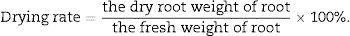Biofertilizer Ning shield was composed of different strains of plant growth promotion bacteria. In this study, the plant growth promotion and root-knot nematode disease control potential on Trichosanthes kirilowii in the field were evaluated. The application of Ning shield significantly reduced the diseases severity caused by Meloidogyne incognita, the biocontrol efficacy could reached up to 51.08%. Ning shield could also promote the growth of T. kirilowii in the field by increasing seedling emergence, height and the root weight. The results showed that the Ning shield could enhance the production yield up to 36.26%. Ning shield could also promote the plant growth by increasing the contents of available nitrogen, phosphorus, potassium and organic matter, and increasing the contents of leaf chlorophyll and carotenoid pigment. Moreover, Ning shield could efficiently enhance the medicinal compositions of Trichosanthes, referring to the polysaccharides and trichosanthin. Therefore, Ning shield is a promising biofertilizer, which can offer beneficial effects to T. kirilowii growers, including the plant growth promotion, the biological control of root-knot disease and enhancement of the yield and the medicinal quality.
The root-knot nematode (Meloidogyne spp.) is one of the most economically important plant parasitic nematodes causing huge damage to a wide variety of plants, including tomato (Lycopersicon esculentum), pepper (Capsicum frutescens), carrot (Daucus carota), potato (Solanum tuberosum), eggplant (Solanum melongena), watermelon (Citrullus vulgaris), cucumber (Cucumis sativus). The disease causes reductions in yield most commonly by 15–25%, however, in some cases it can be up to 75%,1,2 and causes an estimated US $100 billion loss/year worldwide.3
Trichosanthes kirilowii Maxim, a perennial grass liana of Cucurbitaceae is a newly discovered host of root-knot nematode. It belongs to, the seeds, fruit, root of T. kirilowii can be used as medicine to clear heart and to remove phlegm for lung heat cough.4 Over the past few years, root knot nematode disease incidence rate is increasing and had become serious threat to the production of T. kirilowii. Therefore, seeking for an efficient and security measure is required.
The application of chemical nematicides is one of the primary strategies of control against plant parasitic nematodes, but their risks to humans and animals may cause environmental pollution. In addition, the production of medicinal materials is strictly prohibited with the use of toxic pesticides. All these lead to an incremental interest in finding some safe substitutes for the control of plant-parasitic nematodes.5–7
Biological control by using microbial antagonists has attracted much interest as an alternative strategy to chemical methods of controlling pre- and post-harvest plant pathogens. Biological control becomes more familiar due to its environmental compatibility and non-toxic nature.8–10 Therefore, thousands of microbial strains have been screened, and most of them have been found to be antagonistic to plant-parasitic nematodes.3,6,11–13 While, very few biocontrol products are currently commercially available and methods for the biological control of plant-parasitic nematodes are still under discovering and searching. Accordingly, it is very necessary to find different strains of antagonistic bacteria for more efficient biological control against root knot nematode.
Ning shield preparation, which was also called BBS combination, is composed of different bacterial strains of plant growth promotion bacteria, supported by 23 China national patents, 1 European patent of invention, 3 provincial awards and 1 merited product as a biofertilizer. They have been used as alternative soil treatments of chemical pesticides, such as thiadizinone, embamycin and metalaxyl-mancozeb to protect the roots from soil-borne diseases including root-knot nematode, bacterial wilt, Phytophora wilt and Fusarium wilt, and to reduce the dose of chemical fertilizers. In this study, the aim is to evaluate the efficacy of biological control of Ning shield against the root-knot nematode on T. kirilowii under field conditions.
