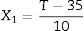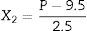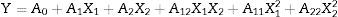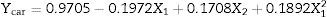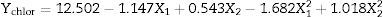Extraction of compounds from microalgae requires cell disruption as a pretreatment to increase extraction yield. Botryococcus braunii is a microalga with a significant content of carotenoids and other antioxidant compounds, such as chlorophylls. Cell disruption of B. braunii using CO2 rapid depressurization was studied as a pretreatment for the extraction of carotenoid and chlorophyll pigments. We studied the effect of temperature (21–49°C) and pressure (6–13MPa) during static compression on pigment recovery with supercritical CO2 at 40°C, 30MPa and solvent flow of 4.7LNPT/min. Within the experimental region, the extraction yield of carotenoids and chlorophylls increased by 2.4- and 2.2-fold respectively. Static compression conditions of high pressure and low temperature increased the extraction of carotenoids and especially chlorophylls. We selected 21°C and 13MPa as the cell disruption condition, which produced 1.91g/kg d.s. of carotenoids and 14.03mg/kg d.s. of chlorophylls. Pretreated microalga gave a 10-fold higher chlorophyll extraction yield compared to the untreated sample. While for carotenoids and tocopherols were 1.25 and 1.14-fold higher, respectively. Additionally, antioxidant activity of pretreated microalga (33.22mmol TE/kg oil) was significantly higher than the value for the untreated samples (29.11mmol TE/kg oil) (p≤0.05). Confocal microscopy images showed morphological differences between micro-colonies with and without disruption treatment, suggesting that partial cell disruption by rapid depressurization improved the extraction of microalga compounds.
Microalgae are a broad group of autotrophic organisms which grow by photosynthesis and can be cultivated as a source of bioactive compounds with high commercial value.1Botryococcus braunii is a unicellular green microalga of the class Chlorophyceae, characterized by the production of chlorophyll pigments. B. braunii cells are held together by an extracellular matrix composed of a cross-linked aldehyde polymer core and are capable of producing large amounts of hydrocarbons, exopolysaccharides and carotenoids.2,3 These hydrocarbons are largely stored in the extracellular matrix.4B. braunii is classified into three races A, B and L, depending on the types of hydrocarbons produced.5 The presence of carotenoids is more pronounced in races B and L.6 The carotenoids found include β-carotene, lutein, violaxanthin, canthaxanthin, astaxanthin, zeaxanthin.7,8B. braunii is an interesting microalga for the extraction of high-value compounds for uses in nutraceutical applications.7,9
One of the characteristics of microalgae is the rigidity of their cell walls. In B. braunii the wall of each cell has an internal fibrillar layer made of polysaccharide and an external trilaminar sheath.4 Cell wall of B. braunii is composed of a cellulose-like polysaccharide (as β-1,4- and/or β-1,3-glucan).10 Cell disruption is therefore necessary to release intracellular compounds and improve extraction solvent access.11 The following methods have been used for microalgal cell disruption: sonication,11 high-pressure homogenizers,12 chemical disruption,13 enzymatic degradation,14 bead milling,11,15 and microwaves.16
Studies comparing methods of microalga cell disruption have been reported in literature. Different methods of cell disruption to identify the most effective method for extracting lipids from microalgae (Botryococcus sp., Chlorella vulgaris, and Scenedesmus sp.) was investigated.13 Among the methods tested (autoclaving, bead milling, microwaves, sonication, and treatment with 10% NaCl solution), the microwave oven was the most efficient for lipid recovery. In other study was investigated different cell disruption methods for extracting lipids from microalgae (Chlorella sp., Nostoc sp. and Tolypothrix sp.), including autoclaving, bead milling, microwave, sonication and treatment with 10% NaCl solution.17 The sonication was the most efficient method for lipid recovery. However, the sonication method has been indicated to be unscalable. Bead milling and high-pressure homogenizing are scalable for industrial use. Cell disruption by bead mill is based on subjecting cells to high stress produced by abrasion during rapid agitation with glass or ceramic beads. This method is effective with different types of microorganism.18 In cell disruption by high-pressure homogenizer, the cell suspension is forced to pass through an adjustable discharge valve with a restricted orifice.19 Castor and Hong,20 pointed out that mechanical cell disruption methods are non-selective in cell wall disruption; this leads to the formation of small fragments of cell wall, increasing the downstream purification burden because these fragments are difficult to separate from the process stream.18
Gaspar et al.21 studied the effect of the decompression rate on disruption efficiency in trichomes from origanum bracts. They observed that as the decompression rate increased, the pressure drop across the gland wall also increased, resulting in higher disruption efficiency. Thus, disruption of these glands was caused by a pressure gradient formed across the gland walls during rapid depressurization. During the CO2 compression stage, glands were slightly permeable to the passage of CO2 by a process akin to diffusion.
