Various chemical compounds, including surfactants, when introduced to culture media may increase the permeability of cellular membranes and thereby affect the quantity of metabolites excreted by cells. The aim of the present study was to evaluate the impact of detergents including Triton X-100, Span 20 and Tween 80 on erythritol production from glycerol by Yarrowia lipolytica Wratislavia K1 in a shake-flask experiment, batch and fed-batch cultures. When Span 20 was added to a fed-batch culture with glycerol as a carbon source (300gL−1), erythritol production increased by 15% compared to the culture without the surfactant where it reached 142gL−1 after 5 days, which corresponded to 0.47gg−1 yield and productivity of 1.1gL−1h−1. Therefore, it was concluded that Span 20 considerably enhanced the production of this polyol from glycerol.
Erythritol is a sugar alcohol from a group of polyols that possesses four carbon atoms and occurs only in the meso-form.1 Its taste profile resembles that of sucrose. It has about 60–80% of the sweetness of sucrose in a 10% (wv−1) solution and in many countries it has been safely used in food as a non-cariogenic sweetener.2,3 Owing to the presence of hydroxyl groups, it additionally displays antioxidative properties.4 Erythritol is produced from glucose by such osmophilic yeast as Moniliella sp.,5Trichosporon sp.,6Aureobasidium sp.,7Torula sp.8 or Pseudozyma tsukubaensis,9 whereas Trichosporonoides megachiliensis SN-G42 yeast are applied for its industrial-scale production.10 Erythritol may also be produced from glycerol by Yarrowia lipolytica yeast.11–13 Different cultivating systems, as well as different factors affecting this biosynthesis has been examined e.g. type of glycerol (pure or crude), various agitation rates, addition of NaCl and supplementation with minerals, however the effect of surfactants on erythritol production has never been studied so far.14–18
Commonly used surfactants like Triton X-100, Span 20 or Tween 80 were successively applied in culture media to modify the permeability of cell membranes of filamentous fungi,19–21 yeast22,23 and bacteria.24 The addition of these compounds caused a significant increase in enzymatic activity, in the quantity of biosynthesis products, in the rate of cell biomass growth as well as in substrate assimilation.25 According to Mirbagheri et al.,26 the Y. lipolytica DSM 3286 and M7 strains were synthesizing 1.4–1.8 times more citric acid in the culture media with Triton X-100 addition.
The aim of the present study was to evaluate the impact of surfactants including Triton X-100, Span 20 and Tween 80 on parameters of erythritol production from glycerol by Y. lipolytica Wratislavia K1 strain in the shake-flask experiment, batch and fed-batch cultures.
Materials and methodsMicroorganismY. lipolytica Wratislavia K1, an acetate-negative mutant, used in this study originated from the yeast culture collection of the Department of Biotechnology and Food Microbiology, Wrocław University of Environmental and Life Sciences in Poland. The yeast strain was stored at 4°C.
SubstratePure glycerol 980gkg−1 (POCH, Gliwice, Poland) was used as a source of carbon and energy.
Media and culture conditionsThe growth medium contained: pure glycerol – 50g; yeast extract (YE) – 3g; malt extract – 3g; bactopeptone – 5g, and distilled water to 1L. Cultures were grown in 0.3-L flasks containing 0.1L of growth medium on an Elpan (Poland) rotary shaker at 30°C and 140revmin−1 for 2 days. Inoculum (10%, vv−1) was added to a bioreactor.
The effect of surfactants on erythritol production was studied in a shake-flask experiment in medium consisted of: pure glycerol – 100g; (NH4)2SO4 – 2.3g; MgSO4×7H2O – 1g; KH2PO4 – 0.22g; yeast extract – 1g; NaCl – 25g; CaCO3 – 3g, and distilled water to 1L.
The shake-flask experiments were carried out with the use of the 24-h bioreactor culture in medium described above. To this end, 0.03L of culture broth was sterile-collected from the bioreactor and transferred to a 0.3-L conical flask. Next, the surfactants, Triton X-100, Tween 80 and Span 20 in the concentration of 0.25, 0.5, 1.0 and 2.0gL−1 were added to the flask. Cultures were run for 7 days on a rotary-shaker Elpan (Poland) at 30°C and 140revmin−1.
The impact of surfactants on erythritol production was studied in batch cultures in medium contained: pure glycerol – 150g; (NH4)2SO4 – 2.3g; KH2PO4 – 0.22g; MgSO4×7H2O – 1g; yeast extract – 1g, and NaCl – 25g; and tap water to 1L. The medium was supplemented with surfactants: Triton X-100, Tween 80 and Span 20 in the concentration of 0.25gL−1.
