Sugarcane straw has become an available lignocellulosic biomass since the progressive introduction of the non-burning harvest in Brazil. Besides keeping this biomass in the field, it can be used as a feedstock in thermochemical or biochemical conversion processes. This makes feasible its incorporation in a biorefinery, whose economic profitability could be supported by integrated production of low-value biofuels and high-value chemicals, e.g., xylitol, which has important industrial and clinical applications. Herein, biotechnological production of xylitol is presented as a possible route for the valorization of sugarcane straw and its incorporation in a biorefinery. Nutritional supplementation of the sugarcane straw hemicellulosic hydrolyzate as a function of initial oxygen availability was studied in batch fermentation of Candida guilliermondii FTI 20037. The nutritional supplementation conditions evaluated were: no supplementation; supplementation with (NH4)2SO4, and full supplementation with (NH4)2SO4, rice bran extract and CaCl2·2H2O. Experiments were performed at pH 5.5, 30°C, 200rpm, for 48h in 125mL Erlenmeyer flasks containing either 25 or 50mL of medium in order to vary initial oxygen availability. Without supplementation, complete consumption of glucose and partial consumption of xylose were observed. In this condition the maximum xylitol yield (0.67gg−1) was obtained under reduced initial oxygen availability. Nutritional supplementation increased xylose consumption and xylitol production by up to 200% and 240%, respectively. The maximum xylitol volumetric productivity (0.34gL−1h−1) was reached at full supplementation and increased initial oxygen availability. The results demonstrated a combined effect of nutritional supplementation and initial oxygen availability on xylitol production from sugarcane straw hemicellulosic hydrolyzate.
Brazil is the largest sugarcane producer in the world, responsible for approximately 38% of global production.1 In the season 2014/2015, the production level reached 634.8 millions metric tons.2 It is estimated that 28% (on a dry weight basis) of the harvested sugarcane corresponds to lignocellulosic byproducts, bagasse and straw (tops, dry and green leaves), which represent two-thirds of the energetic potential of sugarcane.3 Currently, sugarcane bagasse, produced during sugarcane milling, is widely used as a fuel in a cogeneration process for energy production, and as a feedstock in bioprocesses research for production of lignocellulosic ethanol and chemicals.3,4
Also, sugarcane straw is becoming an available lignocellulosic biomass, due to the progressive introduction of the non-burning harvest (green management), which aims to improve the crop sustainability.3,5 According to these authors, this harvest system leads to deposition of 7–25 metric tons/ha of sugarcane straw on the soil, corresponding to a straw-to-stalk ratio of 9–30%. The mulch formed has important agronomic and economic impacts and may potentially cause positive or negative effects on crop yield, soil fertility, fertilizer management, weed control, soil erosion and organic matter dynamics, depending on the characteristics of each area.5
Besides the agronomic benefits of keeping this biomass in the field, sugarcane straw can be used as a feedstock in thermochemical and biochemical conversion processes, making feasible its incorporation in a future sugarcane biorefinery. In fact, it has been proposed that the incorporation of both sugarcane bagasse and straw would be essential for the development of a sugarcane biorefinery with capabilities to produce sugar, first- and second-generation ethanol, electrical and thermal energy, and chemical products, under economic, social and environmental sustainability.6 Currently, the main uses proposed for sugarcane straw are its complete utilization as a fuel for energy generation and its depolymerization for ethanol and energy production.3,4,7
It is also known that the profitability of a low-value biofuel-producing biorefinery can be supported by integrated production of high-value chemicals.8 One of the high-value chemicals as defined by the Department of Energy of the United States (US-DOE), which can fulfill this approach, is xylitol.9 Xylitol is a natural sweetener, which has been highly valued for its important applications in food, odontological, and pharmaceutical industries, and as a platform chemical for the synthesis of other valuable chemicals.10 Currently, commercial production of xylitol is based on catalytic hydrogenation of xylose purified from hemicellulosic hydrolyzates, a process that is considered to be inefficient and expensive due to the complexity of the xylose purification procedure.11,12
Biotechnological production of xylitol from lignocellulosic biomass has been widely studied as an alternative to the commercial chemical process, since neither purification of xylose nor high temperatures and pressures are required for this biologically selective process.11–13 This bioprocess is based on the ability of pentose-assimilating yeasts to reduce xylose to xylitol as the first step of xylose metabolism, with participation of NAD(P)H-dependent xylose reductase (XR, E.C.1.1.1.21).14–16 According to these authors, accumulation of xylitol is caused by a NADH/NAD+ imbalance, i.e., low levels of NAD+ and high levels of NADH, resulting from limited O2 supply and a consequent reduction in cell respiratory rate. This prevents the oxidation of xylitol to xylulose by inhibiting NAD(P)+-dependent xylitol dehydrogenase (XDH, E.C.1.1.1.9), and, as a result, its integration into the central carbon metabolism through the pentoses phosphate pathway (PPP). According to Silva and colleagues17 and Yablochkova and colleagues,15 while some yeasts can compensate for the reduced NAD+ regeneration using NADH as a cofactor for XR, this enzyme is exclusively dependent on NADPH in other yeasts, such as Candida guilliermondii, which makes them good candidates for xylitol production.