Studies of cell disruption using CO2 rapid depressurization to improve the availability of extracted solutes have been reported in literature. This method is based on introducing a pressurized subcritical or supercritical gas into the cells followed by rapid depressurization, causing cell disruption.18 During the stage of static compression, supercritical CO2 is very diffusible and can penetrate cells.21 After the cells are saturated with CO2, a sudden depressurisation is applied and a pressure gradient across the cell wall is generated. They observed that as the decompression rate increased, the pressure drop across the gland wall also increased, resulting in higher disruption efficiency. Thus, disruption of these glands was caused by a pressure gradient formed across the gland walls during rapid depressurization. The cell disruption occurs due to the expansion of the CO2, which improves the speed and increases the extraction yield.22,23 Furthermore, cells are exposed to minimal shear forces, so no heat is generated which might damage heat sensitive compounds, such as carotenoids and chlorophylls.
Temperature, pressure and exposure time during static compression are the operational variables that may affect the efficiency of cell disruption.24 Juhász et al.23 studied the effect of CO2 rapid depressurization on endoglucanase recovery from Escherichia coli. The temperature (32–45°C) and pressure (12–25MPa) affected the recovery of the enzyme, while exposure time (5–60min) had no significant effect. Rapid depressurization has been applied to disruption bacterial cells,25–27 yeast cells,28,29 goji berry seeds,30 rape pollen31 and trichomes.21,32
Extraction with solvents is the traditional technique for lipid extraction from microalgae. Lipids are traditionally extracted using nonpolar solvents, commonly n-hexane.33 However, the United States Environmental Protection Agency listed n-hexane among 187 hazardous air pollutants in the 2002 National-Scale Air Toxics Assessments because of its toxic nature.34 Supercritical fluid extraction has received increasing attention as an extraction technique, because it can provide high solubility, improved mass-transfer rates, and increased selectivity with small changes in the temperature and pressure of the extraction operation.35 Carbon dioxide (CO2) is probably the most widely used supercritical fluid, and has emerged as a substitute for n-hexane for the extraction of nonpolar solutes from biological substrates, due to its inertness, non-toxicity, non-flammability and non-explosiveness.36 Furthermore, its relatively low critical properties make CO2 (Tc=31.1°C, Pc=7.38MPa) an ideal solvent for the extraction of thermally labile components. We studied extraction from B. braunii using CO2 supercritical fluid at 40°C and pressures of 12.5, 20 and 30MPa.37 The extraction yield and the fraction of the hydrocarbons in the extracts both increased with pressure and at 30MPa these compounds were obtained rapidly. The authors reported that chlorophylls were not detected in the extracts. Santana et al.38 studied the extraction of lipids from B. braunii for biodiesel production. These experiments were conducted at temperatures from 50 to 80°C and pressures from 20 to 25MPa. Lipid extraction yield was found to decrease with temperature and to increase with pressure. Carotenoids and chlorophylls are important antioxidants.39,40 These pigments can be extracted from B. braunii using supercritical CO2, and can be used as an indicator of cell disruption in the case of photosynthetic microalgae species containing chlorophyll and carotenoids, because they are released when the cell collapse occurs.41
Cell disruption using rapid depressurization can be carried out in supercritical extraction equipment, so that after pretreatment, temperature and pressure conditions are set to carry out the supercritical extraction of the lipidic compounds. This procedure saves time and minimizes equipment and labor requirements as well as contamination and extract loss.24 Also, it can be scaled up to industrial scale.20 If pretreatment and extraction are conducted in the same extractor, the substrate will be protected from exposure to oxygen and high temperature, avoiding oxidation reactions. Microalga cell disruption using CO2 rapid depressurization has not yet been reported in literature. In this work, we hypothesized that cell disruption of B. braunii using rapid depressurization may enhance the extraction yield of pigments, carotenoids and chlorophylls, by adjusting the temperature and pressure conditions under static compression. The objective of this work was to improve the recovery of pigments from B. braunii with supercritical CO2, through a pretreatment of cell disruption using CO2 rapid depressurization. Response surface design was used to evaluate the effect of the static compression conditions on pigment recovery from B. braunii.
Materials and methodsSubstrateB. braunii UTEX LB572 was supplied by Universidad de Antofagasta (Antofagasta, Chile). This strain corresponds to race B and was cultured under outdoor production in pilot-scale panel reactors.42 Microalga samples consisted of air-dried microalgae, which were carefully milled with mortar and pestle until a particle size of less than Tyler mesh 40 was obtained. Average particle size was 0.244mm. The microalga sample had a moisture content of 7.9±0.2/100g dry substrate (d.s.) (determined gravimetrically by drying in an oven for 10h at 102°C) and an oil content of 106±2g/kg d.s. (determined gravimetrically by extraction with technical grade hexane in Soxhlet apparatus for 10h at 70°C). Samples were stored until use in dry, dark conditions, packed in the absence of oxygen.
Substrate pretreatmentThe initial sample of microalga samples consisted of air-dried microalgae. This sample was divided into two fractions: a fraction without treatment by rapid depressurization (as control), and the other fraction subjected to treatment by rapid depressurization. The pretreatment using CO2 rapid depressurization began by loading ca. 5g of microalgae sample (substrate characterized) into a 50cm3 extraction vessel (14-mm internal diameter). The extraction vessel was placed in an air-convection oven of a Spe-ed SFE unit (Applied Separations, Allentown, PA). Prior to pretreatment, air trapped in the extraction vessel was purged by means of a controlled flow of CO2. The extraction vessel was then pressurized with high-purity (99.95% pure) CO2 (Linde, Santiago, Chile), and kept under static compression for 1h, at different combinations of temperature (21–49°C) and pressure (6–13MPa). The CO2 was then quickly released by opening a valve, which allowed the pressure to diminish to normal atmospheric pressure; the extraction vessel was then kept at atmospheric pressure for a further 10min.
