisms to the extract and fractions from the leaves of C. ambrosioides L. The minimum inhibitory concentrations (MICs) were determined by the microdilution technique, using Mueller–Hinton broth (Difco). The assay was carried out in 96-well microtitre plates. Each extract was mixed with an inoculum prepared in the same medium at a density adjusted per tube to 0.5 of the McFarland scale (1.5×108CFU/mL) and the active extracts/fractions were diluted twofold serially ranging. Microtitre trays were incubated at 37°C and the MICs were recorded after 24h of incubation. The MIC was defined as the lowest concentration of extract that inhibited bacterial growth. This test was performed in triplicate. The 2,3,5-triphenyltetrazolium chloride was used as an indicator of microbial growth.
Antimicrobial assay on MycobacteriumAntimycobacterial activity was evaluated against Mycobacterium avium LR541CDC, Mycobacterium tuberculosis H37Rv (ATCC 25618) and Mycobacterium smegmatis mc2 155 (ATCC 700084). The suspensions were standardized through the range 0.5 Mac Farland scale and then diluted with Middlebrook 7H9 (MD7H9), supplemented with 10% OADC (oleic acid-albumin-dextrose-catalase) and 0.2% glycerol, until the concentration of 105 UFC/mL. Plant extracts, fractions and standard Chrysin were dissolved in DMSO (dimetilsulfoxide), at a concentration of 50.00mg/mL and then diluted in MD7H9 until the desired concentration (2.500μg/mL) for the execution of the test in triplicate. The test was performed by the method of broth microdilution according to CLSI M7-A6. The plates containing M. smegmatis were incubated during 