It is known that the requirement for nutritional supplementation is an important factor for the use of hemicellulosic hydrolyzates as suitable fermentation media for xylose-to-xylitol bioconversion.18–20 It has already been demonstrated that supplementation requirements depend on the raw material characteristics, due to the fact that the composition of a hemicellulosic hydrolyzate varies according to the raw material, presenting different contents of minerals, trace elements, vitamins, proteins, among others, which are compounds that can be used as nutrients for microbial growth and metabolism.20,21 Regarding the fermentative process, it is widely recognized that a limited-oxygen atmosphere is one of the most important factors that must be controlled to increase the process efficiency.16,17 According to Somerville and Proctor,22 oxygen supply in batch cultures at a laboratory scale is a function of the agitation speed and the ratio between the volumes of a culture medium and a flask.
The present work aimed to use the hemicellulosic fraction of sugarcane straw as a feedstock for xylose-to-xylitol bioconversion by C. guilliermondii FTI 20037, as a possible route for its valorization and incorporation in a biorefinery. To the best of our knowledge, this is the first work on biotechnological production of xylitol from sugarcane straw hemicellulosic hydrolyzate. Therefore, nutritional supplementation was studied as a function of initial oxygen availability in order to establish a suitable formulation of the fermentation medium that would favor optimal yeast performance, which in turn affects the cost of this bioprocess.
Materials and methodsSugarcane strawThe sugarcane straw was kindly provided by Usina Santa Adélia, Jaboticabal, São Paulo, Brazil. It was collected directly in the form left in the field after the harvest. The straw was dried until moisture content was 11.25%. The biomass in nature was chemically characterized for cellulose, hemicellulose and lignin contents, according to the methodology proposed by Gouveia and colleagues.23
Preparation of hemicellulosic hydrolyzateThe sugarcane straw was subjected to dilute-acid hydrolysis with 1.0% (w/v) H2SO4 in a 35-L steel reactor at 121°C for 20min at a 1:10 solid/liquid ratio. The hemicellulosic hydrolyzate was filtered and vacuum-concentrated at 70°C to increase the initial concentration of xylose by threefold.24 Next, it was subjected to a detoxification treatment consisting of a pH adjustment to 7.0 with CaO (commercial grade) and to 2.5 with H3PO4, followed by the addition of 1.0% (w/v) activated charcoal (refined powder) and incubation at 60°C, 100rpm for 30min.25 All the precipitates formed during the process were removed by vacuum filtration. The treated hemicellulosic hydrolyzate was autoclaved at 111°C, for 15min to be used as a fermentation medium.
Microorganism and inoculum preparationFor the fermentation experiments, the yeast C. guilliermondii FTI 20037 maintained at 4°C on malt-extract agar slants was used. The medium used for inoculum preparation contained (gL−1): xylose (30.0), rice bran extract (20.0), (NH4)2SO4 (2.0), and CaCl2·2H2O (0.1). The rice bran was kindly provided by the local farm Beneficiadora de Arroz Irmãos Ligabo, Canas, São Paulo, Brazil. For rice bran extract preparation, a mixture of the rice bran (200g) and distilled water (1L) was autoclaved (111°C, for 15min). After cooling, the mixture was centrifuged (2000×g, for 20min) under aseptic conditions and the supernatant, corresponding to the rice bran extract, was aseptically transferred to a sterilized flask. A loopful of cells grown on malt extract agar was transferred to the medium (50mL) used for inoculum preparation in Erlenmeyer flasks (125mL) and the culture was incubated on a rotary shaker at 30°C, 200rpm for 24h. Afterwards, the cells were separated by centrifugation (2000×g, for 20min), rinsed twice with distilled water, and the cell pellet was resuspended in an appropriate volume of distilled water to be used as an inoculum.