Supercritical extractionIt was then re-pressurized and the pretreated substrate was extracted at 40°C (temperature of the air convection oven containing the extraction vessel, controlled automatically) and 30MPa (extraction pressure controlled manually with an air-booster pump), with 4.7L NPT/min of CO2 with a single superficial velocity of 1mm/s. In all cases, the extraction was carried out for 60min (corresponding to a specific consumption of solvent of 101.5kg CO2/kg d.s.), after which the expansion valve (kept at 120°C) was opened. Of this way, rapid depressurization of the microalgae cells and consecutive supercritical CO2 extraction were carried out with the same substrate charge within the extractor vessel. This procedure is similar to that reported in others studies.31,32 The oil recovered was assessed gravimetrically by difference using cleaned and dried glass vials, and the extracted oil yield (Yoil, g/kg d.s.) was measured. Each experimental assay was conducted in duplicate. Recovered oil extracts were used for later analysis.
Analysis of extractsCarotenoid concentration (Ccar, g carotenoids/kg oil) was quantified in an oil sample dissolved in chloroform p.a. (Merck, Darmstadt, Germany). Absorbance was read at 452nm by spectrophotometry in a Genesys 10S UV-Vis spectrophotometer (Thermo Fisher Scientific Inc., Madison, WI).32 The standard for the analysis of carotenoids (β-carotene type I, ≥95% pure) was obtained from Sigma–Aldrich (Saint Louis, MO). The carotenoid extraction yield (Ycar, g/kg d.s.) was obtained from Yoil×Ccar. Chlorophyll concentration (Cchlor, mg chlorophylls a and b/kg oil) was quantified in an oil sample dissolved in ethyl ether p.a. and absorbance was read at 642 and 660nm in the spectrophotometer. Chlorophyll a and b contents were determined using equations reported by Wrolstad et al.43 The chlorophyll extraction yield (Ychlor, mg/kg d.s.) was obtained from Yoil×Cchlor. The tocopherol concentration (Ctoc, g tocopherol/kg oil) of selected oil samples was quantified at 520nm in α-tocopherol equivalents.44 The standard for the analysis of tocopherols α-tocopherol was obtained from Sigma–Aldrich. The tocopherol extraction yield (g/kg d.s.) was estimated from Yoil×Ctoc. Antioxidant activity of selected oil samples was measured using a Trolox equivalent antioxidant capacity assay.45 Antioxidant activity was expressed as millimole Trolox equivalent/kg oil. Trolox standard was obtained from Sigma–Aldrich (Saint Louis, MO).
Confocal microscopyControl sample and microalgae pretreated with rapid depressurization were observed with confocal microscopy. A microalga sample (100mg) was suspended in 1mL of phosphate buffer (pH 7.5 at 25°C) in an Eppendorf tube and filtered through a Millipore filter (Millipore Corp., Bedford, MA). A solution of white calcofluor (Sigma–Aldrich, Saint Louis, MO) in dimethyl sulfoxide (Merck KGaA, Darmstadt, Germany) was prepared in 1:10 ratio v/v. Suspension of microalgae was marked with 100μL of calcofluor solution and incubated at room temperature for 15min. The suspension was centrifuged in a Hitachi centrifuge CT15E (Hitachi Koki Co., Ltd., Tokyo, Japan). Microalgae samples were viewed using a Confocal Laser Microscope FV-1000 (Olympus Corp., Tokyo, Japan). FluoView software FV-10 2.0 (Olympus Corp., Tokyo, Japan) was used for image acquisition (40× magnification).
Experiment designCentral composite rotatable design (CCRD) was used to evaluate the effects of the independent variables: coded temperature (X1, Eq. (1), where T is temperature °C), and coded pressure (X2, Eq. (2), where P is pressure in MPa), both expressed in dimensionless units, on the response variables: extraction yield of carotenoids (Ycar) and chlorophylls (Ychlor).