Materials and methodsField experiment designNing shield is composed of different strains of plant growth promotion bacteria, which was also called BBS. The BBS combination consists of three main bacterial strains (Bacillus cereus AR156, Bacillus subtilis SM21 and Serratia sp. XY21), which were primarily isolated from forest soil in Zhenjiang City, Jiangsu Province, China. The concentration of live bacteria in the final product was 109CFU/mL. The fields selected to carry out this experiment had been cultivated with T. kirilowii for 6 years prior to the trial experiments. The field was heavily infected by root-knot nematode (Meloidogyne incognita) with a initial population of 10.5 (SD±1.41) second stage juveniles (J2) per gram soil.
Two treatments with/without Ning shield biofertilizer were conducted in each field. Each seedling was treated with 500mL of Ning shield suspension (1:100 dilution) at transplanting and reused for each treatment at 60 days after transplanting. Each treatment was replicated three times. Seedlings in control treatment were mock inoculated with an equal volume of water. All plants were watered every 7 days without the use of any fertilizer.
The evaluation of plant growth promotion of biofertilizer Ning shield on T. kirilowiiThe ability of biofertilizer Ning shield to promote the plant growth was evaluated in a field test. Fifteen days post-treatment, seedling emergence was detected. Ninety days post treatment, the stem height, stem diameter, numbers of seedling and area of leaves were measured. Two years post-treatment, the dry and fresh root weights of T. kirilowii and yield were recorded. This experiment was replicated three times.
Yield increase and drying rate were calculated as following:
Determination of the contents of carotenoid pigment and chlorophyll in the leaves of T. kirilowiiThe contents of leaf carotenoid pigment and chlorophylls a, b and a+b were measured at 90 days post treatment (dpt) by using the method of Ashraf and Iram.14 Three leave samples were collected from each treatment, and 1.0g fresh weight for each sample was cut into 0.5cm segments and extracted overnight with 80% acetone at −10°C. The mixture was centrifuged at 12,000×g for 10min, and the absorbance of the resulting supernatant was measured at 450, 645, and 663nm by using a spectrophotometer (Hitachi-220), respectively.9
The contents of leaf carotenoid pigment and chlorophylls a, b, and a+b were calculated as follows:
This experiment was replicated three times.
Determination of the content of available element of nitrogen, phosphorus, potassium, and organic matter in the soilThe contents of organic matter and available nitrogen, phosphorus, potassium were determined by the soil testing instrument (TPY-6A) (Zhejiang Tuopu Instrument Co., Ltd., China) as described by Jiang et al.9 This experiment was replicated three times.
The biocontrol efficacy of biofertilizer Ning shield against the root-knot nematode on T. kirilowiiTo evaluate the biocontrol efficacy of biofertilizer Ning shield to against root-knot nematode, the numbers of nematodes in the soil and disease severity were determined. The nematode numbers in the soil were detected by Baermann funnel apparatus.15 A clamped rubber tube was placed below the funnel, and a piece of window screen (or similar material) was placed in the mouth of the funnel; the funnel was placed into a rack or holder; a tissue-paper wrapped soil sample was placed onto the screen material then add water to the funnel setup until the screen and soil sample were immersed; incubate overnight (or longer if desired). Finally, the first couple of drops of water from the bottom of the tube was gathered by slowly releasing the clamp on the tubing, then the density of worms was examined under the microscope.
For the disease severity detection, the gall indices were recorded 2 years after transplantation on the scale of 0–4, by using a rating chart as follows: 0=no galling, 1=1–25% galling, 2=26–50% galling, 3=51–75% and 4=76–100% galling.16–17 Biocontrol efficacy was calculated as described by Xue et al.18 The T. kirilowii roots were harvested 2 years after transplantation and the marketable yield was determined.
Disease severity, drop rate and biocontrol efficacy were calculated as following:
The impact of biofertilizer Ning shield on the quality of T. kirilowiiTo evaluate the impact of biofertilizer Ning shield on quality of T. kirilowii. The content of polysaccharide and trichosanthinin the harvest root in 2 years after the treatment of biofertilizer Ning shield were detected. The content of polysaccharide were detected according to the method reported by Tu et al.,19 and then the absorbance of the resulting supernatant was measured at 490nm, by using a spectrophotometer (Hitachi-220). This experiment was repeated three times. The content of trichosanthin were detected according to the method reported by Kang et al.,20 then the absorbance of the resulting supernatant was measured at 332nm, by using a spectrophotometer (Hitachi-220). This experiment was replicated three times.