The cultures were run in a 3.5-L stirred-tank reactor Bioflo III (New Brunswick Scientific Co., Edison, USA) with a working volume of 1.5L at 30°C. The specific aeration rate was fixed at 0.2Lmin−1. The stirrer frequency was adjusted to 600revmin−1 and the pH was maintained automatically at 3.0 by the addition of NaOH solution containing 15% (wv−1) of NaOH.
Production of erythritol in fed-batch cultures with SPAN 20 were performed in medium contained: pure glycerol – 300g, (NH4)2SO4 – 5g; KH2PO4 – 0.22g; MgSO4×7H2O – 1g; YE – 1g; NaCl – 25g or none and tap water to 1L. The medium was supplemented with surfactant Span 20 in the concentration of 0.25gL−1.
The fed-batch cultures were run in a 5-L stirred-tank reactor AK-210 (Russia) with a working volume of 3L at 30°C, and a stirring speed of 600revmin−1. The pH was maintained automatically at 3.0 by the addition of 20% (wv−1) NaOH solution. Aeration rate was set at 0.36Lmin−1. In the fed-batch cultures, the initial glycerol concentration was about 130gL−1 and the initial volume of the culture broth was about 2.5L. After 24h of cultivation, Span 20 in concentration of 0.25gL−1 and pure glycerol solution were added (at a constant feeding rate of 1.4gh−1) until a total glycerol concentration of 300gL−1 was obtained.
The shake-flask experiment and all the bioreactor cultures were performed in three and in two replications, respectively, in comparison to control without surfactants.
Analytical methodsSamples (0.01L) from the shake-flask experiment and batch cultures were centrifuged (10min; 4°C; 5.500revmin−1), harvested by filtration on 0.45μm pore-size membranes and washed twice with distilled water. The biomass was determined gravimetrically after drying at 105°C.
Concentrations of glycerol (GLY), erythritol (ERY), mannitol (MAN), were measured in the supernatants by HPLC using a HyperRez Carbohydrate H + Column (Thermo Scientific, Waltham, MA) coupled to a refractive index (RI) detector (Shodex, Ogimachi, Japan). The column was eluted with 25mM trifluoroacetic acid (TFA) at 65°C and a flow rate of 0.6mlmin−1.
Osmotic pressure was measured in supernatants using Osmometer Marcel OS 3000 (Zielonka, Poland).
Calculation of fermentation parameters and list of abbreviationsThe yield of erythritol production from glycerol (YERY), expressed in gg−1, was calculated from: YERY=ERYGLY−1. The volumetric erythritol production rate (QERY), expressed in gL−1h−1, was calculated as: QERY=ERYt−1. The specific erythritol production rate (qERY), expressed in gg−1h−1, was calculated from: qERY=QERYX−1. In all formulas: ERY – erythritol concentration in the culture liquid at the end of the cultivation (gL−1); GLY – total amount of glycerol consumed (gL−1); t – duration of the fermentation process (h); and X – biomass concentration in the stationary phase (gL−1).
Other abbreviation: MAN – mannitol.
ResultsThe impact of surfactants on erythritol production in the shake-flask experimentAt the first stage of the study, the impact of three surfactants: sorbitan monolaurate, octylphenol ethoxylate and polyoxyethylene (20) sorbitan monooleate, on the production of erythritol from glycerol by Y. lipolytica Wratislavia K1 was investigated in the 7-day shake-flask experiment (Fig. 1). These surfactants are well known under commercial names: Span 20, Triton X-100 and Tween 80, respectively. The medium was supplemented with surfactants in the concentrations of 0.25, 0.5, 1.0 and 2.0gL−1. In the control culture without surfactant the yeast produced 19.5gL−1 of erythritol, which corresponded to the yield of 0.18gg−1.
The erythritol production on media with Triton X-100 decreased from 16.0 to 4.3gL−1 with the increasing concentration of surfactant. In contrast to Triton X-100, the overall erythritol production (20.2gL−1) in the culture with 0.25gL−1 of Span 20 was similar in comparison to control without surface-active agent (19.5gL−1). However, the higher concentrations of Span 20 inhibited the production of this polyol. The highest amount of erythritol (21.7gL−1) was observed when Tween 80 was added to the medium in the concentration of 0.25gL−1. Moreover, the erythritol volumetric productivity and yield reached 0.12gL−1h−1 and 0.21gg−1, respectively. However, successive increase of Tween 80 concentration in medium decreased erythritol production (Fig. 1).