The design was based on a two-factor factorial design (n=2), with two levels (coded values −1 and +1. The factors and their levels are shown in Table 1. The CCRD matrix had 4 (2n) cube points (runs 1–4) and 4 (2n) star points (runs 5–8), at an axial distance to the center of 1.41 (α=2n/4), and four replications of the center points (runs 9–12) to determine experimental error (Table 1). Experiments were carried out in a randomized order to minimize the effect of unexpected variability in the observed response due to extraneous factors. A second-order model (Eq. (3)) was used to describe the response variable Y as a function of coded temperature (X1) and coded pressure (X2),
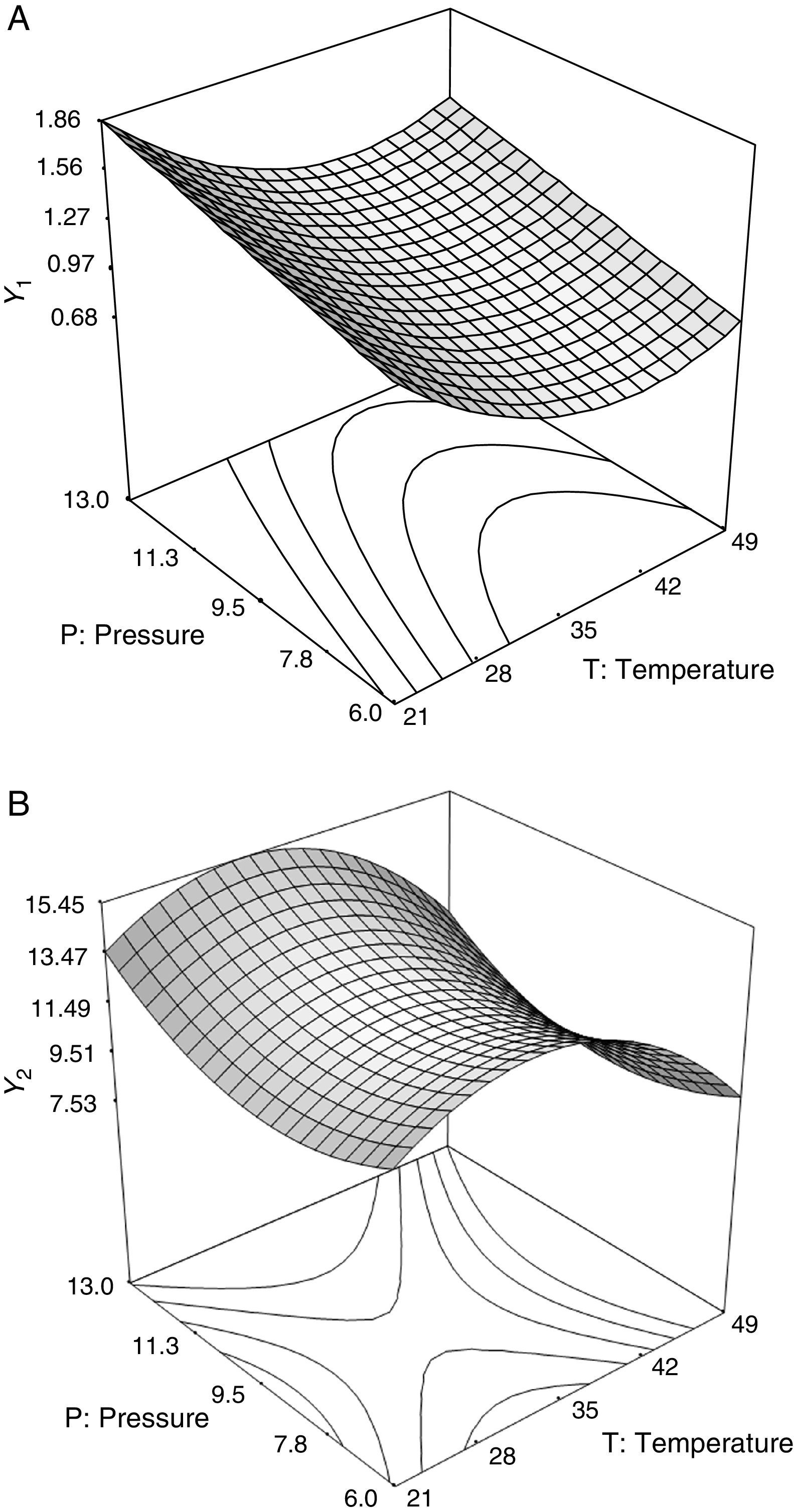
where A0 is a constant; A1 and A2 are linear coefficients; A12 is a cross-product coefficient; and A11 and A22 are quadratic coefficients. Three-dimensional surface response plots were generated by varying the two variables within the experimental range. The model fit was evaluated by analysis of variance (ANOVA). The coefficients of the response surface equation were estimated using Design Expert Design-Expert Software, version 6.0.1 (Stat-Ease, Inc., Minneapolis, MN). The statistical significance was based on the total error criteria with a confidence level of 95%.Extraction yield of carotenoids (Ycar) and chlorophylls (Ychlor) by CO2 at 40°C and 30MPa as a function of temperature and pressure of static compression.