Statistical analysisAll bioassays were conducted three times with 24 seedlings per treatment. Analysis of variance (ANOVA) was carried out using SPSS software version 19.0 (IBM). Mean comparison was conducted using a least significant difference (LSD) test (p<0.05). Standard errors and standard deviations were calculated.
ResultsThe biocontrol efficacy of biofertilizer Ning shield to the root-knot nematode on T. kirilowiiPreviously, thousands of microbial strains have been screened, and most of them have been found to be antagonistic to plant-parasitic nematodes.3,6,11–13 However, few biocontrol products are currently commercially available and methods for the biological control of root-knot nematode were still under discovering. In this field experiment, we evaluated the biocontrol efficacy of biofertilizer Ning shield to the root-knot nematode. As the results shown in Table 1 and Fig. 1, compared with the control treatment, the biofertilizer Ning shield could significantly reduce the population of root-knot nematode in the soil, the drop rate could up to 62.83% (Table 1). It was also found that, the disease severity of biofertilizer Ning Shield treatment was 36%, while the control treatment was 73.5% (Table 1), according to the calculations, the biocontrol efficacy of biofertilizer Ning shield could up to 51.08% (Table 1). All together, the results indicated that, the biofertilizer Ning shield could significantly control the root-knot nematode on T. kirilowii.
The Biocontrol efficacy of biofertilizer Ning shield to the root-knot nematode on T. kirilowii.
| Treatment | RKN number/g soil | Drop rate (%) | Disease severity (%) | Biocontrol efficacy (%) |
|---|---|---|---|---|
| Ning shield | 4.97±1.73 | 62.83 | 36.0±3.79a | 51.08 |
| Control | 13.38±2.45a | – | 73.5±5.18 | – |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
A numbers of microorganisms in the rhizosphere have the ability to promote the growth of plants. In our previous studies, we found that biofertilizer BBS could not only promote the seed germination, but also promote the growth of plants, such as tomato, pepper.21 While Ning shield was preparation with the BBS combination. So we want to know, whether Ning shield also could promote the growth of T. kirilowii in the field. As the results shown in Table 2, Ning shield could significantly promote the growth of T. kirilowii in the field. The seedling emergence rate with Ning shield treatment reached 96.25%, 84.25% for the seedling emergence rate of control treatment. As the results shown in Table 2, the height and diameter of stem, leaves number and area of the seedling in Ning shield treatment was 160.09cm, 8.80mm, 22.05cm2 and 62.16cm2, respectively. Compared with control treatment, the height and diameter of stem, the number and area of leaves was 78.38cm, 6.4mm, 16.30cm2 and 39.54cm2, respectively. The Ning shield treatment significantly (p<0.05) promote the growth of T. kirilowii (Table 2 and Fig. 2).
The effect of biofertilizer Ning shield on the rate and growth parameters of T. kirilowii seedling.
