Very high gravity (VHG) technology was employed on industrial scale to produce ethanol from molasses (fermented) as well as by-products formation estimation. The effect of different Brix° (32, 36 and 40) air-flow rates (0.00, 0.20, 0.40, and 0.60vvm) was studied on ethanol production. The maximum ethanol production was recorded to be 12.2% (v/v) at 40 Brix° with 0.2vvm air-flow rate. At optimum level aeration and 40 Brix° VHG, the residual sugar level was recorded in the range of 12.5–18.5g/L, whereas the viable cell count remained constant up to 50h of fermentation and dry matter production increased with fermentation time. Both water and steam consumption reduced significantly under optimum conditions of Brix° and aeration rate with compromising the ethanol production. Results revealed VHG with continuous air flow is viable technique to reduce the ethanol production cost form molasses at commercial scale.
Due to higher petroleum product prices and air pollution issues, researchers are focusing to explore alternative renewable sources. In this connection, ethanol has been emerged as a potential alternative candidate as it is eco-friendly and useable as blending with gasoline in current combustion engine systems.1 Sugar cane molasses (high fermentable sugars) is the major feedstock for distilleries in Pakistan for ethanol production. The industrial process is well established with 7–8% (v/v) ethanol production from diluted molasses (15–16%).2,3 However, during ethanol production, huge amount of effluent (∼12liter effluent/liter absolute alcohol) with very high biological oxygen demand BOD (45,000–60,000mg/L) and COD (80,000–120,000mg/L) is produced, which is discharged into the environment without proper treatment.4 So for, the ethanol production using current distilleries is a potential source of environmental pollution in Pakistan and ethanol production process needs to be improved and optimized for an environment friendly, fast and cheap ethanol production. The sugar cane molasses (sugar production by-product) is a good option to produce ethanol.5,6 Biomasses are efficient source of energy and are eco-friendly in nature.7–11
The application of VHG fermentation technology (the preparation and fermentation of mashes containing 27g or more dissolved solids per 100g mash) has been proposed for efficient ethanol production and to reduce stillage volume. Among different ethanol production methods, the VHG fermentation technology is very efficient at industrial scale since it offers higher yield (12–15%), low waste generation and can be operated at low cost. Higher ethanol level can reduce the distillation cost as compared to the traditional fermentation, where maximum 7–8% ethanol is produced.2,12 The ethanol percentage production can be enhanced through VHG technology using strains tolerable to high sugar contents. Yeasts are known to tolerate higher sugar contents (up to 40%), however, at higher sugar concentration, the yeast growth is affected, which grows very slowly.13 Yeast growth is regulated by metabolites generated during ethanol production. Generally, industrial ethanol production is performed at low carbohydrate concentration in fermentable sugar.2,14 Previous reports indicated that yeast can grow up to 50% sucrose in fermenting media15 and batch fermentation with 23.8% (v/v) ethanol at laboratory scale was achieved in a simultaneous saccharification and fermentation mode.16 Lab-scale multistage continuous cascade, chemostat fermentation system have been constructed to investigate its applicability toward ethanol production under increased gravity conditions.17 Ethanol production using VHG technology can be enhanced applying various strategies, i.e., vitamin feeding,12 nutrient feeding,13 media supplementations,18 temperature optimization19 and aeration rate12 have been studied well to improve the ethanol percentage yield. Among these strategies, aeration improved the viability of yeast during high-level ethanol production.20
The VHG fermentation technology in alcohol industry (operated in Pakistan) is of great interest, as it saves considerable amount of water with higher alcohol yield, which needs low energy input, less capital cost, more efficient and is also free from bacterial contamination.21 Therefore, this study was aimed to evaluate the ethanol production using Saccharomyces cerevisiae (mutant strain). Moreover, the effects of aeration rate and Brix's on ethanol percentage yield along with by-products estimation in fed-batch mode fermentation at industrial scale (using current distilleries system in Pakistan) was also investigated.