Medium and fermentation conditionsThe fermentation medium consisted of the sugarcane straw hemicellulosic hydrolyzate, with the following composition (gL−1): xylose (50.60), glucose (9.75), arabinose (9.60), acetic acid (2.87), 5-hydroxymethylfurfural (5-HMF) (0.04). The following three nutritional supplementation conditions were evaluated: H1, not supplemented; H2, supplemented with (NH4)2SO4 (2.0gL−1); and H3, fully supplemented with rice bran extract (20.0gL−1), (NH4)2SO4 (2.0gL−1) and CaCl2·2H2O (0.1gL−1). The initial pH was adjusted to 5.5 and was not controlled during runs. Batch fermentations were carried out in 125-mL Erlenmeyer flasks, containing 25 or 50mL of the fermentation medium in order to vary the initial oxygen availability. The initial cell biomass concentration in all the flasks was 1gL−1. The flasks were incubated on a rotary shaker at 30°C, 200rpm for 48h.
Analytical methodsThe concentrations of xylose, glucose, arabinose, acetic acid, xylitol, glycerol, and ethanol were determined by high-performance liquid chromatography (HPLC) (Shimadzu LC-10AD, Kyoto, Japan), using a refractive index detector and a Bio-Rad (Hercules, CA, USA) Aminex HPX-87H column at 45°C, with 0.01N H2SO4 as an eluent, at a flow rate of 0.6mLmin−1. The concentrations of furfural and 5-HMF were also determined by HPLC, using an ultraviolet light detector (SPD-10A UV-VIS, Waters Corp., Milford, MA, USA) and an RP-18 column (Hewlett-Packard, Palo Alto, CA, USA) at 25°C, with acetonitrile:water (1:8) and 10% acetic acid as an eluent and a flow rate of 0.8mLmin−1. Cell growth was monitored by measuring absorbance at 600nm (DU 640B spectrophotometer, Beckman Coulter, Brea, CA, USA) and calculated based on the relationship between absorbance and cell dry weight.
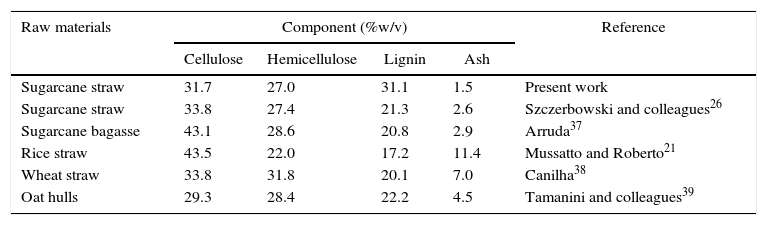
Results and discussionThe suitability of the hemicellulosic fraction of sugarcane straw was studied as a feedstock for the biotechnological production of xylitol. Table 1 shows the structural composition of the sugarcane straw, in comparison with other lignocellulosic biomasses. The proportion of the hemicellulosic fraction (27.0%) determined in this study was similar to that found by Szczerbowski and colleagues26 also in sugarcane straw (27.4%), as well as similar to the values reported for other biomasses that were evaluated for xylose-to-xylitol bioconversion, thus suggesting that sugarcane straw can be an adequate feedstock for this bioprocess.
Structural chemical composition of sugarcane straw and comparison with other lignocellulosic biomasses.