In the shake-flask experiment, Y. lipolytica yeast also produced mannitol in the concentration of 9.9gL−1 in the control culture without detergent (Fig. 1). The addition of Span 20 and Tween 80 to media caused similar effects on mannitol biosynthesis as observed with control culture. However, about two times lower amount of mannitol (4.8gL−1) was detected in the cultures with Triton X-100 addition, regardless of its concentration (Fig. 1).
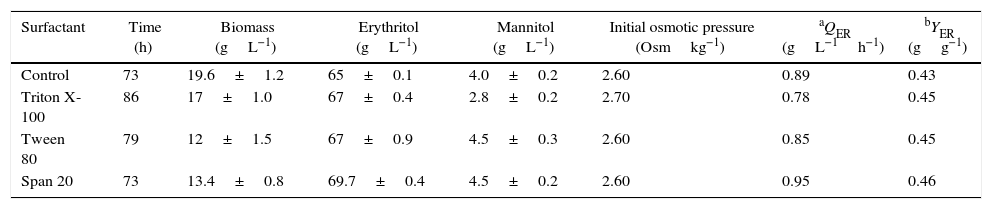
Batch bioreactor culturesIn the next stage of the study, the effect of surface-active agents on biosynthesis of erythritol was examined in bioreactor batch cultures in production medium which was indicated as optimal in our previous study.27 The surfactants were added in the concentration of 0.25gL−1 to the bioreactor for 24-h cultivation. Fermentation parameters of the cultures are summarized in Table 1. The batch cultures were carried out until complete consumption of the carbon source in media, which lasted from 73 to 86h. As shown in Table 1, biomass concentration ranged from 10.5 to 20.8gL−1 and was the highest in the control batch culture. The lowest biomass concentration (10.5gL−1) was observed in the culture with Tween 80.
Influence of surfactants addition on the production of erythritol from glycerol in batch cultures of Y. lipolytica Wratislavia K1.
| Surfactant | Time (h) | Biomass (gL−1) | Erythritol (gL−1) | Mannitol (gL−1) | Initial osmotic pressure (Osmkg−1) | aQER (gL−1h−1) | bYER (gg−1) |
|---|---|---|---|---|---|---|---|
| Control | 73 | 19.6±1.2 | 65±0.1 | 4.0±0.2 | 2.60 | 0.89 | 0.43 |
| Triton X-100 | 86 | 17±1.0 | 67±0.4 | 2.8±0.2 | 2.70 | 0.78 | 0.45 |
| Tween 80 | 79 | 12±1.5 | 67±0.9 | 4.5±0.3 | 2.60 | 0.85 | 0.45 |
| Span 20 | 73 | 13.4±0.8 | 69.7±0.4 | 4.5±0.2 | 2.60 | 0.95 | 0.46 |
In this report, a slight increase in erythritol production to 70.1gL−1, compared to the control culture without detergent, 65gL−1, has been observed upon the use of Span 20 (Table 1). Moreover, the highest volumetric erythritol production rate (0.95gL−1h−1) and the highest yield of erythritol (0.46gg−1) were also observed when Span 20 was added to the production medium (Table 1). As shown in Table 1, the addition of Tween 80 and Triton X-100 to the culture caused a lower amount of erythritol and volumetric production rate in comparison to the control without surfactant.
The initial osmotic pressure in production medium was high and ranged between 2.6 and 2.7Osmkg−1 (Table 1). In these cultures, the value of this parameter was caused by the presence of glycerol and 2.5% of NaCl in the medium.
Mannitol concentration was low and did not exceed 4.5gL−1.
Fed-batch culturesThe production of erythritol in fed-batch mode was investigated using medium with and without NaCl (see Materials and methods). Span 20, which gave the best results in the batch cultivation, was chosen as a surface-active agent.
The presence of salt in the production medium caused higher osmotic pressure i.e. of about 1.4–1.7-fold in comparison to the medium without salt addition (Table 2). The concentration of biomass ranged from 16.2gL−1 in the culture with the addition of Span 20 to 23.3gL−1 in the control culture without detergent (Fig. 2).