| Treatment | Seedling emergence rate (%) | Stem height (cm) | Stem diameter (mm) | Leaves Numbers | Leaves area (cm2) |
|---|---|---|---|---|---|
| Ning shield | 96.25±2.25a | 160.09±5.79a | 8.80±0.98a | 22.05±1.64a | 62.16±1.62a |
| Control | 84.25±4.06 | 78.38±3.32 | 6.40±0.81 | 16.30±1.13 | 39.54±1.75 |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
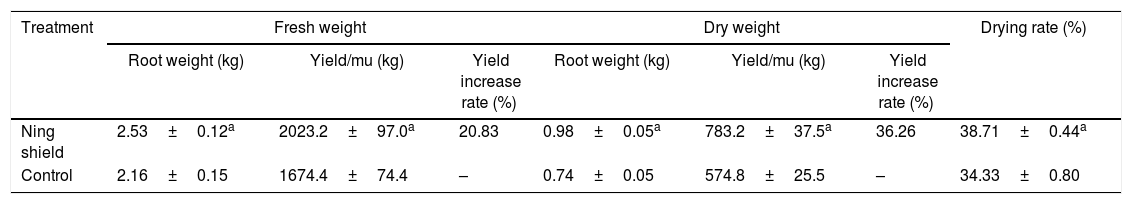
For T. kirilowii production, the final harvest is the root, and use the root as medicine. Therefore, the increase of production depends on the promotion of plant roots. In the process of harvesting in T. kirilowii, we tested the fresh weight and dry weight of T. kirilowii root. As the results shown in Table 3 and Fig. 3, the fresh weight and dry weight of T. kirilowii root in Ning shield treatment was 2.53kg and 0.98kg, respectively, while 2.16kg and 0.74kg for fresh root weight and dry weight of T. kirilowii with control treatments, respectively. These results indicated that Ning shield could significantly (p<0.05) promote the growth of T. kirilowii. We also analyzed the yield in different treatment, as the results shown in Table 3, the fresh yield and dry yield of Ning shield treatment was 2023.2kg/mu and 783.2kg/mu, respectively, the fresh yield and dry yield of control 1674.4kg/mu and 574.8kg/mu, respectively. These results suggesting that Ning shield treatment was could significantly (p<0.05) increase the yield of T. kirilowii and the yield increase rate could reach up to 20.83% or 36.26 and up to 38.71% for the drying rate (Table 3).
Effect of biofertilizer Ning shield on production of T. kirilowii ground and underground parts.
| Treatment | Fresh weight | Dry weight | Drying rate (%) | ||||
|---|---|---|---|---|---|---|---|
| Root weight (kg) | Yield/mu (kg) | Yield increase rate (%) | Root weight (kg) | Yield/mu (kg) | Yield increase rate (%) | ||
| Ning shield | 2.53±0.12a | 2023.2±97.0a | 20.83 | 0.98±0.05a | 783.2±37.5a | 36.26 | 38.71±0.44a |
| Control | 2.16±0.15 | 1674.4±74.4 | – | 0.74±0.05 | 574.8±25.5 | – | 34.33±0.80 |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
As is known to all, the efficiency of photosynthesis determined to the plant biomass, so the efficiency of photosynthesis of T. kirilowii was analyzed in this research. We first determined the content of leaf carotenoid pigment, chlorophyll in the leaves of T. kirilowii plants at 90 days post treatment. The contents of leaf carotenoid pigment, chlorophylls a, b, a+b in Ning shield-treated plants were increased by 18.4%, 8.7%, 17.4%, and 10.3%, respectively, compared with the control plants (Table 4), which indicated that Ning shield maintained carotenoid pigment and chlorophyll contents in T. kirilowii leaves. The result of SPAD value was consistent with the previous results (Table 4). The result was consistent with the above observation that Ning shield- treated T. kirilowii plants had much darker green leaves than control plants (Fig. 2).
The Effect of biofertilizer Ning shield on the chlorophyll content of T. kirilowii.
| Treatment | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a+b (mg/g) | Chlorophyll a/b | Carotenoid pigment (mg/g) | SPAD value |
|---|---|---|---|---|---|---|
| Ning shield | 1.61±0.01a | 0.54±0.00a | 2.14±0.01a | 2.97±0.052 | 0.45±0.01a | 56.11±3.70a |
| Control | 1.48±0.005 | 0.46±0.005 | 1.94±0.009 | 3.17±0.03a | 0.38±0.002 | 48.15±3.33 |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
To better understand the mechanisms of the Ning shield growth promotion to T. kirilowii, we determined the contents of organics matter and available nitrogen, phosphorus, potassium in the soil at 90 days post treatment. As the results shown in Table 5, Ning shield treatment could significantly (p<0.05) increase the content of organic matter and available nitrogen, phosphorus, potassium in the soil. The contents of organics matter and available nitrogen, phosphorus, potassium, and in Ning shield-treated plants increased by 27.27%, 28.9%, 41.8%, and 50.9%, respectively, in comparison with the control plants, especially to the content of available potassium (Table 5).