| Raw materials | Component (%w/v) | Reference | |||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Ash | ||
| Sugarcane straw | 31.7 | 27.0 | 31.1 | 1.5 | Present work |
| Sugarcane straw | 33.8 | 27.4 | 21.3 | 2.6 | Szczerbowski and colleagues26 |
| Sugarcane bagasse | 43.1 | 28.6 | 20.8 | 2.9 | Arruda37 |
| Rice straw | 43.5 | 22.0 | 17.2 | 11.4 | Mussatto and Roberto21 |
| Wheat straw | 33.8 | 31.8 | 20.1 | 7.0 | Canilha38 |
| Oat hulls | 29.3 | 28.4 | 22.2 | 4.5 | Tamanini and colleagues39 |
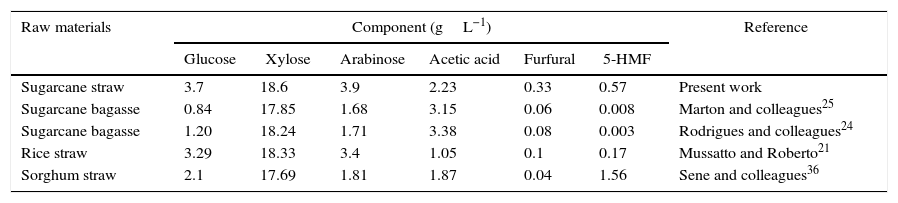
The chemical composition of the sugarcane straw hemicellulosic hydrolyzate obtained by dilute-acid hydrolysis (not concentrated and not detoxified) is shown in Table 2 in comparison with other hemicellulosic hydrolyzates obtained under similar hydrolysis conditions. Xylose was the major monosaccharide, representing 71% of the sugar content, and its concentration (18.6gL−1) was in the range of other hemicellulosic hydrolyzates. Glucose and arabinose constituted 14.1 and 14.9%, respectively. For comparison, Szczerbowski and colleagues26 found that xylose represented 75% of the hemicellulosic fraction of sugarcane straw. The high xylose content of the sugarcane straw hemicellulosic hydrolyzate supported the suitability of sugarcane straw as a feedstock for biotechnological xylitol production.
Composition of sugarcane straw hemicellulosic hydrolyzate and comparison with other hemicellulosic hydrolyzates.
| Raw materials | Component (gL−1) | Reference | |||||
|---|---|---|---|---|---|---|---|
| Glucose | Xylose | Arabinose | Acetic acid | Furfural | 5-HMF | ||
| Sugarcane straw | 3.7 | 18.6 | 3.9 | 2.23 | 0.33 | 0.57 | Present work |
| Sugarcane bagasse | 0.84 | 17.85 | 1.68 | 3.15 | 0.06 | 0.008 | Marton and colleagues25 |
| Sugarcane bagasse | 1.20 | 18.24 | 1.71 | 3.38 | 0.08 | 0.003 | Rodrigues and colleagues24 |
| Rice straw | 3.29 | 18.33 | 3.4 | 1.05 | 0.1 | 0.17 | Mussatto and Roberto21 |
| Sorghum straw | 2.1 | 17.69 | 1.81 | 1.87 | 0.04 | 1.56 | Sene and colleagues36 |