Materials and methodsMicroorganism and culture mediaThe Saccharomyces cerevisiae parental strain was obtained from Shakarganj distillery (Instant-France). The mutant strain of Saccharomyces cerevisiae UAF-1 was used for fermentation (S. cerevisiae SAF-INSTANT strain were exposed to gamma radiation (500 Krad using Co-60 gamma radiation source) and resultantly, 1135 mutant strains were recorded. All survivors were tested for sugar tolerance and ethanol production. The survivor (GAMMA-11) furnished higher tolerance to sugar as well as ethanol production, which was named as Saccharomyces cerevisiae UAF-1 and used for ethanol production). The selected strain was cultured in medium containing (g/L): sucrose (10.0), yeast extract (3.0), (NH4)2SO4 (2.0), and MgSO4 (0.5). We found that gene creAdelta4 was significantly mutated and levels of mRNA of CreA protein in the wild strain were more in quantity than those of the mutant derivative (creA delta4 gene is useful in enhancing the production of extracellular invertase (protein) level in the medium as well as take part in carbon catabolite repression. The Sko1 gene has role in activation and repression of protein transcription involved in osmotic and oxidative stress and also suppression off kinase overexpression is controlled by Sko1 gene). Moreover, copy number of sko1 gene in the mutant derivative was sufficiently decreased to cause repression of transcription of CreA motif as done in wild strain. Good quality molasses having 89 °Bx, total reducing sugars (TRS) (50% w/v) with addition of nitrogen and phosphorus contents was used for the inoculate preparation and very high gravity technique was used for fermentation of molasses.
Molasses pre-treatmentFor molasses pretreatment, diluted molasses was settled in tanks of conical bottom type, especially designed for the removal of sludge, ash contents and other particulates. Sodium hexameta phosphate was added and pH was adjusted at 4.0–4.5 with commercial sulfuric acid which converted Ca2+ into calcium sulfate.22 Brix° of the substrate was adjusted at 32, 36 and 40 and sugar contents were 21%, 24%, and 27% (w/v) in pre-treatment molasses, respectively.
Inoculum preparationInitially, the propagation of yeast was started in 1m3 vessel and cell count reached to 3.00×106CUF/mL; it was transferred to 60m3 vessel to maintain appropriate cell count2 and this inoculum was used for fermentation of molasses.
FermentationFed-batch experiments were performed in fermenters of 300m3 capacity. The temperature was maintained at 32±1°C and stirred by circulation of mash with the help of electric pump at 300rpm. To control the foaming, silica based antifoaming agent was used. Air was supplied by the air blowers. The aeration rates used were 0.0, 0.1, 0.3, 0.6 and 0.9 (vvm). The fermenter was filled in 16h after the transfer of inoculum (25%) with appropriately diluted molasses. Level rise of the fermenter was kept at 5%/h (15m3 molasses were fed to fermenter/h (Fed-batch mode), which is 5% of total fermenter molasses volume used for fermentation). After transferring of inoculum (100m3), the substrate (200m3) was supplied continuously. Five fermenters were run for each treatment in order to check reproducibility.
DistillationFermented mash was distilled in double effect distillation plant (Firlli, Italy) having 40,000 liters processing capacity/day.2 Fermented mash was transferred to the mash column and steam was applied from bottom in such a way that temperature maintained in the range of 78–80°C. Vacuum was applied through condensers. Vapors traveled from column to condensers were condensed and cooled. The resultant liquid was re-boiled in depuration column for removing impurities and finally, fed to rectification column. Rectification of ethanol was done in the range of 96.0–96.4% and this column was operated at 1.5kgm/s2 pressure.
Determination of cell count/viabilityFor cell count determination hematocytometer was used, fermenter broth sample was serially diluted with a sterile saline solution (0.89% (w/v) NaCl) to a point where reasonable number of cells could be counted (3.00×106CUF/mL). The cell viability was determined by the Methylene Blue technique.12
Brix, reducing sugars, ethanol and by-products determinationConcentration of TRS in diluted molasses and fermented mash (after sucrose inversion using HCl) was measured by Fehling-Soxhlet method.23 Brix was measured with the help of ATAGO densimeter (model 2312; ATAGO Co. Ltd., Tokyo, Japan). Ethanol and by-products in fermented samples were determined using GC (Shimadzu GC-17A, 3.0) equipped with flame ionization detector (FID) as reported earlier.2 The aldehyde content was estimated through ASTM-D-1363 method.