Parameters of fed-batch erythritol biosynthesis by Y. lipolytica Wratislavia K1 on pure glycerol media with and without Span 20 addition.
| Surfactants | NaCl (%) | Initial osmotic pressure (Osmkg−1) | Osmotic pressure after 24h (Osmkg−1) | Osmotic pressure after 24h and addition of glycerol (Osmkg−1) | aQER (gL−1h−1) | bYER (gg−1) |
|---|---|---|---|---|---|---|
| Control I | 0 | 1.8 | 0.9 | 3.0 | 1.17 | 0.39 |
| Span 20 | 0 | 1.8 | 1.1 | 3.2 | 1.29 | 0.42 |
| Control II | 2.5 | 2.5 | 2.1 | 4.4 | 0.82 | 0.40 |
| Span 20 | 2.5 | 2.5 | 2.2 | 4.3 | 1.10 | 0.47 |
Generally, surfactant addition enhanced erythritol biosynthesis. The highest erythritol concentration, 142.6gL−1, and production yield, 0.47gg−1, was observed in the medium with salt. However, the highest volumetric production rate, 1.29gL−1h−1, was achieved in the variant with Span 20 and without NaCl (Fig. 3 and Table 2).
Mannitol production was influenced especially by NaCl presence in the medium, addition of salt strongly decreased the concentration of this polyol. The lowest amount of mannitol was determined in culture broth (3.7gL−1) when the production medium with salt and Span 20 was used (Fig. 3).
DiscussionIt has previously been shown that Y. lipolytica is able to produce some interesting compounds from glycerol as a sole carbon source, such as organic acids, polyols and invertase.12 The present study reports erythritol production from glycerol by Y. lipolytica Wratislavia K1 in the shake-flask, batch and fed-batch cultures with or without surfactants. Various concentrations of the surfactants including Triton X-100, Tween 80 and Span 20 were tested in the flask experiment. The best results were achieved when 0.25gL−1 of surfactants was added to the culture media. The highest concentration of erythritol, 21.7gL−1 and 20.5gL−1, was obtained in the variant with Tween 80 and Span, respectively. These results were slightly lower than erythritol production (23gL−1) by Y. lipolytica Wratislavia K1 strain noted in the study by Tomaszewska et al., on screening Y. lipolytica strains for sugar alcohols biosynthesis from pure glycerol in a 10-day shake-flask experiment.13 During flask culture with 200gL−1 of glycerol, the ATCC 8661 strain of Y. lipolytica produced 43.3gL−1 of erythritol, which corresponded to the yield of 0.21gg−1, i.e. the same as in our study, which was reported in a patent of Mitsubishi Chemical Corporation Chiyodaku.28 Generally glucose has been applied as a source of carbon and energy for erythritol biosynthesis. In the study conducted by Yang et al., a mutagenized strain of Candida magnoliae produced 25gL−1 of erythritol after 83h of the flask culture in a medium containing 10% (wv−1) of glucose, which corresponded to a 25% increase in erythritol concentration and a 30% increase in erythritol productivity compared with the wild type strain.29 In other experiment, Hajny et al. have also reported that Torula sp. produced a high amount of erythritol from glucose in the shake-flask experiment (35.2gL−1).30 Moreover, Aoki et al. reported that Trichosporonoides sp. produced 43.0 and 37.4gL−1 erythritol with the yield of 0.43 and 0.37gg−1, respectively, when glucose and sucrose were used as sources of carbon.31
Based on our results obtained in the flask experiment, the surfactant in the concentration of 0.25gL−1 was chosen for further research conducted in bioreactor batch cultures. The highest concentration of erythritol (70.1gL−1) was observed in the batch culture with the addition of Span 20. The parameters of erythritol production, productivity and yield, reached 0.95gL−1h−1 and 0.46gg−1, respectively. Tomaszewska et al. reported that volumetric erythritol production rate in the medium with crude glycerol and salt addition (2.5%) was comparable and reached 1.0gL−1h−1.13
It should be noted that the amount of erythritol obtained from glucose in batch cultures has been reported to vary from 18 to 243gL−1 depending on the type of microorganism and medium composition.9,8,29,32–36 The results obtained in our study are significantly higher than these obtained when Moniliella sp. was used for erythritol biosynthesis from glucose, i.e. yield of 0.37gg−1.34 However, the higher value of erythritol production yield (0.49gg−1) was obtained for Torula sp.37 The highest value of this parameter, 0.63gg−1, was noted by Lin et al. in the case of Moniliella sp. N61188-12.5