It is known that the presence of glucose in a fermentation medium can affect, positively or negatively, xylitol production, depending on the glucose/xylose ratio.27,28 According to Table 2, the glucose concentration in the sugarcane straw hemicellulosic hydrolyzate was higher than in other hemicellulosic hydrolyzates; nevertheless, the glucose/xylose ratio in the sugarcane straw hemicellulosic hydrolyzate was approximately 1/5, which had been found by Silva and Felipe28 to be the optimum ratio for xylitol production by C. guilliermondii FTI 20037 from a sugarcane bagasse hemicellulosic hydrolyzate. Regarding toxic compounds, it was confirmed that the concentrations of acetic acid, furfural and 5-HMF in the sugarcane straw hemicellulosic hydrolyzate were in most cases higher than those found in the hydrolyzates of other biomasses, except for acetic acid in sugarcane bagasse and 5-HMF in sorghum straw (Table 2). Nevertheless, the determined values for all the toxic compounds were lower than the reported inhibitory concentrations for xylose consumption and xylitol production by C. guilliermondii in semi-synthetic media29–31 and hemicellulosic hydrolyzates.30,32
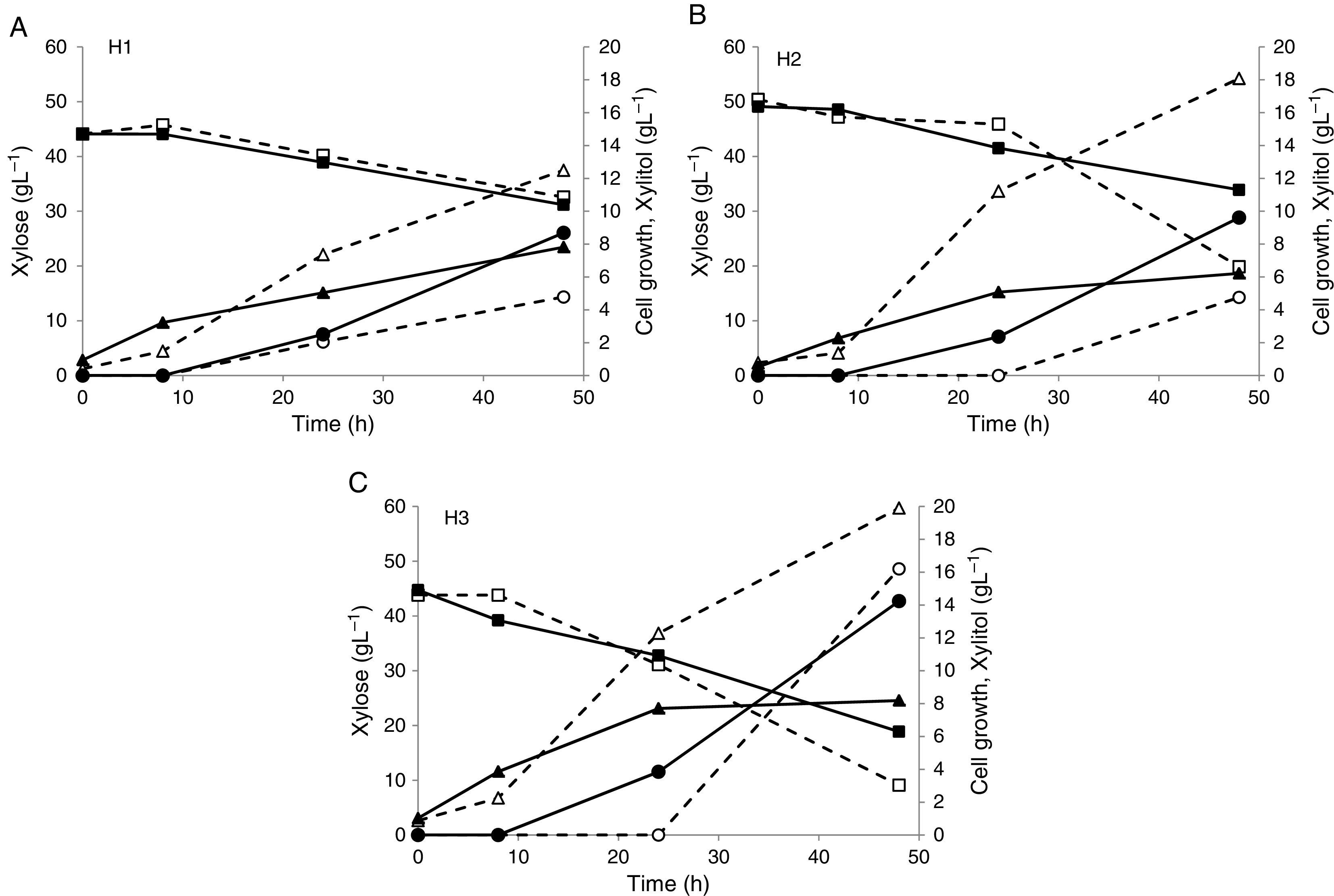
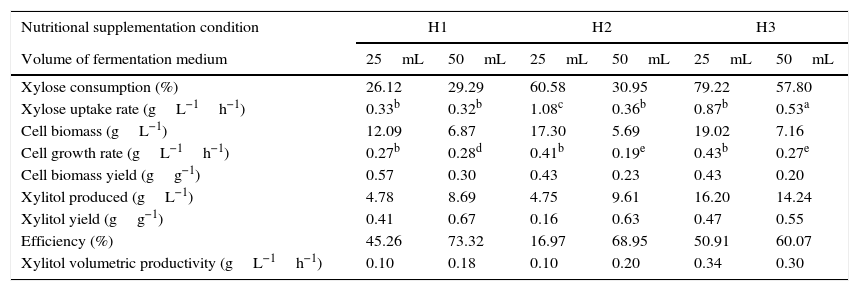
The profiles of xylose consumption, cell growth and xylitol production by C. guilliermondii FTI 20037 growing in the sugarcane straw hemicellulosic hydrolyzate are presented in Fig. 1A–C as functions of nutritional supplementation and initial oxygen availability. Table 3 summarizes the fermentative parameters for all the experiments. In general, the results obtained in the present work showed that xylose consumption, cell growth and xylitol production were improved by nutritional supplementation. Furthermore, it was observed that xylose utilization for cell growth or xylitol production was dependent on the initial oxygen availability, since the experiments were performed under conditions of increased and reduced initial oxygen availability, which were established by the use of different medium volumes (25 and 50mL, respectively) in the Erlenmeyer flasks (150mL).