48h, M. avium for 5 days and for M. tuberculosis for 7 days, at 37°C. In order to verify the existence, or not, of microbial growth, MTT dye was used. Following this procedure, it was considered as MIC the lowest concentration of the extract under test capable of inhibiting the growth of the microorganisms used in the assay.
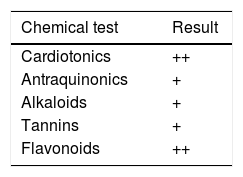
ResultsPhytochemical screeningPhytochemical screening of hydroalcoholic extract from the leaves of C. ambrosioides L. revealed the presence of important secondary metabolites showed in Table 1.
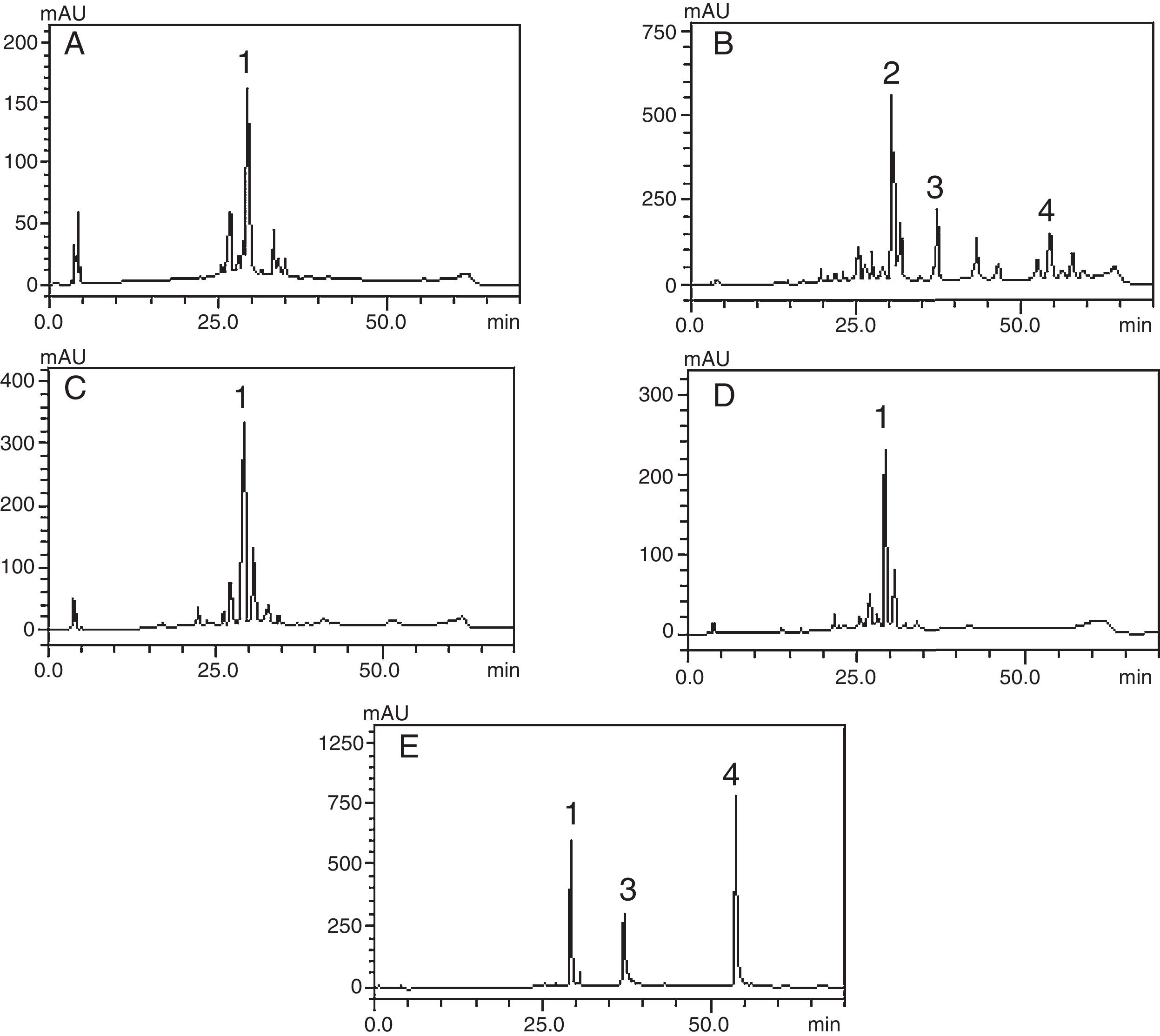
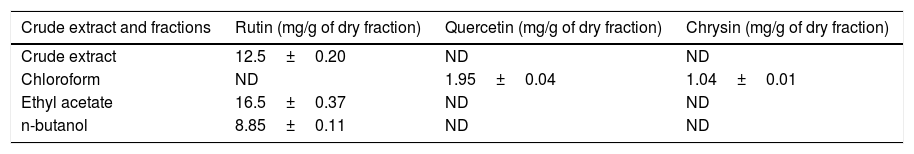
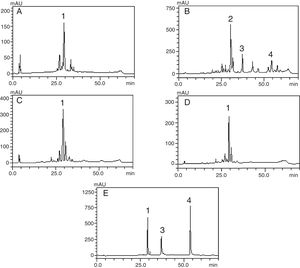
HPLC–DAD analysis of polyphenols compoundsThe crude extract and chloroform, ethyl acetate and butanol fractions from the leaves of C. ambrosioides L. were analyzed by HPLC–DAD. Chromatographic profile of the crude extract, ethyl acetate and butanol fractions presented rutin, chloroform fraction furnished quercetin and chrysin (Fig. 1). Rutin, quercetin and chrysin were quantified by HPLC–DAD, based on the reference standard curves (Table 2), where calibrations curves were: rutin y=72823x−1901 (r=0.9982), quercetin y=187893x−163671 (r=0.9984) and chrysin y=148116x+62256 (r=0.9988).
Phenolic profile of C. ambrosioides leaves: Crude extract (A, λ=365nm), chloroform fraction (B, λ=313nm), ethyl acetate fraction (C, λ=365nm), n-butanol fraction (D, λ=365nm) and standard compounds (e, λ=254nm): rutin (1), unidentified peak (2), quercetin (3) and crhysin (4). Chromatographic conditions described in the experimental section.
