Quantification of bacteria being grazed by microzooplankton is gaining importance since they serve as energy subsidies for higher trophic levels which consequently influence fish production. Hence, grazing pressure on viable and non-viable fraction of free and particle-associated bacteria in a tropical estuary controlled mainly by protist grazers was estimated using the seawater dilution technique. In vitro incubations over a period of 42h showed that at the end of 24h, growth coefficient (k) of particle-associated bacteria was 9 times higher at 0.546 than that of free forms. Further, ‘k’ value of viable cells on particles was double that of free forms at 0.016 and 0.007, respectively. While bacteria associated with particles were grazed (coefficient of removal (g)=0.564), the free forms were relatively less grazed indicating that particle-associated bacteria were exposed to grazers in these waters. Among the viable and non-viable forms, ‘g’ of non-viable fraction (particle-associated bacteria=0.615, Free=0.0086) was much greater than the viable fraction (particle-associated bacteria=0.056, Free=0.068). Thus, grazing on viable cells was relatively low in both the free and attached states. These observations suggest that non-viable forms of particle-associated bacteria were more prone to grazing and were weeded out leaving the viable cells to replenish the bacterial standing stock. Particle colonization could thus be a temporary refuge for the “persistent variants” where the viable fraction multiply and release their progeny.
Bacteria are capable of consuming more than 50% of primary production in the form of dissolved organic matter.1,2 Thus, they play an important role as energetic subsidies for higher trophic levels. In recent years, studies focusing on grazing of bacteria by bacteriophagous microfauna have gained importance. Predation by microzooplankton in aquatic habitats is known to decrease bacterial abundance and stimulate mineralization of nutrients.3 The process could influence the morphological structure, taxonomic composition4 and physiological status of bacterial communities.5 The grazers (microzooplankton) consist of holoplanktonic (protozoa, ciliates, flagellates, copepod nauplii, etc.) and meroplanktonic organisms (larval stages of benthic invertebrates: trochophores, veligers, etc.). Protozoans are known to play a major ecological role in the aquatic environment due to their effectiveness in consuming a wide range of prey size classes and types.6 They are capable of consuming other protozoa,7 phytoplankton8 and bacteria.9–11 Their grazing ability depends on the concentration of bacteria and digestion capacity of the grazer. Some ubiquitously distributed protists like ciliates occasionally form a major component of the microzooplankton community12,13 and have a key position in plankton food webs as major consumers of pico- and nano-plankton.14,15 Availability and concentration of the prey size and type, attached vs. unattached cells and viable vs. non-viable cells are some of the factors affecting ciliate grazing rates.
Among the planktonic bacterial community, particle-associated bacteria (PAB) have received more emphasis than free-living forms because of their relatively easier accessibility to filter feeders16,17 and their ability to improve the nutritional quality of the particles.18 The PAB can be directly grazed by larger metazoans, bypassing consumption by protozoan grazers and short-circuiting the microbial loop.16 Reports on pelagic bacterial community have demonstrated that it is unwarranted to club particle-attached or unattached bacteria as a single unit.19 To understand grazing preferences on the bacterial community in a tropical estuary dominated by protists, we conducted a seawater dilution experiment to measure the coefficients of increase ‘k’ and grazing ‘g’.20 The following queries were put forth: (1) do grazers exhibit selective preference for free or particle-associated bacteria (PAB) and (2) which physiological state (viable or non-viable) of free or particle-associated bacteria of the bacterioplankton will be eliminated by the grazers. Though previous work has highlighted the importance of PAB as food for grazers like microzooplankton16,17,21 for the first time we have demonstrated elevated grazing pressure on non-viable cells of PAB and conservation of their viable forms for stock replenishment.
Materials and methodsStudy area and samplingA Niskin sampler was used to collect water samples at 3m depth in Dona Paula Bay (15°27′N, 73°48′E; Fig. S1) which is located at the terminus of Zuari estuary in west coast of India. This semi-enclosed bay is undisturbed by seaport and riverine traffic. Details on the climatic conditions, annual variation in hydrographic parameters and biotic variables in the study area have been described elsewhere.22 The temperature, salinity and pH of seawater during sampling were 24°C, 21psu and 6.3, respectively.
Grazing studiesThe dilution technique23 was used for grazing studies in the present work. The technique has been widely used to reduce the number of predators by creating a gradient.24 The method has also been used to estimate growth rates of phytoplankton/bacterioplankton25,26 as well as grazing pressure exerted by microzooplankton on these forms.26,27 We set up incubations with whole and diluted seawater to determine bacterial abundance (total bacterial cells, PAB and free-living). A schematic representation of the experimental design is shown in Fig. S2.
For preparing particle-free water (used for diluting whole seawater), the filtration assembly was washed with 50% HCl and rinsed with Milli-Q water. Two litres of seawater was then filtered through a 0.22μm filter (pressure<100mmHg). Thus, to prepare ‘diluted’ samples for incubation, untreated ‘whole’ sample water was added to particle-free water in the ratio 1:4.
The PAB were considered as those that would be retained on a 3μm pore-size filter (Fig. S2). Bacteria that passed through these filters were considered as free-living bacteria. An estimate of the total and free-living bacterial population in the whole and diluted seawater was carried out. The abundance of PAB was derived by subtracting the free-living from the total bacterial population. Controls were maintained for bottle effect and these were used to subtract from the experimental values. Diluted and whole water samples were incubated for up to 42h at 27±2°C under static conditions. Sub-samples were removed from the whole and diluted seawater bottles at 6h intervals for the estimation of TC (total bacterial cells), the total, viable and non-viable fraction of PAB and free-living forms. For the enumeration of total bacterial cells (TC), a 5mL aliquot of water sample was fixed using 250μL of buffered formalin (2% final concentration) as described by Hobbie et al.28 For enumeration of viable cells (VC), 5mL of seawater samples were amended with 0.001% final concentration of yeast extract and 0.0016% final concentration of antibiotic cocktail solution containing piromedic, pipemedic and nalidixic acid which were in the ratio of 1:1:1.29 Samples were incubated statically in dark for 6h. At the end of the incubation, the aliquots were fixed with 2% of buffered formalin. For TC and VC, 1mL of sub-sample was filtered over a 0.2μm black Isopore polycarbonate filter paper (Millipore Corp., MA, USA) and stained with acridine orange (final concentration 0.01%, w/v). The samples were incubated for 2min and then filtered. Bacterial cells retained on the filter papers were counted using Nikon 50i epifluorescence microscope equipped with a 100× oil immersion objective. Viable cells refer to those which enlarge using the direct viable count (DVC) procedure.30 These enlarged cells comprise of a mixture of cells which have increased in size and actively dividing cells. Thus, in this study, only cells that enlarge or replicate are counted as viable while the non-viable forms are considered as the ones which did not enlarge/replicate. Bacterial abundance has been expressed as cellsL−1.
The specific rate of increase in bacterial cells ‘k’ and mortality/decrease ‘g’ of bacterioplankton were estimated. The constants k and g are coefficients of population increase and grazing mortality, respectively.23 These constants also refer to increase and removal not necessarily linked to growth and death. Therefore, it would mean an increase in one pool i.e., particle association by colonization, and decrease in another pool i.e., free-living forms. Both these coefficients may vary with time of day without affecting our comparisons of increase in bacterial numbers in the dilution over a fixed period of incubation. Thus, the abundance and grazing mortality of bacterioplankton was inferred from observed changes in population.
Microzooplankton density and diversityDue to low density of plankton in the study area, 10L of seawater was obtained for the enumeration of microzooplankton density and diversity. The water sample was filtered through a 200μm mesh to exclude mesozooplankton. Further, this filtered water was slowly passed through a net of 20μm mesh size. The seawater was then concentrated to 1L by siphoning out excess water with the help of a Tygon tubing (Saint-Gobain Performance Plastics Corporation, USA). The dipped end of the tubing was covered with 20μm mesh. Thereafter, the sample was preserved in 1% acid Lugol's solution and left to settle for 24h and further concentrated to 10mL by siphoning out the excess water. One millilitre of concentrated sample was transferred into a Sedgwick-Rafter chamber to enumerate microzooplankton abundance. Counting was done using an inverted phase microscope fitted with a whipple grid. About 30–40 fields representing 150–200 individuals were counted. The microzooplankton were identified to genus and/or species level. Their density has been expressed as individuals L−1.
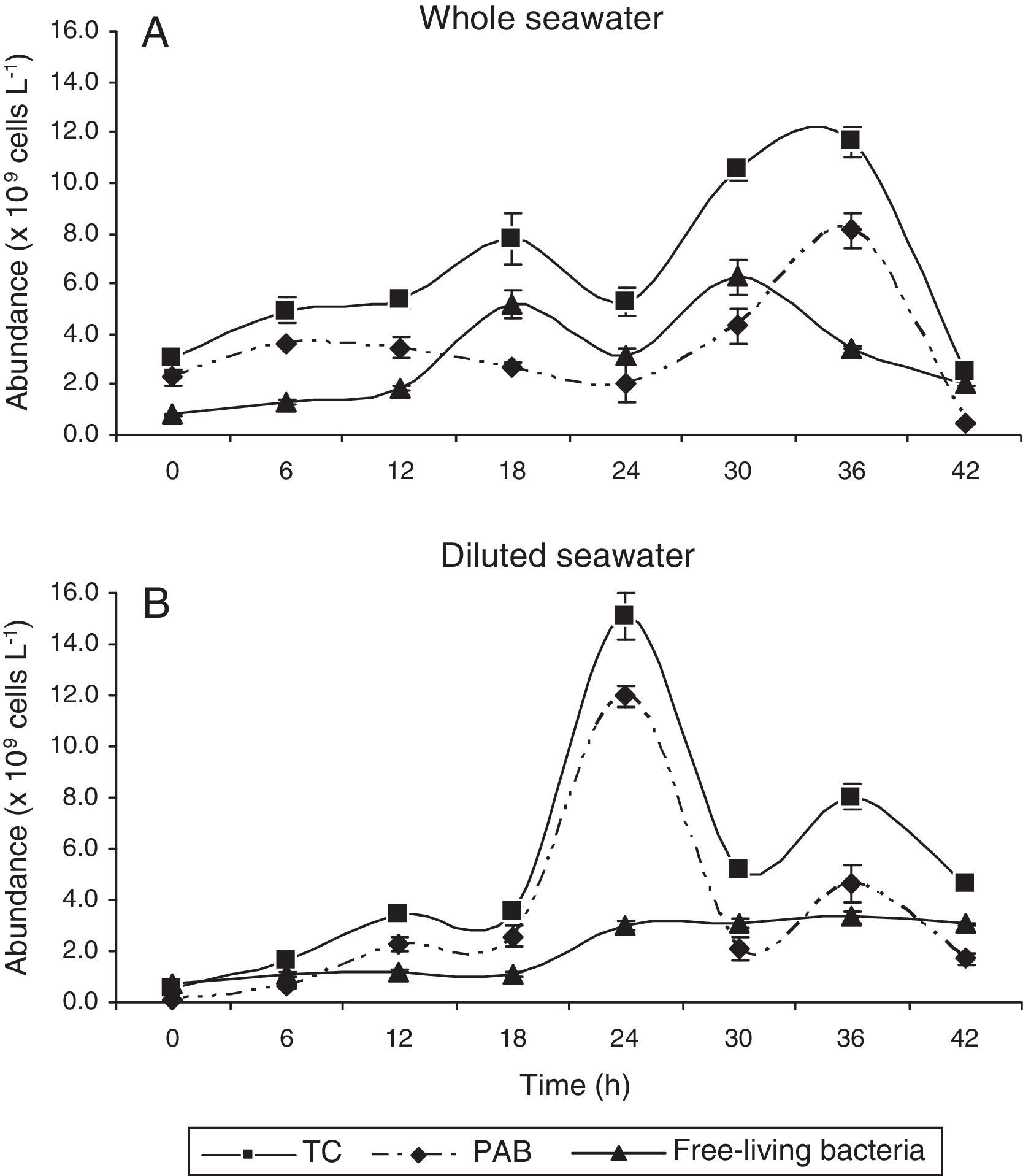
ResultsGrazing of particle-associated and free-living bacteriaThe abundance of bacterial cells in whole seawater increased steadily over time and peaked at the end of 36h to reach 1.16±0.06×1010cellsL−1 (Fig. 1A). In the diluted sample, peak in cell abundance was observed at 24h of incubation and was marginally higher at 1.51±0.09×1010cellsL−1 (Fig. 1B).
Likewise, the peak in PAB abundance in whole and diluted seawater was observed at the end of 36 and 24h, respectively. The maximum abundance of PAB in whole seawater was 8.10±0.73×109cellsL−1 (Fig. 1A).
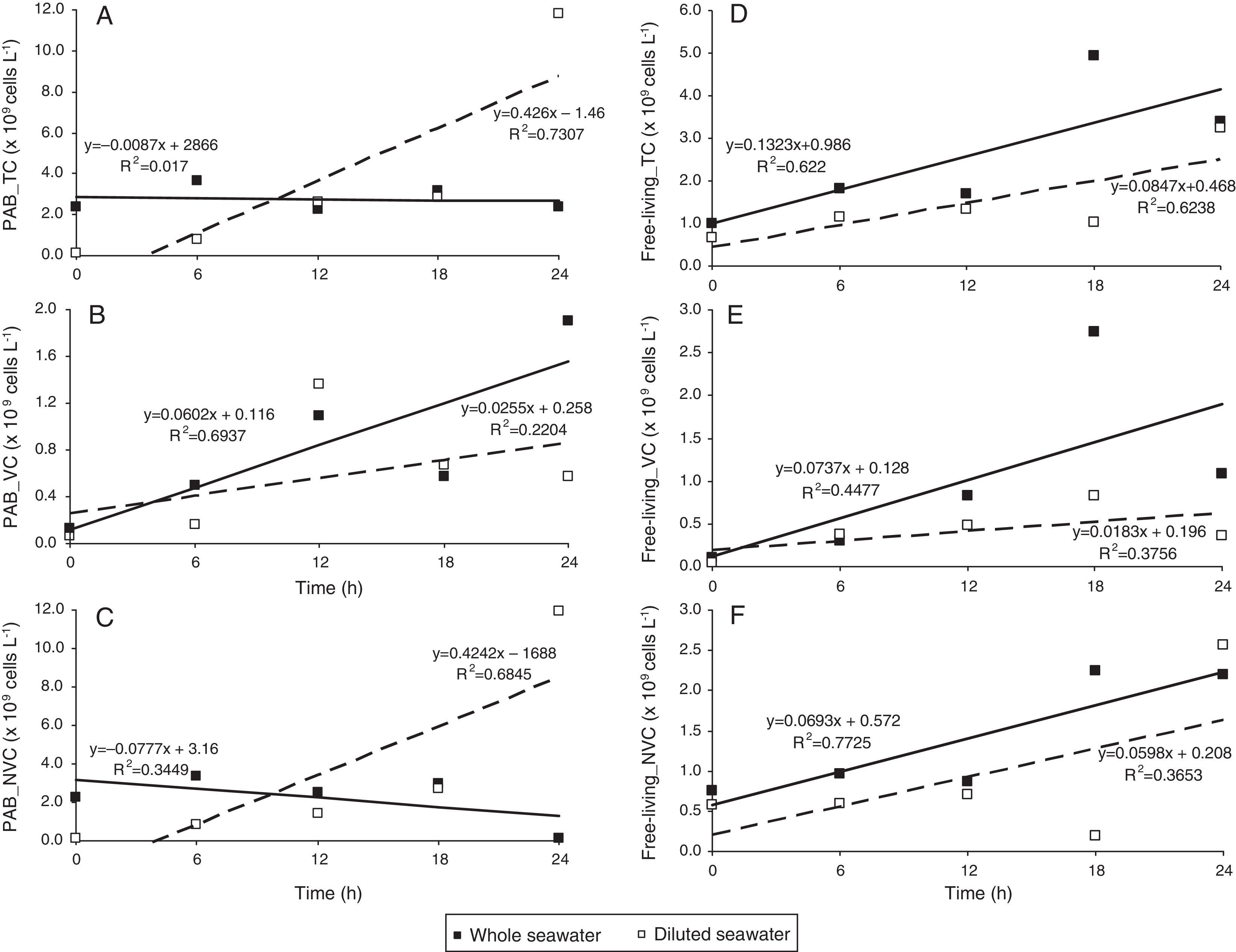
The viable PAB abundance was not affected both in whole (R2=0.6937, p<0.05, n=5; Fig. 2B) and diluted samples (R2=0.2204). In diluted seawater, PAB abundance was higher by one order i.e., 1.20±0.04×1010cellsL−1 (Fig. 1B).
Regression analysis of total (A), viable (B) and non-viable (C) particle-associated bacteria (PAB) in whole (■) and diluted (□) seawater. Regression plots for total, viable and non-viable free-living bacteria are shown in (D), (E) and (F), respectively. Linear regression line for whole seawater samples has been depicted using a solid line whereas the dashed lined is for diluted seawater samples. The R2 values denoted in the figures have been converted to the correlation coefficient (r). Subsequently, the level of significance (p) for a two-tailed test was inferred from a standard table (Wheater and Cook, 2000)43 and was accepted at p<0.05.
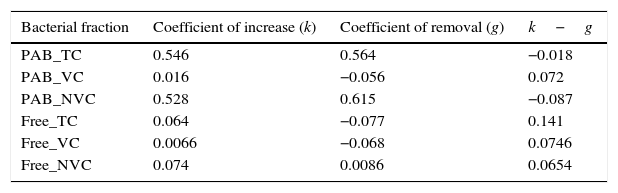
The non-viable fraction increased over time (R2=0.6845, p<0.05, n=5; Fig. 2C) with a k value of 0.528 (Table 1) in comparison to the viable forms (k=0.016). However, removal of non-viable PAB by grazers was far greater (g=0.615; Table 1) than that of viable cells (Table 1). Free-living cells were far lesser in abundance compared to PAB and were slow growers as observed from the low k values (Table 1). Their numbers in whole seawater increased from 0.81±0.07×109cellsL−1 at the beginning of the incubation period to a maximum of 6.25±0.66×109cellsL−1 at the end of 30h (Fig. 1A). The increase in abundance of free-living non-viable cells was seen to be strongly dependent on the incubation time (R2=0.7725, p<0.05, n=5; Fig. 2F). In diluted seawater, a steady increase in free-living bacterial cells was observed up to 36h where they numbered about 3.34±0.22×109cellsL−1 (Fig. 1B). Increase in the abundance of viable free-living cells over time was marginally higher (R2=0.3756, n=5; Fig. 2E) than the non-viable fraction (R2=0.3653, n=5; Fig. 2F). Removal of free-living forms by grazers was found to be negligible (Table 1).
Coefficients of increase and removal of bacterioplankton.
| Bacterial fraction | Coefficient of increase (k) | Coefficient of removal (g) | k−g |
|---|---|---|---|
| PAB_TC | 0.546 | 0.564 | −0.018 |
| PAB_VC | 0.016 | −0.056 | 0.072 |
| PAB_NVC | 0.528 | 0.615 | −0.087 |
| Free_TC | 0.064 | −0.077 | 0.141 |
| Free_VC | 0.0066 | −0.068 | 0.0746 |
| Free_NVC | 0.074 | 0.0086 | 0.0654 |
Note: PAB, particle-associated bacteria; TC, total bacterial cells; VC, viable bacterial cells; NVC, non-viable cells. Coefficient of increase includes actively replicating/enlarged cells and net colonization/exchange from the free to the particle-associated pool and vice versa. The coefficient of increase of non-viable forms was higher than that of viable ones as these include non-replicating/non-enlarged cells.
Leprotintinnus nordquisti and Stenosemella sp. dominated the microzooplankton community in the Dona Paula bay at an abundance of 25 and 24% of the total, respectively (Fig. S3). Other important microzooplankton included Favella sp. (12%), Dictyocysta sp. (8%), Codonellopsis sp. (8%) and Tintinnopsis uryguayensis (5%).
DiscussionBacteria are relevant members of the planktonic food web, both in terms of biomass and production share. However, bacterial mortality/loss occurs largely due to protist grazing31 whereas, viral lysis accounts for ∼10–20% of the loss.32 In aquatic systems, PAB are known to constitute about 60% of the total bacterial counts. In the present study, microscopic examination revealed that PAB formed up to 76% of the TC (Fig. 1B) in whole seawater whereas, the free-living bacterial cells varied between 26 and 82% (Fig. 1C). Our attempt to demonstrate the effects of grazing on particle-attached and free bacterial population in natural samples using dilution method, estimated an increase and removal coefficients of PAB and free-living bacterial cells. This approach minimized the grazing pressure on bacterial cells as evident from a peak in their abundance within 24h of sample incubation as compared to the undiluted sample where the peak was at 36h (Fig. 1B). Based on the g values (Table 1), the grazing pressure on PAB was far greater than on free-living cells. In aquatic systems, bacteria attached to detrital particles serve as an important food source for zooplankton33 e.g., ciliates which transfer significant amount of bacterial production to higher trophic levels.34 At the time of sampling in the Dona Paula bay, tintinnid ciliates viz., L. nordquisti and Stenosemella sp. were dominant (Fig. S3). There was a considerable bacterial load in the study area which implies that the bacterivorous ciliates did not face any limitation for food. It may be noted that the experimental approach used in the present study did not directly measure grazing. Therefore, all references to grazing are inferred from indirect observations. We intend to study the impacts of grazing on bacteria by ciliates in our future research.
An increase of viable cells in both diluted and whole water samples (Fig. 2B and E) was observed. The increase was more prominent in the latter with a negative coefficient of removal suggesting avoidance/escape. More viable cells perhaps get added from the free pool besides the increase by multiplication on particles. In contrast, the coefficient of increase for PAB_NVC was marginally lesser than their rate of removal (Table 1) indicating their elimination as soon as they accumulate. This in turn leaves the viable cells relatively intact.
Not only do the free viable cells but also the particle-associated viable ones escape grazing. We observed negative grazing coefficients for viable cells of PAB and free-living bacteria. Attached bacterial communities are known to release progeny into the surrounding water.35 Consequently, a general exchange of attached and free bacteria36 in the viable state could be expected. As the coefficient of increase of PAB was more than 2× higher than that of free-living forms (Table 1), it is apparent that free cells attach and multiply on particles. The adherence of bacteria to detrital particles and ability to rapidly reproduce is an important response for survival in the pelagic marine environment.37 It is possible that bacterial cells first lose their viability and when the accumulation reaches a threshold, grazing eliminates them. From our experiments, it can be noted that the percentage of non-viable PAB and free-living cells is relatively more than the VC (Fig. 2). For example, at the end of 6h of incubation (Fig. 2B), the viability of PAB in whole and diluted seawater is <21% of the TC; whereas, the NVC can constitute almost 90–99%. When the NVC reaches such a threshold, the low numbers of remaining viable cells i.e., the persistent variants detach into the free pool to start a new cycle on fresh particles. De-colonization has been suggested as a means of avoiding sinking.38 It is also a means of quitting the less labile, over-aged particles for the relatively more labile fresher particles. Previous studies have shown a positive correlation between abundance and production of free and attached bacteria indicating that production by attached bacteria supplies ‘new’ free bacteria to the pelagic microbial community.39 Though large free-living active cells have been shown to be prone to grazing,40 some of these forms could escape being grazed temporarily by dividing or colonizing on detrital particles. It is suggested that fresh particulate organic matter would not attract grazers till they are sufficiently aged while the most complex polymers get converted to simpler ones by bacterial action. Bacterial association with particles can be active and dynamic.41 Our study demonstrates that grazing pressure can control the physiological state of the bacterial community besides other known factors like genotype and phenotypic composition.42
ConclusionThis study demonstrates that PAB are significantly grazed and the non-viable fraction is more prone to removal by grazers like tintinnid ciliates which are abundant in tropical estuaries. The viable bacterial forms (persistent variants) which are generally much lower in abundance in both the attached and free-state escape getting grazed. Therefore, it is suggested that accumulation of the non-viable cells on particles up to a threshold could signal directly (1) the remaining viable or “persistent variants” to detach or (2) indicate particle maturity to grazers. In contrast to the general contention that active cells are more prone to being grazed upon, it now seems more apparent that the non-viable cells generally get eliminated, leaving the viable less disturbed to replenish the stock.
Conflicts of interestThe authors declare no conflicts of interest.
This paper was supported by grants from the Office of Naval Research, Washington, D.C., USA. The authors are thankful to the Director, NIO for the facilities. The authors thank the two anonymous reviewers for their valuable comments. This is NIO contribution no. 5885.