The Effect of biofertilizer Ning shield on soil ammonium nitrogen, available phosphorus, available potassium and organic matter content.
| Treatment | Ammonium nitrogen (mg/kg) | Available phosphorus (mg/kg) | Available potassium (mg/kg) | Organics matter (%) |
|---|---|---|---|---|
| Ning shield | 15.86±1.76a | 42.20±2.72a | 249.6±19.6a | 0.644±0.063a |
| Control | 12.085±0.67 | 29.76±3.15 | 165.4±10.7 | 0.506±0.063 |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
Previous reports showed that, the content of polysaccharide and trichosanthis in the T. kirilowii determined its medicinal value. So in this study, we also detected the content of polysaccharide and trichosanthis in the T. kirilowii, and evaluate whether Ning shield can increase the content of polysaccharide and trichosanthis and raise the quality of T. kirilowii. As the results shown in Table 6, Ning shield treatment could significantly (p<0.05) increase the content of polysaccharide and trichosanthis in the T. kirilowii. It was found that the content of polysaccharide and trichosanthis in Ning shield treatment was 184.67mg/g and 7.75mg/g, compared with the control treatment in which the content of polysaccharide and trichosanthis was 164.51mg/g and 6.64mg/g, the content of polysaccharide and trichosanthis in Ning shield treatment increased by 12.3% and 16.7%, respectively, which suggested that Ning shield can increase quality of T. kirilowii.
The impact of biofertilizer Ning shield on the quality of T. kirilowii.
| Treatment | Polysaccharide content (mg/g) | Trichosanthin content (mg/g) |
|---|---|---|
| Ning shield | 184.67±4.34a | 7.75±0.16a |
| Control | 164.51±1.63 | 6.64±0.19 |
Note: Data are presented as means of three replicates±SD, and error bars represent SD for three replicates.
Biological control by using microbes that are associated with plants was an environmental-friendly and efficient strategy for controlling root-knot nematode.16,22,23 A number of mechanisms of rhizobacteria biocontrol against root-knot nematodes have been studied. Jung and associates reported that chitinase produced by Paenibacillus illinoisis KJA-424 caused the lysis of M. incognita eggshell and resulted in the inhibition of egg hatching in vitro.5,24 Siddiqi and Shaukat (2003) have reported production of metabolites, including HCN and 2,4-diacetylphloroglucinol (DAPG) by a rhizo-bacteriaum, Pseudomonas fluorescens strains CHA0, induce mortality in J2 of root-knot nematodes and inhibit egg hatch.25 According to Nielsen and Sorensen (1997) Paenibacillus polymyxa exhibited in vitro antagonism against the fungi.26 Van Loon and associates reported the ability of plants to develop ISR in response to root colonization by non-pathogenic bacteria depends on the interactions between the colonizing rhizobacterium and host plant.27–28 The rhizobacterial strain P. fluorescensWCS417r (WCS417r here after) has been shown to trigger ISR in serials of plant species.28 In our study, we found that biofertilizer Ning shield could significantly control root-knot nematode in T. kirilowii (Table 1 and Fig. 1). It was found that the biofertilizer Ning shield could decease the population of J2 in the soil. Therefore, the disease severity of Ning shield treatment could be lower than the control treatment. While, how the Ning shield can biocontrol the root-knot nematode in T. kirilowii. As we all known, Ning shield was the BBS combination, which was consist of three bacterial strains (B. cereus AR156, B. subtilis SM21 and Serratia sp. XY21). Niu and associates reported that B. cereus AR156 could induced systemic resistance to pathogens in plants,29 and AR156 also has some abilities to kill J2 of root-knot nematodes and inhibit egg hatch.13 Therefore, we can know that the reason why biofertilizer Ning shield could significantly biocontrol root-knot nematode in T. kirilowii may due to the function of B. cereus AR156.