Composition of Chenopodium ambrosioides leaves.
| Crude extract and fractions | Rutin (mg/g of dry fraction) | Quercetin (mg/g of dry fraction) | Chrysin (mg/g of dry fraction) |
|---|---|---|---|
| Crude extract | 12.5±0.20 | ND | ND |
| Chloroform | ND | 1.95±0.04 | 1.04±0.01 |
| Ethyl acetate | 16.5±0.37 | ND | ND |
| n-butanol | 8.85±0.11 | ND | ND |
ND, not detected.
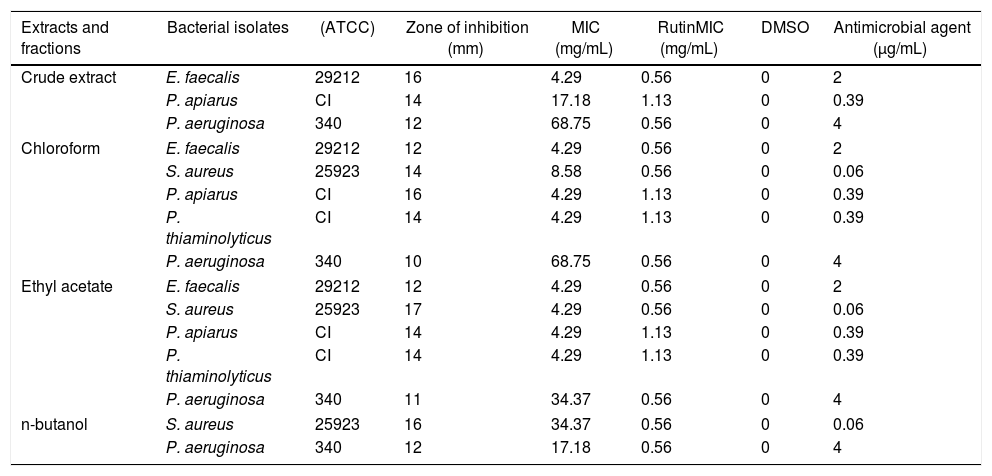
The extract and fractions were tested at a standardized concentration of 275mg/mL and the results are shown in Table 3. All fractions were active against important microorganisms; however, the most promising results were obtained with the ethyl acetate fraction, which inhibited a greater number of microorganisms and presented the lowest values of MIC against the S. aureus, P. aeruginosa, E. faecalis, Paenibacillus apiarus and P. thiaminolyticus (ranging from 4.29 to 34.37mg/mL).
Correlation between the halo of inhibition from each microorganism associated to MIC value and microbial activity of rutin (compound detected from HPLC analysis).
| Extracts and fractions | Bacterial isolates | (ATCC) | Zone of inhibition (mm) | MIC (mg/mL) | RutinMIC (mg/mL) | DMSO | Antimicrobial agent (μg/mL) |
|---|---|---|---|---|---|---|---|
| Crude extract | E. faecalis | 29212 | 16 | 4.29 | 0.56 | 0 | 2 |
| P. apiarus | CI | 14 | 17.18 | 1.13 | 0 | 0.39 | |
| P. aeruginosa | 340 | 12 | 68.75 | 0.56 | 0 | 4 | |
| Chloroform | E. faecalis | 29212 | 12 | 4.29 | 0.56 | 0 | 2 |
| S. aureus | 25923 | 14 | 8.58 | 0.56 | 0 | 0.06 | |
| P. apiarus | CI | 16 | 4.29 | 1.13 | 0 | 0.39 | |
| P. thiaminolyticus | CI | 14 | 4.29 | 1.13 | 0 | 0.39 | |
| P. aeruginosa | 340 | 10 | 68.75 | 0.56 | 0 | 4 | |
| Ethyl acetate | E. faecalis | 29212 | 12 | 4.29 | 0.56 | 0 | 2 |
| S. aureus | 25923 | 17 | 4.29 | 0.56 | 0 | 0.06 | |
| P. apiarus | CI | 14 | 4.29 | 1.13 | 0 | 0.39 | |
| P. thiaminolyticus | CI | 14 | 4.29 | 1.13 | 0 | 0.39 | |
| P. aeruginosa | 340 | 11 | 34.37 | 0.56 | 0 | 4 | |
| n-butanol | S. aureus | 25923 | 16 | 34.37 | 0.56 | 0 | 0.06 |
| P. aeruginosa | 340 | 12 | 17.18 | 0.56 | 0 | 4 | |
CI, clinical isolate.