ResultsThe VHG technology with and without aeration was evaluated on industrial scale to improve the traditional fermentation for ethanol production. VHG technology resulted in higher alcohol production (11–12%, v/v) as compare to 7–8% (v/v) in conventional process and overall, variation among replicates was in the range of ±2–3% throughout the experimentation. High gravity molasses media containing different concentration of dissolved solid, ranging from 32 to 40° Brix were prepared and fermented for 60h in 300m3 vessel. Maximum ethanol was produced (12.2%) from 27% TRS at 0.2vvm aeration rate. The higher sugar content results in higher ethanol production and these findings are in line with reported finding, i.e., pilot-scale ethanol production from rice straw hydrolysates using xylose-fermented with Pichia stipites was studied24 and influence of aeration on bioethanol production from ozonized wheat straw hydrolysates using Pichia stipitis.25
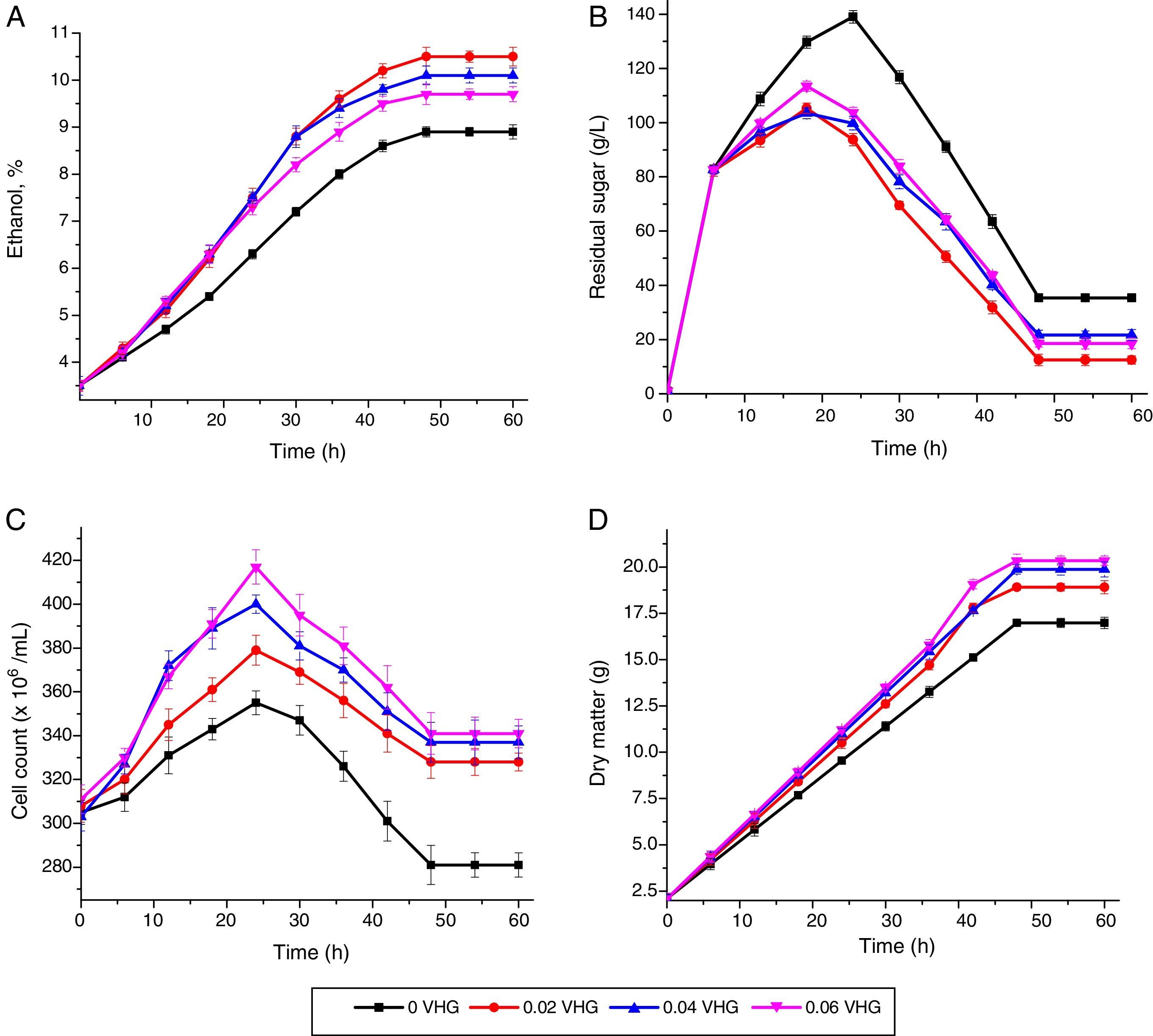
Viable cell count, ethanol formation, residual sugar content and dry matter generation were determined during the fermentation period. By-products, i.e., methanol, acetic acid, higher alcohols (1-propanol, 1-butanol, 2-butanol) production and aldehyde appearance time were recorded and results are depicted in Table 2. Ethanol (%, v/v) and residual sugar (g/L) at 32° Brix with different aeration rates are shown in Fig. 1A and B, respectively. Residual sugar showed increasing trend up to the completion of batch. Without aeration, the ethanol production was 8.9%, with 35.41g/L residual sugar contents. Fig. 1C shows the viable cell count at 32° Brix with different aeration rates. Maximum viable cell count remained 417 million/mL at 0.60vvm. High oxygen concentration exerted strong influence on yeast growth during fermentation and this trend was in line with previous findings that oxygen supplement has significant effect on ethanol production as well as cell viability.26 The viable cell count increased to 16%, 20% and 22% at 0.2, 0.4 and 0.6vvm, respectively. The final biomass (Fig. 1D) and ethanol production was recorded considerably at higher sugar level, which indicates that increasing level of sugar, the yeast cope the osmotic stress due in response of ethanol production in fermenter. The studied parameters (Table 1) during ethanol fermentation clearly showed that the aeration affected the process at higher sugar level.
