The pathogenic bacterium Listeria monocytogenes can persist in food processing plants for many years, even when appropriate hygienic measures are in place, with potential for contaminating ready-to-eat products and, its ability to form biofilms on abiotic surfaces certainly contributes for the environmental persistence. In this research, L. monocytogenes was grown in biofilms up 8 days attached to stainless steel and glass surfaces, contributing for advancing the knowledge on architecture of mature biofilms, since many literature studies carried out on this topic considered only early stages of cell adhesion. In this study, biofilm populations of two strains of L. monocytogenes (serotypes 1/2a and 4b) on stainless steel coupons and glass were examined using regular fluorescence microscopy, confocal laser scanning microscopy and classic culture method. The biofilms formed were not very dense and microscopic observations revealed uneven biofilm structures, with presence of exopolymeric matrix surrounding single cells, small aggregates and microcolonies, in a honeycomb-like arrangement. Moreover, planktonic population of L. monocytogenes (present in broth media covering the abiotic surface) remained stable throughout the incubation time, which indicates an efficient dispersal mechanism, since the culture medium was replaced daily. In conclusion, even if these strains of L. monocytogenes were not able to form thick multilayer biofilms, it was noticeable their high persistence on abiotic surfaces, reinforcing the need to focus on measures to avoid biofilm formation, instead of trying to eradicate mature biofilms.
The ability to form biofilms is a key factor for the persistence of Listeria monocytogenes in processing plants and they represent a potential source of contamination of food products, especially ready-to-eat items.1,2 Biofilm architectural characteristics, such as thickness, density and spatial arrangement are important to define the functional properties of these biological structures, including resistance and persistence.1,3
Many studies indicated L. monocytogenes present weak to moderate ability to form biofilms, although it has been shown some isolates can adhere strongly to abiotic surfaces, depending also on the material they are made of.1,2,4 Sessile L. monocytogenes populations can reach 4–6logCFUcm−2 but it not always forms thick multilayer biofilms (9–12logCFUcm−2) as many other bacteria do.5,6
In the present study, biofilms formed by two strains of L. monocytogenes on abiotic surfaces during prolonged incubation times (up to 8 days – 192h) are described, using culture method and differential staining with viability dyes, combined with observations under Confocal Laser Scanning Microscopy (CLSM) and regular fluorescence microscopy.
Materials and methodsBacterial strains and culture conditionsTwo strains of L. monocytogenes were used for biofilm experiments: serotype 1/2a represents the main one recovered from food and from environmental areas; serotype 4b strains are the major cause of human listeriosis outbreaks.7L. monocytogenes ATCC 19115 (serotype 4b) and L. monocytogenes IAL 633 (serotype 1/2a) were acquired from Adolfo Lutz Institute (São Paulo, Brazil) and stored at −80°C in Brain Heart Infusion broth (BHI, Oxoid, UK) supplemented with 20% (v/v) glycerol (Synth, Brazil). Working cultures were made in BHI broth incubated at 37°C for 24h.
Biofilm formation by L. monocytogenes on stainless steel and glass couponsBiofilms of L. monocytogenes were grown on stainless steel (AISI 304, 2.45cm2) and glass coupons (2.65cm2). Prior to inoculation, the coupons were cleaned and prepared according to Winkelströter et al.,8 placed in 24-wells polystyrene microtiter plates (TPP, Switzerland) and the wells were filled with 2ml of an overnight culture of L. monocytogenes in Brain Heart Infusion (BHI) broth (Oxoid). The load of initial inoculum (ca. 108CFU/ml) was checked at every experiment by plate counting on BHI agar plates (37°C/24h). A static pre-incubation of biofilms was done at 25°C for 3h,to favor bacterial adhesion9 and, incubation proceeded under orbital shaking (120rpm) up to 8 days (192h).
After 3, 24 and 96h of incubation, to remove non-adherent bacterial cells, coupons were double rinsed with 1ml of phosphate buffered saline (PBS) pH 7.0, transferred to clean wells and further incubated up to 192h in 2ml of fresh BHI broth, to promote bacterial growth.
At the selected times, planktonic cells of L. monocytogenes in the remnant BHI broth were plated onto BHI agar (BHI broth plus 1.5% bacteriological agar, Oxoid) and incubated at 37°C for 24h. To enumerate L. monocytogenes cells attached to the coupons, each coupon was rinsed three times with PBS to eliminate non-adhered cells, transferred to test tubes containing 10ml of PBS, treated for 2min in ultrasound bath (50–60kHz) and vortexed for 1min according to Leriche and Carpentier,10 with modification – it was necessary to use a shorter time of sonication to maintain cell viability, since in the original publication a less potent equipment was used (4min in a 28kHz). Tenfold dilutions of the biofilm suspensions were done in PBS, drop plated (10μl) on BHI agar and incubated at 37°C for 24h, according to Herigstad et al.,11 with modifications.