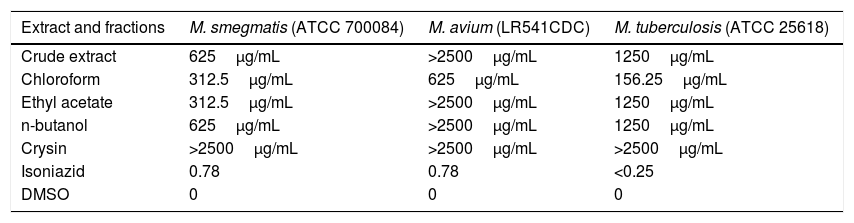
Considering the activities of the extract and fractions against Mycobacterium genus, the best results and the lowest MIC were obtained by chloroform fraction against M. tuberculosis, M. smegmatis, and M. avium (MIC ranging from 156.25 to 625μg/mL, respectively, Table 4).
Minimal inhibition concentration (μg/mL) of crude extract and fractions from the leaves of C. ambrosioides, against M. smegmatis, M. tuberculosis and M. avium.
| Extract and fractions | M. smegmatis (ATCC 700084) | M. avium (LR541CDC) | M. tuberculosis (ATCC 25618) |
|---|---|---|---|
| Crude extract | 625μg/mL | >2500μg/mL | 1250μg/mL |
| Chloroform | 312.5μg/mL | 625μg/mL | 156.25μg/mL |
| Ethyl acetate | 312.5μg/mL | >2500μg/mL | 1250μg/mL |
| n-butanol | 625μg/mL | >2500μg/mL | 1250μg/mL |
| Crysin | >2500μg/mL | >2500μg/mL | >2500μg/mL |
| Isoniazid | 0.78 | 0.78 | <0.25 |
| DMSO | 0 | 0 | 0 |
In 2009, the Brazilian Ministry of Health elaborated the National List of Medicinal Plants of Interest in the Health System (RENISUS), which encourages research on medicinal plants widely used among the Brazilian population. C. ambrosioides L. integrates this list; thus motivating the identification of its compounds with pharmacological potential. Phytochemical screening of C. ambrosioides revealed the presence of cardiotonics and antraquinonics, alkaloids, tannins and flavonoids; these results were similar to those reported by Nedialkova et al.8
The compounds verified by HPLC analysis such as rutin, quercetin and chrysin can be explained by the differences of polarity between the fractions and the compounds, since rutin is a glycoside of quercetin, being more polar than its aglycone. In the same way, sequential extractions with solvents of crescent polarity can provide a selective extraction, based on chemical characteristics of secondary metabolites.16 In a previous study that traced a chromatographic profile of the crude extract from aerial parts (leaves and flowers) of C. ambrosioides L. by HPLC–DAD, trans p-coumaric acid, quercetin dirhamnoside, kaempferol-O-rhamnosyl-glucuronide and rutin were identified and held responsible for the antimicrobial activity verified.17 The present study reports the presence of quercetin and chrysin for the first time in this plant. It is of particular interest the presence of chrysin, which is reported to have several biological activities, including antioxidant, anti-allergic and anti-inflammatory, besides a strong inhibitory effect on PGE2 and TXB2 production in human blood, and this property could be used to search its chemical structure as a lead compound in the development of COX inhibitors.18 The aglycones quercetin and chrysin were quantified only in the chloroform fraction, whereas the rutin was detected and quantified ranging from 4.5 to 8.5 times greater than quercetin in butanolic fraction, crude extract and ethyl acetate fraction, and this last one furnished the largest amount of rutin per gram of dry fraction.