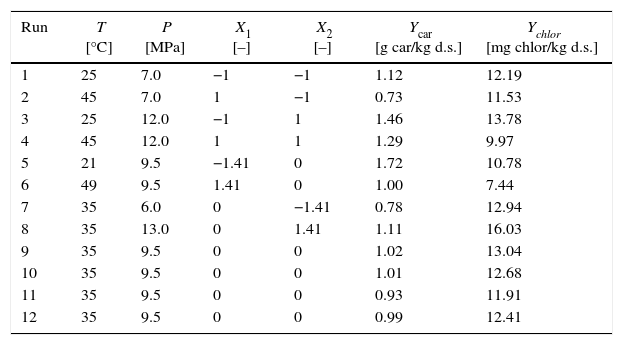
| Run | T [°C] | P [MPa] | X1 [–] | X2 [–] | Ycar [g car/kg d.s.] | Ychlor [mg chlor/kg d.s.] |
|---|---|---|---|---|---|---|
| 1 | 25 | 7.0 | −1 | −1 | 1.12 | 12.19 |
| 2 | 45 | 7.0 | 1 | −1 | 0.73 | 11.53 |
| 3 | 25 | 12.0 | −1 | 1 | 1.46 | 13.78 |
| 4 | 45 | 12.0 | 1 | 1 | 1.29 | 9.97 |
| 5 | 21 | 9.5 | −1.41 | 0 | 1.72 | 10.78 |
| 6 | 49 | 9.5 | 1.41 | 0 | 1.00 | 7.44 |
| 7 | 35 | 6.0 | 0 | −1.41 | 0.78 | 12.94 |
| 8 | 35 | 13.0 | 0 | 1.41 | 1.11 | 16.03 |
| 9 | 35 | 9.5 | 0 | 0 | 1.02 | 13.04 |
| 10 | 35 | 9.5 | 0 | 0 | 1.01 | 12.68 |
| 11 | 35 | 9.5 | 0 | 0 | 0.93 | 11.91 |
| 12 | 35 | 9.5 | 0 | 0 | 0.99 | 12.41 |
The experimental results of the extraction yields of carotenoids (Ycar) and chlorophylls (Ychlor) as a function of the temperature and pressure applied during static compression are shown in Table 1. Experimental data is the average of two measurements. We note that the pressure drop occurred in the first 10s. For cell disruption of rape pollen collected by bees, was reported that the pressure (treatment at 45MPa) was quickly released within 1min.31 For treatment of microorganism with compressed CO2, was reported that a rapid release of the CO2 pressure (1.5–5MPa) was within 4s.26 Oil extraction yield (Yoil) ranged from 84.43 to 103.01g/kg d.s. (full data not shown). Carotenoid extraction yield (Ycar, g/kg d.s.) ranged from 0.73 to 1.72g/kg d.s., 2.4-fold differences. Chlorophyll extraction yield (Ychlor, mg/kg d.s.) ranged from 7.44 to 16.03mg/kg d.s., 2.2-fold differences.
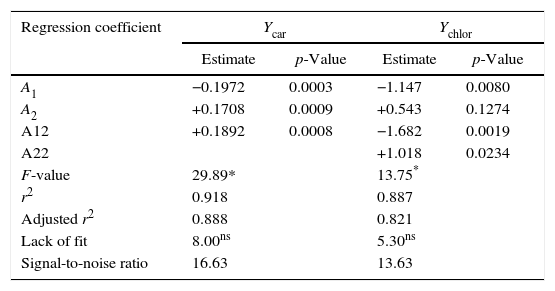
Table 2 summarizes the statistical indicators obtained from the analysis of variance applied to the second-order model selected (Eqs. (4) and (5)). The model was considered adequate because of the significance of the model (p≤0.001), the non-significance of the lack of fit (p>0.05) relative to pure error, the high signal-to-noise ratio (>4) and high coefficient of determination (r2). For instance, this coefficient indicates that the model explains 91.8% of the variability in the carotenoid extraction yield Ycar. The information provided by the statistical indicators was complemented by a good correlation between predicted and experimental responses within the experimental range investigated, for both responses, since the plot shows a close fit of the experimental with the predicted values (data non shown).
Analysis of variance of regression coefficients and statistical indicators of appropriateness of the second order model selected.
| Regression coefficient | Ycar | Ychlor | ||
|---|---|---|---|---|
| Estimate | p-Value | Estimate | p-Value | |
| A1 | −0.1972 | 0.0003 | −1.147 | 0.0080 |
| A2 | +0.1708 | 0.0009 | +0.543 | 0.1274 |
| A12 | +0.1892 | 0.0008 | −1.682 | 0.0019 |
| A22 | +1.018 | 0.0234 | ||
| F-value | 29.89* | 13.75* | ||
| r2 | 0.918 | 0.887 | ||
| Adjusted r2 | 0.888 | 0.821 | ||
| Lack of fit | 8.00ns | 5.30ns | ||
| Signal-to-noise ratio | 16.63 | 13.63 | ||
Analysis of variance was used to evaluate the significance of the model's regression coefficients (Table 2). A large regression coefficient and a small p-value would indicate a more significant effect on the response variable. Significant coefficients (p>0.05) were used to write the second order models. The variable with the largest effect on the carotenoid extraction yield was the linear term of the temperature (p=0.0003), followed by the quadratic term of the temperature (p=0.0008) and the linear term of the pressure (p=0.0009). There was no significant effect of the quadratic term of the pressure (p=0.5212), nor of the interaction term between temperature and pressure (p=0.2708). Thus the second-order model (Eq. (4)) establishes a statistically significant relationship between carotenoid recovery and the temperature and pressure conditions when carrying out rapid depressurization in the selected experimental range (6≤P≤13MPa; 21≤T≤49°C). Coded variables are important because they give a direct, quantitative indication of the effect of the independent variables on any dependent variable as a function of the selected experimental range. The term A2 (=+0.1782), which multiplies the linear term X2, indicates that the carotenoid extraction yield increases by 0.1782g/kg d.s. when the compression pressure increases by 2.5 [=0.5(12–7)]MPa while operating at 35°C (X1=0).