Based on the results from batch cultures, Span 20 was selected as a stimulating agent of erythritol production in fed-batch cultivations. Many research efforts of erythritol biosynthesis have been focused on the optimization of operation strategies in fed-batch fermentation processes, which are very promising systems, easy to use for pilot experiments, and verifying media compounds or supplements.8,9,18 To compare biosynthesis of erythritol in different cultivation systems, fed-batch cultures were also conducted in our study. Noteworthy is that a significantly higher value of volumetric erythritol production yield was observed in the fed-batch mode in comparison to batch culture. As mentioned before, the highest productivity in this study, 1.29gL−1h−1, was observed during fed-batch culture in the medium without salt and with Span 20 addition. This parameter was higher than that achieved by the same strain, growing on crude glycerol (300gL−1) during fed-batch cultivation, i.e. 1.0gL−1h−1.15 Up to date, the highest value of this parameter in this system, 2.86gL−1h−1, was noted by Jeya et al.9
The yield of erythritol production in the culture with Span 20 was similar in batch and fed-batch cultures, 0.46 and 0.47gg−1, respectively. In repeated fed-batch culture on glycerol conducted by Mirończuk et al., Y. lipolytica Wratislavia K1 strain produced on average 208gL−1 of erythritol, which corresponded to 0.41gg−1 yield.14 Our results are higher to those obtained with other strains, reported for glucose. For comparison, in the study conducted by Ryu et al., 0.41gg−1 yield of erythritol production by the mutant of C. magnoliae was achieved when the initial glucose concentration was 400gL−1.35 The repeated fed-batch culture of Trichosporon sp. showed a 0.45gg−1 total erythritol conversion yield on glucose.6 However, Jeya et al. reported that P. tsukubaensis KN75 produced 245gL−1 of erythritol from 400gL−1 of glucose which corresponded to 0.61gg−1 yield.9
Other polyols such as mannitol, ribitol, arabitol or glycerol were produced during biosynthesis with yeasts species of e.g. Trichosporon, Aureobasidium and Candida genera, which led to a decrease in the erythritol conversion yield.6,7 In our study, the lowest amount of mannitol was detected in batch culture with Triton X-100 (3.0gL−1) and in fed-batch culture with Span 20 and salt addition (3.7gL−1).
Up till now, literature lacks reports on the production of erythritol by Y. lipolytica in the medium with the presence of surfactants. However, it has been reported that some surfactants like Tween 80 or Span 20 have a positive effect on the growth rate and metabolites production of some yeast. They may be used to damage the cytoplasmic membrane of microorganisms and to improve the yield of many components produced via fermentation.38 Experiments on the permeabilization of microbial cells have also been carried out using Triton X-100 as a disrupting agent. These processes are employed to determine intracellular enzyme activities as well as protein release from cells or to enhance the quantity of metabolites excreted by cells. Interestingly, the addition of Tween 80 to the culture medium significantly enhanced pullulan production (from 38gL−1 in control culture to 55gL−1 in culture with 0.5% Tween 80), but had no impact on biomass accumulation.38 It has also been reported that Span 20, a non-ionic surfactant, significantly enhanced β-carotene production (from 0.15 to 2.16gL−1) in Blakeslea trispora, which could be probably attributed to the increase in substrate uptake arising from better cell permeability.20 Moreover, Musiał et al. found that the addition of Span 20 in the concentration of 0.75gL−1 improved the oxalic acid production by Aspergillus niger in the medium containing 30gL−1 of post-refining fatty acids.39 Therefore, surfactants and their concentrations have to be chosen carefully, as some of them are toxic to the microorganisms and promote or inhibit the production of metabolites.40 Triton X-100 in the concentration of 0.5% (vw−1) had a negative effect on the production of phytase and reduction of phytic acid in rapeseed meal by Aspergillus niger A-98 (local isolate) during solid state fermentation.41
SummaryIn view of our results, the highest erythritol production of the three surfactants: Span 20, Triton X-100 and Tween 80, was observed with Span 20 addition. This surfactant increased production of erythritol in the fed-batch culture by 15%, compared to the control culture without the surfactant. The production reached 142.6gL−1 after 5 days and corresponded to 0.47gg−1 yield and productivity of 1.1gL−1h−1. Therefore, the results indicate that Span 20 may be applied as a stimulating agent of erythritol production by Y. lipolytica yeast. On the other hand, the non-ionic surfactant Span 20 affords the possibility of using a surfactant to enhance the production of value-added substances from yeast cultures, which often require improved mass transfer environments.
Conflicts of interestThe authors declare that they have no competing interests.
This work was co-sponsored by European Union under Project No. POIG 01.01.02-00- 074/09 and by the Ministry of Science and Higher Education of Poland (grant No. N N312 256640).