Profiles of xylose (square), xylitol (circle) and cell biomass (triangle) in batch fermentation with C. guilliermondii FTI 20037 in sugarcane straw hemicellulosic hydrolyzate with different nutritional supplementation and the two conditions of initial oxygen availability: (A) without supplementation (H1); (B) supplementation with (NH4)2SO4 (H2); (C) full supplementation with (NH4)2SO4, rice bran extract and CaCl2·2H2O (H3). Dashed line (---) and open style: increased initial oxygen availability (25mL of fermentation medium). Solid line (—) and solid style: Reduced initial oxygen availability (50mL of fermentation medium).
Parameters of C. guilliermondii FTI 20037 fermentation for each evaluated condition.
| Nutritional supplementation condition | H1 | H2 | H3 | |||
|---|---|---|---|---|---|---|
| Volume of fermentation medium | 25mL | 50mL | 25mL | 50mL | 25mL | 50mL |
| Xylose consumption (%) | 26.12 | 29.29 | 60.58 | 30.95 | 79.22 | 57.80 |
| Xylose uptake rate (gL−1h−1) | 0.33b | 0.32b | 1.08c | 0.36b | 0.87b | 0.53a |
| Cell biomass (gL−1) | 12.09 | 6.87 | 17.30 | 5.69 | 19.02 | 7.16 |
| Cell growth rate (gL−1h−1) | 0.27b | 0.28d | 0.41b | 0.19e | 0.43b | 0.27e |
| Cell biomass yield (gg−1) | 0.57 | 0.30 | 0.43 | 0.23 | 0.43 | 0.20 |
| Xylitol produced (gL−1) | 4.78 | 8.69 | 4.75 | 9.61 | 16.20 | 14.24 |
| Xylitol yield (gg−1) | 0.41 | 0.67 | 0.16 | 0.63 | 0.47 | 0.55 |
| Efficiency (%) | 45.26 | 73.32 | 16.97 | 68.95 | 50.91 | 60.07 |
| Xylitol volumetric productivity (gL−1h−1) | 0.10 | 0.18 | 0.10 | 0.20 | 0.34 | 0.30 |
H1: without supplementation; H2: supplementation with (NH4)2SO4; H3: full supplementation with (NH4)2SO4, rice bran extract and CaCl2·2H2O.
25mL of fermentation medium: increased initial oxygen availability; 50mL of fermentation medium: reduced initial oxygen availability.
Xylose uptake rate and cell growth rate were quantified during the period of maximum xylose consumption and cell growth, respectively, as is denoted by the following symbols:
It is important to point out that in the experiments without nutritional supplementation (H1, Fig. 1A) the yeast was able to completely consume glucose (data not shown) and partially consume xylose, resulting in cell growth and xylitol production (Table 3). This result suggested that the sugarcane straw hemicellulosic hydrolyzate could supply a basal level of nutrients to support the microbial metabolism. A similar observation was made for the same yeast grown in hemicellulosic hydrolyzates of rice straw20 and brewer's spent grain.21 The authors suggested that, in the case of the rice straw, this fact was associated to its high ash content (11.4%), indicating that the ash contained minerals, trace elements and vitamins20; and, in the case of the brewer's spent grain, due to its elevated protein content (15.25%) and a large variety of minerals.21 In the present work, the ash content of the sugarcane straw (1.5%) was lower than the values reported for the above-mentioned materials (Table 1), while the protein and mineral contents of the sugarcane straw were not determined. Hence, further studies are necessary to establish the feasibility of developing a technology for xylitol production from sugarcane straw hemicellulosic hydrolyzate without nutritional supplementation.
Without supplementation (H1), xylose consumption was 26.12% and 29.29% under conditions of increased and reduced initial oxygen availability, respectively (Table 3). In the experiments with increased initial oxygen availability, xylose consumption was incremented by 132% relative to H1 with the supplementation of (NH4)2SO4 (H2). An additional increase of xylose consumption by 31% relative to H2 was obtained with the full supplementation of (NH4)2SO4, rice brain extract and CaCl2·2H2O (H3), corresponding to a total improvement of 200% between H3 and H1 (Table 3). On the other hand, in the experiments with reduced initial oxygen availability, the supplementation of (NH4)2SO4 did not lead to any improvement in xylose consumption; however, a 87% increase relative to H1 was achieved with full supplementation (Table 3). Thus, it can be stated that under the condition of reduced initial oxygen availability full supplementation was necessary to achieve an improvement in xylose consumption similar to that reached with (NH4)2SO4 alone under the condition of increased initial oxygen availability.