Fermentation kinetic parameters at different Brix°.
| Parameters | 32° | 36° | 40° |
|---|---|---|---|
| Fermentation time (h) | 60 | 60 | 60 |
| Residual sugars (g/L) max | 35.4 | 39.7 | 60.4 |
| Residual sugars (g/L) min | 12.5 | 14.5 | 18.7 |
| μmax (h−1) | 0.38 | 0.34 | 0.35 |
| μmin (h−1) | 0.31 | 0.42 | 0.43 |
| Xmax (g/L) | 20.3 | 22.2 | 22.7 |
| Xmin (g/L) | 16.1 | 18.4 | 19.0 |
| Ethanol max (g/L) | 8.2 | 8.9 | 9.6 |
| Ethanol (ming/L) | 7.18 | 7.30 | 7.50 |
Ethanol production (%) was recorded to be 9.3% (v/v) at 36° Brix without aeration and reached to 11.3% (v/v) with aeration (Fig. 2A). Aeration rates of 0.20, 0.40 and 0.60vvm increased the ethanol production by 20%, 16% and 14% over the non-aerated fermentation, respectively. The residual sugar increased up to 30h of fermentation and then, decreased sharply up to 60h (Fig. 2B). Maximum viable cell count was enhanced with increasing sugar level and 499million/mL cell count was recorded at 36° Brix (Fig. 2C). Similarly, the biomass increasing trend was observed as the time of fermentation is increased at different VHG levels (Fig. 2D). The reduction is residual sugar and production of biomass is a good indication of ethanol production and Brix level significantly affected the ethanol production. The maximum ethanol production of 12.2% (v/v) was recorded at 40° Brix with 0.2vvm aeration rate (Fig. 3A). The residual sugar levels were 18.7g/L, 24.8g/L and 21.2g/L at 0.2, 0.4 and 0.6vvm, respectively (Fig. 3B), whereas in non-aerated fermenter, the residual sugar was recorded to be 60.4g/L, which indicates that aeration along with Brix° level is also important factor in ethanol production. The cell count was recorded to be 514 million/mL at 40° Brix (Fig. 3C), which was maximum cell count as compared to other Brix levels. The biomass production trend at 40° Brix was recorded similar to other Brix levels, which increased with the passage of time.
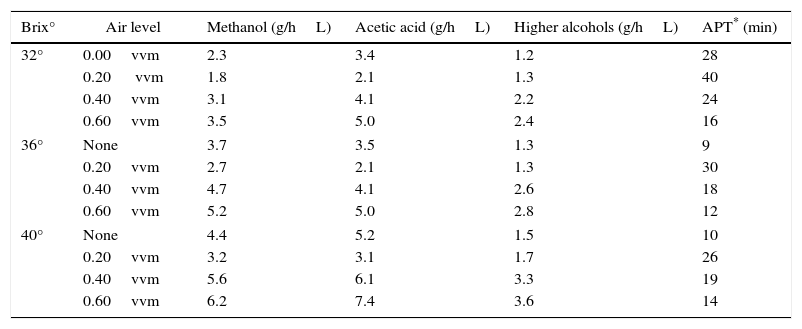
Different by-products, i.e., methanol, acetic acid, higher alcohols (1-propanol, 1-butanol, 2-butanol) were recorded and by-products production was recorded to be minimum at 0.2vvm air flow-rate (Table 2) as compared to control (0.00vvm aeration rate). The aldehyde appeared after longer time of fermentation when aeration rate was 0.2vvm versus all other air flow-rates. Higher alcohols, i.e., 1-propanol, 1-butanol, 2-butanol productions were recorded to be 2.4, 2.8 and 3.6g/hL in high aerated (0.6vvm) fermenter in comparison with non-aeration medium (1.2, 1.3 and 1.5g/hL), respectively. It was observed that increased ethanol production inhibited the cell growth. Cell count viability declined up to 25% in non-aerated culture at 32° Brix. Overall, at Brix° three levels used, residual sugars were very high in non-aerated fermenters. Aeration improved the ethanol production by 18% at aeration level of 0.2vvm, however, the ethanol production decreased at higher aeration rates. Sugar fermentation by S. cerevisiae is generally inhibited in the presence of oxygen, but small amount of dissolved oxygen enhances ethanol production compared to highly aerobic condition.27 Residual sugar recorded to be 39.7% in non-aerated fermenter and only 14.5% in aerated fermenter. Maximum residual sugar during feeding was reached to 130.5g/L. The viability of cells maintained 80% even after 48h of fermentation as compared to control (60%). Higher alcohols production along with other by-products was higher in aerated fermentation and acidity was low at 0.2vvm as compared to non-aerated process and high aerated cultures at all Brix° levels. The formation of by-products took place under certain unfavorable fermenting conditions. Therefore, significant reduction in the by-products was observed at 0.2vvm controlled aeration at all high gravity fermenting medium. The production of oxidized metabolites (acetaldehyde, acetate, and acetoin) is always favored,2 however, under aerobic conditions and acetic acid production was recorded to be high.