The variable with the largest effect on the chlorophyll extraction yield was the quadratic term of the temperature (p=0.0019), followed by the linear term of the temperature (p=0.0080) and the quadratic term of the pressure (p=0.0234). There was no significant effect of the linear term of the pressure (p=0.1274) or of the interaction term between temperature and pressure (p=0.0670). Thus, a statistically significant relationship between chlorophyll recovery and the temperature and pressure conditions has been established with the following second-order model (Eq. (5)). The linear term of the pressure (A2=+0.543) was not removed from Eq. (5) to maintain the hierarchy of the model. To better visualize the effect of the temperature and pressure on the recovery of carotenoids and chlorophylls in the experimental range, surface response graphs were generated using the second-order models
Effects of temperature and pressureCarotenoid recovery decreased with increasing temperature in the lower temperature range (<40°C). There was a negative linear effect (A1=−0.1972) (Eq. (4)) of temperature. Thus, we observed that carotenoid recovery decreases from 1.86 to 1.16g/kg d.s. when temperature increases from 21 to 40°C at 13MPa. In the upper temperature range, over 40°C, the positive non-linear effect of temperature (A12=+0.1892) becomes important and carotenoid recovery increases slightly with temperature. Similar behavior for Ycar was observed in the lower pressure range.
To chlorophylls recovery, the negative linear effect of temperature (A1=−1.147) (Eq. (5)) was observed in the upper temperature range (over ∼35°C). When the temperature increased from 35 to 49°C at 13MPa, Ychlor decreased from 15.26 to 10.36mg/kg d.s. The negative non-linear effect of temperature (A12=−1.682) becomes important for temperatures below 35°C. This was reflected in the plateau for temperatures between 30 and 35°C, where there was a minimal change in Ychlor and 15.41 and 15.26mg/kg d.s. respectively were obtained. Similar behavior for Ychlor for temperature changes was observed in the lower pressure range. According to analysis of variance, the quadratic term of temperature contributed 28% and 37% to explaining the behavior of Ycar and Ychlor, respectively. The quadratic term of pressure contributed 13% to explaining the behavior of Ychlor.
Carotenoid recovery increased with pressure (A2=+0.1708) (Eq. (4)) to whatever temperature during static compression. There was a positive effect of pressure on Ycar. For instance Ycar increased from 1.38 to 1.86g/kg d.s. (1.3-fold increase) when the pressure increased from 6 to 13MPa at 21°C. Similar behavior was observed for Ychlor for pressure increases, in the upper pressure range over 9.5MPa. There was a positive effect of pressure on Ychlor. For instance Ychlor increased from 10.81 to 13.57mg/kg d.s. (1.3-fold increase) when the pressure increased from 9.5 to 13MPa at 21°C. For pressures below 9.5MPa, the non-linear effect of pressure (A22=+1.018) becomes important, and we observed slight changes in Ychlor with pressure. Supercritical extractions were performed under constant temperature and pressure conditions, therefore the solvent power of CO2 should not change within the extraction assays. However, microalga extracts are complex mixtures of several interacting compounds. Probably the effect of quadratic terms would be explained by the existence of solute–solute interactions (anti-solvency and co-solvency effects) and/or solute–matrix interactions that affect the solubility behavior of a compound of the mixture in the supercritical phase and its recovery yield46 (Fig. 1).
Cell disruption studies using CO2 rapid depressurization have been reported in literature. The cell disruption of Ralstonia eutropha to extract poly (β-hydroxybutyrate) (PHB) was studied.27 A multipurpose SFE-SFC system was used for the cell disruption, and its efficiency was measured as PHB recovery. Extraction yield increased with increasing pressure from 15 to 20MPa, but decreased at 30MPa. The authors attribute the negative effect of high pressure to the fact that disruption of the cytoplasm membrane occurs faster than disruption of the cell wall at 30MPa, resulting in cell wall shrinkage, which hinders the extraction of intracellular compounds. In a similar study on the recovery of PHB from R. eutropha cells, it was reported that PHB recovery decreased with increasing pressure from 25 to 35MPa at constant temperature.47 Was studied the cell disruption of E. coli using CO2 rapid depressurization for endoglucanase enzyme recovery.23 The pressure during static compression (10–25MPa) had a significant positive effect on enzyme recovery. Higher pressure resulted in better enzyme recovery. Therefore, the positive effect of pressure during static compression on cell disruption has been observed at pressures below 20MPa. With respect to the compression temperature, Hejazi et al.27 reported that biopolymer recovery decreased when the compression temperature rose from 40 to 70°C. The authors attributed this effect to the supercritical CO2 being closer to its liquid state at lower temperatures: when rapid depressurization occurs, the CO2 reaches its gas state with maximum volume change, which in turn causes more extensive cell wall disruption. Also, Khosravi-Darani et al.47 reported that PHB recovery decreased with increasing temperature from 30 to 40°C at constant pressure. Thus a negative effect of temperature during static compression on cell disruption using rapid depressurization has been observed.