Biofertilizer B. subtilis BEB13-bs has the ability to promote some aspects of tomato fruit quality. This study was the first report on physical changes in fruit due to PGPR root treatment. Root fresh weight and root dry weight increased significantly in plants treated with the BS13, in comparing with the control treatment.9 These results were also shown in our study, as the results shown in Figs. 2 and 3 and Tables 2 and 3. It was also found that root fresh weight, dry weight, and other biomass data increased significantly in plants of Ning shield treatment in comparing with the control treatment.
We also analyzed the mechanisms of plant growth promotion of Ning shield on T. kirilowii from two parts, one is from plant photosynthesis, and the other part is from plant nutrition aborting. As is known to all, the efficiency of photosynthetic decided to plant biomass, ultimately determine the output of plants. Therefore, the efficiency of photosynthesis of T. kirilowii was detected in our study. As results shown in Table 4, the contents of leaf chlorophylls and carotenoid pigment in Ning shield-treated group increase by 10.3% and 18.32% respectively, compared with the control plants. Beside photosynthesis affection, we also evaluate the impact on plant nutrition aborting. So, in this study, we also detect the available nitrogen, phosphorus, potassium and organics matter in the soil. As the results shown in Table 5, it was found that the content of organics matter and available nitrogen, phosphorus, potassium in Ning shield-treated group increase by 27.27%, 28.9%, 41.8%, and 50.9%, respectively. The results indicated that Ning shield could accelerate the decomposition of nutrients in the soil, So as to favor the plant nutrient absorption.
Compared with chemical fertilizer, biological fertilizer also has the effect of improving the quality of crops. Bacillus amyloliquefaciens 54 could not only promote the growth of plant, but also improve the quality of the fruit by increasing the contents of nutrients such as vitamin, protein and sugar.9 As a kind of Chinese traditional medicine, the content of polysaccharide and trichosanthis in the T. kirilowii determinedits medicinal value. So in this study, we also detected the content of polysaccharide and trichosanthis in the T. kirilowii, and evaluate whether Ning shield can increase the content of polysaccharide and trichosanthis and raise the quality of T. kirilowii. As the results shown in Table 6, the content of polysaccharide and trichosanthis in Ning shield treatment increased by 12.3% and 16.7%, respectively, it was suggesting that biofertilizer Ning shield has ability to enhance the medicinal compositions of T. kirilowii, so as to enhance its quality. Our study also provides a new way to improve the quality in the production of Chinese herbal medicine.
ConclusionIn this study, we evaluated the abilities of biofertilizer Ning shield on the biocontrol to root-knot nematode and plant growth promotion in T. kirilowii. This study was first time to report that one biofertilizer can efficiently control root-knot nematode disease, and promote the growth of T. kirilowii. We also reported that biofertilizer could increase medicinal ingredient content, so as to enhance the quality of T. kirilowii. Therefore provides a new strategy to improve the quality in the production of Chinese herbal medicine. This study has profound significance for the production of Chinese herbal medicines.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the National Postdoctoral Program for Innovative Talents (BX201600074), China Postdoctoral Science Foundation (2017M611839), National Natural Science Foundation of China (31471812, 31672075, 31701829), Natural Science Foundation of Jiangsu Province (BK20170709), Jiangsu Province Agricultural Science and technology independent innovation fund project (CX (15)1044), Science and technology project of Jiangsu Province (BE2015364, BY2014128-05, BY2015071-04).