Effect of aeration on by-products formations at different Brix° levels.
| Brix° | Air level | Methanol (g/hL) | Acetic acid (g/hL) | Higher alcohols (g/hL) | APT* (min) |
|---|---|---|---|---|---|
| 32° | 0.00vvm | 2.3 | 3.4 | 1.2 | 28 |
| 0.20 vvm | 1.8 | 2.1 | 1.3 | 40 | |
| 0.40vvm | 3.1 | 4.1 | 2.2 | 24 | |
| 0.60vvm | 3.5 | 5.0 | 2.4 | 16 | |
| 36° | None | 3.7 | 3.5 | 1.3 | 9 |
| 0.20vvm | 2.7 | 2.1 | 1.3 | 30 | |
| 0.40vvm | 4.7 | 4.1 | 2.6 | 18 | |
| 0.60vvm | 5.2 | 5.0 | 2.8 | 12 | |
| 40° | None | 4.4 | 5.2 | 1.5 | 10 |
| 0.20vvm | 3.2 | 3.1 | 1.7 | 26 | |
| 0.40vvm | 5.6 | 6.1 | 3.3 | 19 | |
| 0.60vvm | 6.2 | 7.4 | 3.6 | 14 | |
The inhibitory effect of by-products in ethanol production is main factor in ethanol production.28 It was observed that under aerated conditions, decline in cell count viability was much lower as compared to non-aerated culture at all sugar levels. Residual sugar under non aerated condition and aerated process as well as the viability of cells after 48h of fermentation clearly indicates that the yeast cells need some aeration in order to overcome the osmotic stress at initial stage and ethanol induced oxidative stress at the end of fermentation. These findings are in line with.29 Authors reported that aerated fed batch process on glucose medium is useful in ethanol production. Similarly, Maemura30 also found increase in viable cell count with the aeration level. The total number of cells reached maximum level after incubation for about 24h and cell growth cultivation was found to be dependent on the aeration. More aeration enhanced the dissolved oxygen and at higher Brix°, the viable cell count decreased and this is in agreement with observations reported previously.31 It has been reported that the cell viability decreased as the concentration of ethanol increased using S. cerevisiae during fermentation.16 In present investigation, ethanol production was maximum at the lowest aeration rate and Seo32 also observed similar trend. Moreover, they reported that the growth and ethanol production may decrease at later stages of fermentation, when the ethanol concentration reached ∼100g/L. Overall, ethanol production was higher in the aerated fermenters and these findings are in line with Alfenore et al.12 and in another study, production of higher alcohols was markedly enhanced under oxidative conditions maintained by agitation or sparging with air.2 So for, based on current finding, it is concluded that the ethanol production using VHG technology along with aeration is efficient method since at optimized condition a significantly higher ethanol production was achieved along with minimum by-products production.
ConclusionsEthanol production along with other by-products was evaluated at different aeration rates and Brix levels. Aeration increased the ethanol production up to 12.0% (v/v) in high gravity medium versus 7–9% (conventionally produced ethanol). Since ethanol production is the major objectives in fermentation using easily controlled distillation processes at reduced costs. Therefore, the use of high gravity medium (40° Brix) under aerated condition is useful in reducing water consumption up to 35%, which would ultimately decrease the effluents generation. Moreover, with ease in distillation process, this method also help in cutting down the steam consumption, resulting in lower distillation costs and improved ethanol production at industrial scale.
Conflict of interestThe authors declare no conflicts of interest.
The Jhang sugar mill Authorities are highly acknowledged for providing research facilities at Jhang sugar mill laboratories