With the exception of the experiments conducted without nutritional supplementation (H1), in which the profiles of xylose consumption were similar (Fig. 1A, Table 3), the two conditions of initial oxygen availability used in this work promoted differences in the profiles and rates of xylose consumption. In the experiments with the reduced initial oxygen availability, xylose began to be consumed at a constant rate sooner than in those with the increased initial oxygen availability (Fig. 1B and C). Nonetheless, the xylose consumption rates (calculated during the phase of maximum xylose consumption for each experiment) were higher in the experiments with the increased initial oxygen availability (by 200% with supplementation of (NH4)2SO4 and by 53% with full supplementation). Consequently, the xylose consumption levels were higher than in the experiments with the reduced initial oxygen availability (by 96% in H2 and 37% in H3) (Table 3).
The results obtained on xylose consumption suggested that the yeast capacity to assimilate xylose was limited in the experiments with reduced initial oxygen availability, which could be due to a limited supply of NADPH necessary for XR activity.28,33 It has previously been indicated that under conditions of oxygen limitation the carbon flux from xylose assimilation could be insufficient to promote NADPH regeneration through the PPP,17,29,33 which is an important factor that can affect the xylitol production.16,17,27
The cell growth and xylitol production were improved in a similar way as xylose consumption. In regards to cell biomass, in the experiments with increased initial oxygen availability the supplementation of (NH4)2SO4 (H2) led to a 43% improvement relative to the medium without supplementation (H1), and an additional increase of 9% was achieved relative to H2 with full supplementation (H3), corresponding to a total improvement of 57% between H3 and H1 (Table 3). In the experiments with reduced initial oxygen availability, no improvement in cell growth was observed with nutritional supplementation (Table 3). It should be emphasized that supplementation with (NH4)2SO4 alone did not increase xylitol production under either condition of initial oxygen availability. On the other hand, full supplementation led to 240% and 48% improvements in xylitol production under conditions of increased and reduced initial oxygen availability, respectively (Table 3).
Considering the results obtained on xylose consumption, cell growth, and xylitol production in the experiments with reduced initial oxygen availability, it can be stated that simple supplementation with an inorganic nitrogen source such as (NH4)2SO4 was not sufficient to improve xylitol production. On the other hand, with the full supplementation of the sugarcane straw hemicellulosic hydrolyzate with (NH4)2SO4, rice brain extract and CaCl2·2H2O, increased xylose consumption and xylitol production were achieved under both conditions of initial oxygen availability. These facts suggested the necessity of supplementation with a complex nitrogen source such as the rice bran extract, an agro-industrial by-product, which can also supply minerals and vitamins.34 Similarly to our results, it has been demonstrated that the xylitol production by C. guilliermondii FTI 20037 was improved in hemicellulosic hydrolyzates from sugarcane bagasse24 and wheat straw19 by the supplementation with rice bran extract.
Regarding the effect of initial oxygen availability, changes were observed not only in xylose consumption, as discussed earlier, but also in the use of the xylose consumed for cell growth or xylitol production. With the reduction of the initial oxygen availability, an improvement in xylose-to-xylitol bioconversion was observed to occur in detriment to cell growth. In the experiments with reduced initial oxygen availability, the xylitol yield was higher and the cell biomass yield was lower compared to those obtained under conditions of increased initial oxygen availability (Table 3). These results can be explained by limited respiratory metabolism due to limited oxygen availability, which not only reduces the regeneration of NAD+, promoting a NADH/NAD+ imbalance that causes a partial inhibition of XDH and xylitol accumulation, but also decreases the energy production and formation of carbon intermediates, affecting the cell growth.16,27,33
Interestingly, a slight reduction in xylitol yield was observed under the condition of reduced initial oxygen availability with the increase of nutritional supplementation, corresponding to a 20% difference between the full supplementation and no supplementation (Table 3), and, in fact, the highest xylitol yield (0.67gg−1) was obtained under the latter condition. This value can be compared with the respective values obtained under similar fermentation conditions in other studies, which used the same yeast but other feedstocks, with a different composition of the fermentation media, as well as different processes of concentration and detoxification of hemicellulosic hydrolyzates. Compared with a sugarcane bagasse hemicellulosic hydrolyzate detoxified with active charcoal, the highest xylitol yield achieved in the present work was similar to that (0.66gg−1) obtained in the study of Marton and colleagues,25 in which xylose and glucose concentrations in the fermentation medium were higher and lower, respectively, than those in this work. Arruda and colleagues,35 who worked with a sugarcane bagasse hemicellulosic hydrolyzate detoxified using ion-exchange resins, reported a higher xylitol yield (0.81gg−1) than the one obtained in this study. It is worth noting that in the latter work the fermentation medium contained a higher concentration of xylose and lower concentrations of glucose and acetic acid than these found in the present study. Mussatto and Roberto,21 who used a rice straw hemicellulosic hydrolyzate detoxified with active charcoal and with higher concentrations of xylose and glucose and lower of acetic acid than those found in this study, obtained a higher xylitol yield (0.72gg−1) than the one achieved in the present work. On the other hand, in the case of a sorghum straw hemicellulosic hydrolyzate with lower concentrations of xylose and glucose than in the sugarcane straw hemicellulosic hydrolyzate in this work, Sene and colleagues36 reported a lower xylitol yield (0.44gg−1) than the one obtained in the present study.