Many studies highlight the potential of natural compounds against pathogens that cause severe infections such as S. aureus, P. aeruginosa, and E. faecalis, this last being considered, one of the most prevalent species cultured from humans; isolation of Enterococcus resistant to multiple antibiotics has become increasingly common in the hospital setting. Consequently, microbial resistance demands new pharmacological alternatives, which led to the testing of the crude extract and fractions from the leaves of C. ambrosioides against some pathogenic microorganisms.
Standard rutin showed the best results against these microorganisms, corroborating the assumption that this glycoside contributes, at least in part, to the antimicrobial activity observed in ethyl acetate fraction of the C. ambrosioides L.; furthermore, rutin is able to increase the antibacterial activity of other compounds.19,20 Commercial standard rutin was tested at a concentration of 4.53mg/mL, since this value is proportional to the concentration of rutin obtained in the fraction that presented the highest antimicrobial activity (ethyl acetate fraction).
Previous studies with C. ambrosioides extracts also revealed antimicrobial potential against pathogens such as S. aureus, P. aeruginosa, Brevibacillus agri, Trichophyton mentagrophytes, C. albicans (MICs ranging from 0.25 to 0.80mg/mL).21 Moreover, the capacity of the plant extract to inhibit M. tuberculosis growth was tested, affording a MIC value of 0.5.mg/mL.22 The aforementioned results are in accordance with the results of the study, in fact the extract from this research exhibited a stronger antimycobacterial activity than the extract presented in the previous work mentioned.
Chromatographic profile of this fraction evidenced the presence of the aglycones quercetin and chrysin. Quercetin possesses antimicrobial properties against Gram-negative and Gram-positive bacteria and shows variable inhibitory activity against mycobacteria.23 In this study, chrysin was evaluated and showed no activity against mycobacteria (Table 4), furnishing a MIC value above the highest tested concentration of 2.500μg/mL. Also, the research group previously tested standard quercetin against M. smegmatis, obtaining MIC=625μg/mL, which indicated that other different compounds might be acting to increase the activity of this fraction. In line with these findings, rutin whose presence was evidenced in crude extract, ethyl acetate and butanol fractions showed a MIC of 625μg/mL when assayed against M. smegmatis4; therefore, it is unlikely that rutin and crisin are the only compounds exerting the observed antimicrobial activity.
The phenolic compounds present in medicinal plants are responsible for a range of biological activities, including antimicrobial action. Flavonoids are a group of heterocyclic organic compounds and constitute an important class of polyphenols. Many biological properties of flavonoids have been reported on their antimicrobial potential. Studies report the antimicrobial potential of phenolic compounds such as rutin, quercetin and chrysin through of the permeation and destabilization of the bacterial membrane, resulting in the disturbance of the proton motive force, the electron flow, coagulation and leakage of the cellular content causing a bactericidal effect.24–26
Crude extract and fractions from C. ambrosioides L. leaves possess important phytochemical compounds (rutin, quercetin and chrysin) which could be related to its popular medicinal use. Additionally, this species proved to be active against pathogens of clinical interest, such as S. aureus, E. faecalis, P. aeruginosa and M. tuberculosis. The herb of Santa Maria is widely used in folk medicine for the most diverse purposes,27 mainly as natural antimicrobial aiding in the process of wound healing, for this reason was inserted in RENISUS.
The results confirm, in part, the popular medicinal use this species, due to antimicrobial activity, especially against cutaneous opportunistic pathogens. In addition, relevant pharmacological activity against strains of M. tuberculosis were showed, contributing, this way, in the elucidation of its use in tuberculosis. Taking together, these results encourage the continuation of our studies aiming to obtain pure bioactive compounds against resistant microorganisms and biofilm formers.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Prof. Dra. Margareth Linde Athayde for the orientation and support to conduct of this work, and Prof. Dr. Renato Aquino Záchia for providing the identification of Chenopodium ambrosioides L. The authors thank the financial support of CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.