The assays were performed as biological triplicates and the results were expressed as colony forming units (CFU) per cm2 or per ml, respectively, for sessile and planktonic cells.
Microscopic evaluation of biofilms formed by L. monocytogenes on stainless steel coupons and glassArchitectural features of L. monocytogenes biofilms, formed on stainless steel were observed by regular fluorescence microscopy. Additionally, biofilms of L. monocytogenes were grown in a special glass chamber (Nunc™ Lab-Tek™ II 8 Chambered Coverglass Nagle Nunc Int., USA) with optical characteristics adequate for studies with confocal laser scanning microscopy (CLSM). For that, 0.5ml of overnight cultures of L. monocytogenes on BHI broth (Oxoid) were placed in the wells of the glass chamber, followed by incubation at 25°C (room temperature) up to 192h. Supernatants were replaced by fresh BHI broth (Oxoid) at times 3, 24 and 96h.
After 24, 96 and 192h of incubation, stainless steel coupons and the glass chamber wells were rinsed three times with PBS to remove planktonic cells and stained with Live/Dead BacLight® kit (Molecular Probes, USA). This kit contains two fluorescent dyes: Syto 9, that stains in green live cells with intact membranes and propidium iodide (PI), which marks in red cells with a compromised membrane, that are considered to be dead or dying.12
Biofilms on stainless steel coupons were observed using an epifluorescence microscope (Nikon 80i, Japan) equipped with filters to detect Syto 9 (Nikon B-2A, 450–490nm) and PI (Nikon G-2A, 510–560nm), combined with the software NIS-Elements AR 3.2 (Nikon).
Biofilms formed on the glass chamber were observed by CLSM (Leica TCS SP5-AOBS, Germany) equipped with argon laser (488nm) for detection of cells stained with Syto 9 and helium laser (543nm) for detection of PI marked cells. Analyses of images were performed using the software LAS AF version: 2.6.0 build 7266 (Leica), adjusted to capture images with a z-series of scans (xyz) of 0.55μm thickness and standardized stacks of 0.13μm. The software ImageJ MacBiophotonic was used to analyze the data (available <http://www.macbiophotonics.ca/imagej/installing_imagej.htm>). The results were expressed as fluorescence units per volume (FU/μm3), and the total volume of the biofilm was calculated by multiplying the value of the area observed by the depth value.
The images of L. monocytogenes biofilms grown on stainless steel coupons and on glass chamber were acquired as independent duplicate experiments, and ca. 3–5 images were captured each time, for both systems and strains.
Statistical analysisExperimental data were analyzed by two-way ANOVA test, followed by Bonferroni and, P<0.05 was considered for significant difference (GraphPadPrism, version 5.0, USA). Bonferroni test is adequate when few comparisons are done at once, being simple to understand and versatile. It is suitable to compare two values and to compute confidence intervals for each comparison (https://www.graphpad.com/guides/prism/6/statistics/index.htm?stat_the_bonferroni_method.htm).
ResultsBoth strains of L. monocytogenes adhered to stainless steel and glass coupons since 3h of contact (105–106CFU/cm2), and reached 106–108CFU/cm2 after 24h, with no further increase of sessile populations, despite incubation for up to 192h. Planktonic cells of L. monocytogenes were abundant (107–1010CFU/ml) over the course of experiments.
For both strains, under fluorescence microscopy (data not shown), green stained (viable) single cells or clusters of L. monocytogenes cells were seen in biofilms formed on stainless steel coupons and they were surrounded by a yellowish background. Only sparse non-viable/dying (red cells) were seen in all assays, except for 96h. The yellowish color may result from overlapping of Syto 9 and PI dyes, as observed for L. monocytogenes ATCC 19115 and, it indicate the presence of EPS, extracellular DNA (e-DNA) or cell injury.