The results summarized in Table 3 show that the highest xylitol production (16.20gL−1) and volumetric productivity (0.34gL−1h−1) were obtained in the experiment with increased initial oxygen availability and full supplementation. This is consistent with the highest cell concentration also found in this experiment but not with the xylitol yield, as discussed previously (Table 3). These results suggested that, even though the control of oxygen availability is a critical factor on xylose-to-xylitol bioconversion, it may be possible to improve this bioprocess, particularly the volumetric productivity, by increasing xylose consumption under conditions of oxygen limitation. Another possibility to consider would be the use of conditions favoring of cell growth in a previous phase, before the xylose-to-xylitol bioconversion begins. In comparison with the above-mentioned studies, which used other lignocellulosic biomasses but the same yeast and similar fermentation conditions, the highest xylitol volumetric productivity obtained in this study (0.34gL−1h−1) was higher than the one reported by Sene and colleagues36 (0.19gL−1h−1) and lower than those obtained by Arruda and colleagues35 (0.60gL−1h−1), Marton and colleagues25 (0.50gL−1h−1), and Mussatto and Roberto21 (0.57gL−1h−1).
ConclusionThe hemicellulose content of sugarcane straw, similar to that of other biomasses evaluated for xylitol production, and the high concentration of xylose in the hemicellulosic hydrolyzate suggested the suitability of this biomass as a feedstock for xylose-to-xylitol bioconversion. The ability of C. guilliermondii FTI 20037 to partially consume xylose and produce xylitol in a non-supplemented sugarcane straw hemicellulosic hydrolyzate can be considered a starting point for the development of technologies for xylitol production from this substrate without supplementation through the study of fermentation conditions. At the same time, our study confirmed a combined effect of nutritional supplementation and initial oxygen availability on xylose consumption and xylitol production. It was found that the supplementation of an inorganic nitrogen source, such as (NH4)2SO4, was sufficient to achieve improvements in xylose consumption and cell growth under a condition of increased initial oxygen availability. However, in order to improve these two parameters under a condition of reduced initial oxygen availability and to increase xylitol production under both oxygen conditions, addition of a complex nitrogen source, as rice bran extract, along with (NH4)2SO4 and CaCl2·2H2O, was necessary. Although the reduced initial oxygen availability increased the xylose-to-xylitol bioconversion in detriment to the cell growth, it also affected the xylose consumption, which, in turn, hindered improvements in the xylitol volumetric productivity. Whereas the highest xylitol yield was obtained at reduced oxygen availability and without nutritional supplementation, the highest volumetric productivity was achieved at increased oxygen availability and full supplementation. Considering that the conditions of nutritional supplementation and initial oxygen availability required to achieve the maximum values of xylitol yield and volumetric productivity were different, further studies focused on the relations between the parameters evaluated and other process parameters, such as the type of nutrients, supplementation strategy and control of the oxygen availability, are necessary for biotechnological production of xylitol to become a competitive biochemical route for the integration of sugarcane straw in a biorefinery.
Conflicts of interestThe authors declare no conflicts of interest.
This work was financially supported by the Brazilian research funding agencies FAPESP (Fundação do amparo à pesquisa do estado de São Paulo, Brazil, process 2013/27142-0) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil).