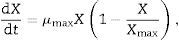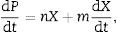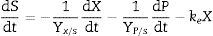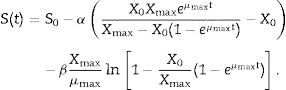Pullulan is a natural exopolysaccharide with many useful characteristics. However, pullulan is more costly than other exopolysaccharides, which limits its effective application. The purpose of this study was to adopt a novel mixed-sugar strategy for maximizing pullulan production, mainly using potato starch hydrolysate as a low-cost substrate for liquid-state fermentation by Aureobasidium pullulans. Based on fermentation kinetics evaluation of pullulan production by A. pullulans 201253, the pullulan production rate of A. pullulans with mixtures of potato starch hydrolysate and sucrose (potato starch hydrolysate:sucrose=80:20) was 0.212h−1, which was significantly higher than those of potato starch hydrolysate alone (0.146h−1) and mixtures of potato starch hydrolysate, glucose, and fructose (potato starch hydrolysate:glucose:fructose=80:10:10, 0.166h−1) with 100gL−1 total carbon source. The results suggest that mixtures of potato starch hydrolysate and sucrose could promote pullulan synthesis and possibly that a small amount of sucrose stimulated the enzyme responsible for pullulan synthesis and promoted effective potato starch hydrolysate conversion effectively. Thus, mixed sugars in potato starch hydrolysate and sucrose fermentation might be a promising alternative for the economical production of pullulan.
Pullulan is an extracellular water-soluble homo-polysaccharide composed of linear maltotriose-units interconnected via α-1,6-glycosidic linkages. These linkages endow pullulan with structural flexibility and high aqueous solubility.1,2 Pullulan is usually biosynthesized by strains of the yeast-like polymorphic fungus Aureobasidium pullulans.3–5 Because of its strictly linear structure, pullulan has been used in applications such as blood plasma substitutes and as an additive for food, adhesives, and cosmetics.1,6 Despite these applications, pullulan is more costly ($25kg−1) than other exopolysaccharides, which is a major limiting factor for its effective application.7
A primary strategy to solve the problem of high production costs is to search for cheap carbon and nitrogen sources, which are nutritionally rich enough to support growth of the microorganism and the production of pullulan.8 Leathers concluded that an agricultural waste product, maize residue, can be used as a carbon source for pullulan production by A. pullulans.1 Other waste products from the agriculture and food industries, such as deproteinized whey, cassava starch residue, beet molasses, sugar cane juice, and sweet potato and peat hydrolysates, have also been considered as economical substrates for pullulan production.8,9
Potato, a cheap and available agricultural product, contains a large amount of starch, which is a suitable feedstock for industrial fermentation. Potato is also a major crop in Shaanxi Province of China. However, low productivity of pullulan fermentation using potato starch as a substrate is a drawback that has not yet been solved.10 Available data show that both the amount of exopolysaccharide produced and the rate of carbohydrate source consumption are clearly influenced by the particular carbohydrate used, and Duan proposed that high pullulan yield is related to high activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase, and glucosyltransferase in A. pullulans Y68 grown on different sugars.11 Based on these considerations, mixed-sugar fermentation might be an efficient fermentation strategy that leads to high productivity by providing more than one substrate.
In our previous research, sucrose was demonstrated to be superior to other sugars, based on pullulan yield, and a mixture of sucrose and potato starch hydrolysate (PSH) was found to significantly enhance the pullulan formation rate of A. pullulans 201253 when using a carbon source in a flask. Finally, based on fermentation kinetics evaluation for pullulan formation by A. pullulans 201253, the model parameters associated with polysaccharide formation (m, n, and (dP/dt)/X) and substrate consumption α, β and (dS/dt)/X were assayed to elaborate the fermentation kinetic processes using a mixture of potato starch hydrolysate and sucrose.
Material and methodsMicroorganism and cultureA. pullulans 201253 was obtained from the American Type Culture Collection (Rockville, MD, USA) and was maintained at 4°C on potato dextrose agar (PDA) slants and sub-cultured every 2 weeks. For long-term storage, cultures were maintained at −80°C in a 20% glycerol solution. For inoculum preparation, A. pullulans was grown at 28°C for 48h in a medium containing 50g glucose, 2.5g yeast extract, 0.6g (NH4)2SO4, 1g NaCl, 5gK2HPO4, and 0.2g MgSO4·7H2O per liter of deionized water, at an initial pH of 6.5 with agitation at 230rpm.
Basic fermentation medium consisted of 50g of carbon source, 2.5g yeast extract, 0.6g (NH4)2SO4, 1g NaCl, 5gK2HPO4, and 0.2g MgSO4·7H2O per liter of deionized water,12 at an initial pH of 6.5 with agitation at 230rpm. To evaluate the effects of mixed carbon sources on pullulan production, the carbon source was varied in this study.
Preparation of potato starch hydrolysatePotato starch was purchased from a local agricultural market. The pH of a slurry of potato starch at a concentration of 40% (w/v) was adjusted to pH 6.5 and instantly heated to 70°C within 3min in the presence of 0.1% α-amylase. After liquefaction, the temperature was quickly reduced to 55°C within 1min, and the pH was adjusted to 5.0. Amyloglucosidase was then added at a concentration of 0.3% (w/w) and incubated for prolonged hydrolysis. After hydrolysis, the hydrolysates were filtered.8
Analytical methodsThe culture was centrifuged at 10000×g for 10min to remove the microorganism. The biomass (mycelia and yeast-like cells) dry weight (BDW) was determined by washing the sediment with distilled water and drying at 105°C overnight. Fifteen milliliters of the supernatant was transferred into a test tube, and then 30mL of cold ethanol was added to the test tube and mixed thoroughly. The solution was then held at 4°C for 12h to precipitate the exopolysaccharide, which was separated by centrifugation at 8000×g for 10min. The precipitated material was dried at 80°C, and the final weight determined as that of exopolysaccharide. The total concentration of reducing sugar was determined by the dinitrosalicylic acid (DNS) method.13 FTIR Fourier transform infrared spectroscopy (FTIR) spectra of pullulan samples for composition analysis were directly acquired using the Smart-iTR setup without further preparation.14
Batch fermentation of pullulan in a 10-L bioreactorBatch fermentation was carried out in a 10-L bioreactor (Yangge Bioengineering Equipment Co., Ltd, Shanghai, China) containing 7.0L fermentation medium at 28°C with agitation of 500rpm. The inoculum volume was 5.0% (v/v). Samples were taken periodically to determine the concentration of pullulan, biomass, and reducing sugar.
Determination of parameters during the fermentation processBiomass formationThe logistic equation was used to fit the biomass curves. A common autonomous rate equation is the differential form of the logistic equation:
where μmax and Xmax are the initial specific growth rate and the maximum biomass concentration, respectively. The integrated form using X0=X(t=o) gives a sigmoidal variation X(t) that may empirically represent both an exponential and a stationary phase:Product formationProduct formation is described by Luedeking–Piret kinetics. The product formation rate has been shown to depend upon both the instantaneous biomass concentration, X, and the growth rate, dX/dt, in a linear manner:
where m and n are empirical constants (for growth and non-growth associated polysaccharide formation, respectively) that may vary with formation conditions. Integration of (3) using (2) gives an equation with two initial concentrations (X0, P0), a final condition (Xmax), and three parameters (μ, n, and m):Substrate consumptionThe substrate balance for polysaccharide formation may be written as follows (modification of Luedeking and Piret):
where Yx/s and Yp/s are the yield coefficients for the biomass and product, respectively, and ke is the specific maintenance rate (i.e., the substrate used to support cell activity even in the absence of growth). From (3) and (5):where α and β are constants for substrate consumption at growth and non-growth phases, respectively. Substituting (2) into (6) and integrating,The 1stOpt (First Optimization, 7D-Soft High Technology Inc.) software was applied to solving the parameters of biomass formation, pullulan formation, and substrate consumption. OriginPro 8 software was applied to build the kinetic curves.
Results and discussionKinetics of biomass formationWe cultivated A. pullulans in a 10-L stirred-tank bioreactor on sucrose, PSH, PSH:sucrose=80:20, and PSH:glucose:fructose=80:10:10. Samples were taken at different time intervals and analyzed.
The logistic equation was used to describe the biomass formation, and the calculated model parameters associated with biomass (Xmax, μ, and X0) are summarized in Table 1 and represented in Fig. 1.
Kinetic parameters for the fermentation.
| Parameter | Carbon source | |||
|---|---|---|---|---|
| A | B | C | D | |
| Specific growth rate μm (h−1) | 0.046 | 0.043 | 0.042 | 0.044 |
| Maximum attainable biomass Xmax (gL−1) | 13.12 | 15.72 | 14.5 | 14.73 |
| Calculated initial biomass X0 (gL−1) | 1.38 | 1.72 | 1.64 | 1.29 |
| Growth associated constant m (gproductg−1biomass) | 6.37 | 3.405 | 5.054 | 3.779 |
| Non-growth associated constant n (gproductg−1biomassh) | −0.154 | −0.478 | −0.291 | −0.232 |
| Calculated initial product P0 (gL−1) | 0.809 | 1.473 | 0.857 | 1.816 |
| Growth associated constant for substrate consumption α (gsubstrateg−1biomass) | 6.539 | 4.359 | 6.308 | 5.152 |
| Non-growth associated constant for substrate consumption β (gsubstrateg−1biomassh) | 0.474 | 0.263 | 0.294 | 0.206 |
| Calculated initial substrate S0 (gL−1) | 95.06 | 91.09 | 97.77 | 98.6 |
Note: (A) Sucrose; (B) PSH; (C) PSH:sucrose=80:20; (D) PSH:glucose:fructose=80:10:10.
The value of Xmax showed a peak of 15.72gL−1 with 100gL−1 PSH, while the specific growth rate, μ, showed a peak of 0.046h−1 with 100gL−1 sucrose. The value of Xmax varied from 13.12gL−1 to 15.72gL−1, and the specific growth rate, μ, varied from 0.042h−1 to 0.046h−1 with different carbon sources (all at a concentration of 100gL−1) in this study. Thus, we concluded that the type of carbon source does not lead to a significant difference in the biomass. The results might be attributed to the presence of the same amounts of nitrogen source available for assimilation in the medium during different cultivations.15 According to the results of previous studies, nitrogen source levels could shift the pattern of carbon efflux during the fermentation of pullulan by A. pullulans, and pullulan production decreased but biomass accumulation increased as the level of nitrogen source increased.16,17
Kinetics of pullulan formationProduct formation is described by Luedeking–Piret kinetics and the calculated model parameters associated with polysaccharide formation (m, n, P0, and (dP/dt)/X) are presented in Table 1 and Fig. 2(a).
Growth of A. pullulans 201253 with different carbon sources. (a) Comparison of experimental data (■) with simulations using the logistic equation (□). (b) Comparison of the simulation using the Luedeking–Piret model (□) with the experimental polysaccharide clonal (■). (c) Comparison of the experimental data (□) of the consumption by A. pullulans 201253 and the simulation using the modified Luedeking–Piret model (■). (A) Sucrose; (B) PSH; (C) PSH:sucrose=80:2; (D) PSH:glucose:fructose=80:10:10.
Variations in the specific production rate (dP/dt)/X showed a peak of 0.243h−1 with 100gL−1 sucrose as the carbon source. The results also indicated that the specific production rate (dP/dt/X, h−1) on mixtures of PSH and sucrose (PSH:sucrose=80:20) was 0.212h−1, which was significantly higher than those of PSH (0.146h−1) and mixtures of PSH, glucose, and fructose (PSH:glucose:fructose=80:10:10, 0.166h−1) with 100gL−1 total carbon source.
The values of m and n (Table 1) indicate that polysaccharide formation by a non-growth associated mechanism is much less important compared to that by a growth-associated mechanism, regardless of the initial carbon source. Similar results were reported by Klimek, Boa, and Mulchandani.18–20
Fig. 2(b) also indicates that a mixed substrate of PSH and sucrose can increase the rate of pullulan formation and shorten the fermentation period. The highest yield of pullulan was 110h when PSH was used as a sole carbon source; however, to achieve the same yield, sucrose, PSH:sucrose=80:20, and PSH:glucose:frutcose=80:10:10 required 50, 60, and 80h, respectively.
According to previous studies, co-feeding fermentation is an efficient fermentation strategy that enables high productivity with low cost by providing more than one substrate. Xu et al. adopted a co-feeding strategy to produce l-lactic acid based on cane molasses/glucose carbon sources, with the aim of removing substances from cane molasses that inhibit fermentation.21 Li et al. applied a mixed-sugar strategy for highly efficient and economic production of docosahexaenoic acid by Aurantiochytrium limacinum SR21.22 Our results also indicated that a mixture of PSH (a low cost substrate) and sucrose, which was demonstrated to be superior to other sugars with respect to the titer of pullulan produced in our study and previous reports,15,23,24 can significantly enhance the pullulan formation rate of A. pullulans 201253 at a low cost.
Kinetics of substrate consumptionSubstrate consumption is described by modification of Luedeking and Piret kinetics, and the calculated model parameters associated with substrate consumption are given in Table 1.
Variations in the specific substrate consumption rate (dS/dt)/X showed a peak of 0.675h−1 with 100gL−1 sucrose as the carbon source. The specific substrate consumption rate (dS/dt)/X on mixtures of PSH and sucrose (PSH:sucrose=80:20) was 0.559h−1, which was significantly higher than those of PSH (0.45h−1) and mixtures of PSH, glucose, and fructose (PSH:glucose:fructose=80:10:10, 0.52h−1) with 100gL−1 of total carbon sources. As Fig. 2(c) shows, after 96h of fermentation, the carbon source in the fermentation broth was almost entirely consumed when sucrose only was used as the substrate, and only 1.15gL−1 sugar was not converted. Residual sugar (17.40gL−1, 6.32gL−1, and 12.24gL−1) remained when PSH, PSH:sucrose=80:20, and PSH:glucose:fructose=80:10:10, respectively.
These results show that sucrose was the most efficient carbon source for substrate consumption, which was in agreement with a previous study.15 Additionally, these results indicate that mixtures of PSH and sucrose can significantly enhance the utilization rate of A. pullulans for the carbon source. The reason may be that A. pullulans could simultaneously consume different carbon sources and eliminate the carbon source repression effect. Similar results have been widely reported for the fermentation of a variety of products.25,26
IR characteristicsFig. 3 shows the FT-IR spectra for pullulan obtained from a mixture of sucrose and PSH. The peaks appearing at 3400cm−1 and 2930cm−1 were attributed to OH stretching and CH stretching, respectively. Further characteristic signals obtained at 1368, 1155, and 1080cm−1 were attributed to C–OH bending, C–O–C stretching, and C–O stretching, respectively.27 Other features of the biopolymer were also found from the spectra, including O–C–O stretch (1656cm−1), and C–O–C stretch (1155cm−1), as reported previously.28 The absorption that appeared at 755cm−1 and 931cm−1 was attributed to α(1, 4) and α(1, 6) linkages between glucose units within pullulan, respectively.29 These results suggest that the exopolysaccharide produced from the mixture of PSH and sucrose is pullulan.
ConclusionsIn our research, sucrose was the best carbon source for pullulan production by A. pullulans 201253, while the potato is a relatively cheap raw material and a major crop in Shaanxi Province. Hence, a novel mixed-sugar strategy was developed for economical pullulan production by A. pullulans. Eventually, the yield of pullulan increased to 54.57gL−1 when the ratio of PSH to sucrose was 20:80, which is slightly below the yield of pullulan used by sucrose, but higher than that with PSH and a sugar mixture (PSH:glucose:fructose=80:10:10). The results suggest that sucrose can promote pullulan synthesis, because a small amount of sucrose might stimulate the enzyme responsible for pullulan synthesis and promote effective PSH conversion effectively. Overall, this study provides an efficient and economical option for the utilization of raw materials in microbial production.
Conflicts of interestThe authors declare no conflicts of interest.
This project was funded by the Scientific and Technologic Research Program of Shaanxi Academy of Sciences, China (No. 2013k-06, 2014k-01), the WesternChinese Academy of Sciences (2013DF01), the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2014JM2-2015), Shaanxi Science & Technology Co-ordination & Innovation Project (2014SZS07-K03), and the Science and Technology Program of Xi’an (NC1505(3)).