According to our results, the extraction of carotenoids was best at 21°C and 13MPa with Ycar at 1.87g/kg d.s., while the extraction of chlorophyll was best at 32°C and 13MPa, with Ychlor at 15.45mg/kg d.s. In other words higher pigment extraction was obtained in the experimental range of high pressure and low temperature, which agrees with the observations of previous authors.23,27,47 This range is characterized by higher CO2 density (about 810–880kg/m3). When the CO2 is denser, its solvent power increases and in consequence so does the permeation capability of CO2 within the cell. Furthermore, when the CO2 density increases, the net amount of gas absorbed into the cells increases and this increases the force caused by rapid depressurization.47 A major change in the volume occurs when the CO2 returns to the gaseous state due to rapid depressurization.27 This sudden volume change results in more effective cell disruption and improves the availability of pigments by supercritical extraction.
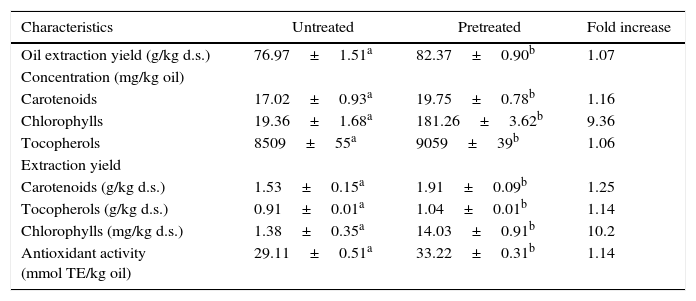
Comparison between untreated and pretreated microalgaeTable 3 shows a comparison of supercritical CO2 extraction (40°C and 30MPa) using untreated microalgae and microalgae pretreated using rapid depressurization. For rapid depressurization pretreatment, we selected 21°C and 13MPa conditions to favor recovery of carotenoids and chlorophylls. The static time was kept constant at 60min. The extraction yields for carotenoid and chlorophyll reported in Table 3 confirm the predictive power of the selected second-order models (Eqs. (4) and (5)). Table 3 shows that there was significant difference (p≤0.05) in compound recovery and antioxidant activity between oils extracted with supercritical CO2 from untreated and pretreated samples using rapid depressurization. We included the measurement of antioxidant activity and quantified total tocopherols, which are important antioxidants. The highest difference was observed in the extraction of chlorophylls, 1.38 and 14.03mg/kg d.s., for untreated and pretreated samples respectively. Chlorophyll pigments are produced and stored intracellularly in the chloroplasts.48 Mendes et al.37 reported that in supercritical extracts (at 40°C and 12.5–30MPa) obtained from freeze-dried samples of B. braunii, chlorophylls were not detected. Therefore, the higher extraction of chlorophylls from pretreated samples with rapid depressurization would be a very good indicator of cell disruption. To support these results, observations were made under confocal laser microscopy.
Comparison of supercritical CO2 extraction (40°C and 30MPa) between microalgae untreated and pretreated with rapid decompression (21°C and 13MPa).
| Characteristics | Untreated | Pretreated | Fold increase |
|---|---|---|---|
| Oil extraction yield (g/kg d.s.) | 76.97±1.51a | 82.37±0.90b | 1.07 |
| Concentration (mg/kg oil) | |||
| Carotenoids | 17.02±0.93a | 19.75±0.78b | 1.16 |
| Chlorophylls | 19.36±1.68a | 181.26±3.62b | 9.36 |
| Tocopherols | 8509±55a | 9059±39b | 1.06 |
| Extraction yield | |||
| Carotenoids (g/kg d.s.) | 1.53±0.15a | 1.91±0.09b | 1.25 |
| Tocopherols (g/kg d.s.) | 0.91±0.01a | 1.04±0.01b | 1.14 |
| Chlorophylls (mg/kg d.s.) | 1.38±0.35a | 14.03±0.91b | 10.2 |
| Antioxidant activity (mmol TE/kg oil) | 29.11±0.51a | 33.22±0.31b | 1.14 |
Different letters in same row indicate significant difference at p≤0.05.
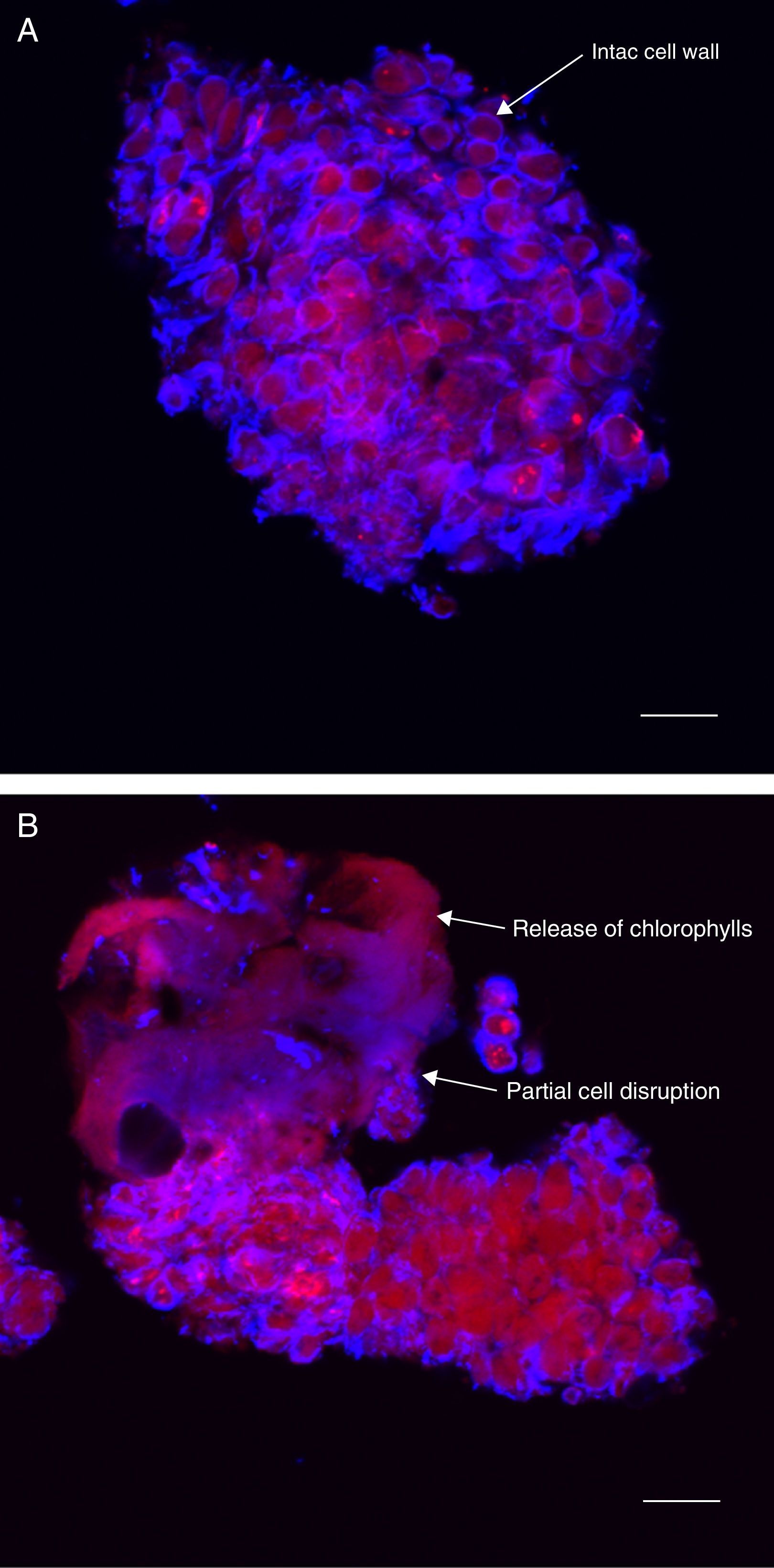
Confocal microscopy images in Fig. 2 show the effect of CO2 rapid depressurization pretreatment on the morphology of microalgae cells. Fig. 2A shows a micro-colony of B. braunii not treated with rapid depressurization as control sample. Intact cell walls can still be distinguished in the micro-colony. This is clear when we observe the walls surrounding the cells stained blue (emission 450nm), due to the reaction of calcofluor with the cellulose components of the cell wall. Fig. 2B shows major destruction of cell walls in the micro-colony to which CO2 rapid depressurization was applied: large cell wall integrity was lost. Cell units are not clearly differentiated, since some cells have fused together. In addition, red areas appear due to an increase in the release of chlorophyll pigments (emission 650–750nm), which would permit higher extraction of chlorophyll pigments with supercritical CO2 as compared to the control sample (without treatment with rapid depressurization). There was therefore an observable difference in cell wall integrity between the untreated and pretreated samples, which resulted in increased extraction of carotenoids, chlorophylls and tocopherols, and in higher antioxidant activity in pretreated samples. These observations suggest that pretreatment using CO2 rapid depressurization improved pigment extraction due to partial cell disruption of the microalgae.
ConclusionsThis work investigated cell disruption using CO2 rapid depressurization as a substrate pretreatment for pigment extraction from the microalga B. braunii. According to the response surfaces we suggest a region of low temperature and high pressure for the static compression condition, which favors the extraction of carotenoids and especially chlorophylls. The combination of high pressure (13MPa) and low temperature (21°C) during static compression was selected for the pretreatment of microalgae with purposes of comparison with untreated microalgae. Oil from pretreated microalgae presented better antioxidant activity due to the higher concentration of carotenoids, chlorophylls and tocopherols. From confocal microscopy images, it was observed that partial cell disruption of microalgae improved pigment extraction. CO2 rapid depressurization is a simple and efficient method that can be employed to recover important intracellular components, such as the pigments from B. braunii. Moreover, this pretreatment is performed at moderate temperatures which would protect these heat sensitive compounds. This is the first report on cell disruption using CO2 rapid depressurization of a microalga.
Conflicts of interestThe authors declare no conflicts of interest.
This research was funded by the Universidad de La Frontera (project DI11-0055) and Innova-CORFO (project 09CTEI-6860). Our acknowledgments to Agriaquaculture Nutritional Genomic Center, CGNA (R10C1001).