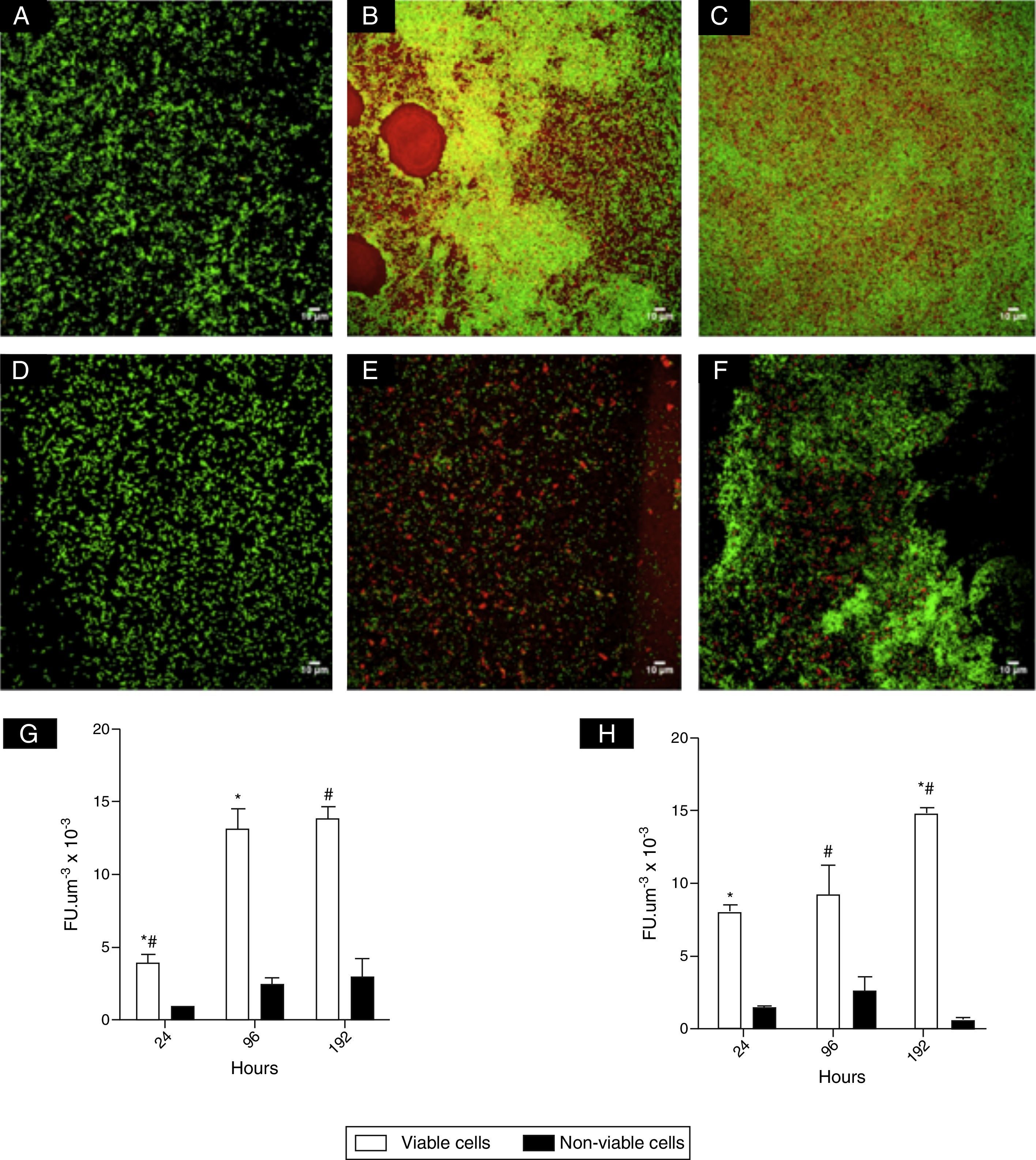
CLSM images (Fig. 1) revealed clusters and microcolonies of L. monocytogenes at 24h of incubation, for both strains (Fig. 1A, D and E). However, honeycomb-like structures with channels predominated with advanced incubation (Fig. 1B, C and F). Fig. 1G and H shows the estimates of the number of viable cells of L. monocytogenes calculated from CLSM data and it was shown at 96 and 192h there were significantly higher populations compared to 24h. It was also noted that the biomass of non-viable cells did not increase significantly in mature biofilms.
Photomicrographs of biofilms of L. monocytogenes IAL 633 serotype 1/2a (A–C) and L. monocytogenes ATCC 19115 serotype 4b (D–F) acquired with CLSM (Leica SP5), ocular 10× and objective 63×, using immersion oil. Cells stained with SYTO 9 were green and cells stained with PI were red: 24h (A and D); 96h (B and E); 192h (C and F). Estimates of viable and non-viable cells (G and H) respectively for L. monocytogenes ATCC 19115 and L. monocytogenes IAL 633 are also shown, expressed as fluorescence units per volume (FUμm−3). *#Denote significant statistical difference in viable cells.
Biofilm formation by L. monocytogenes is influenced not only by lineage and origin of the strain, but also by intrinsic and extrinsic food factors. Under laboratorial conditions, Kadam et al.13 observed that nutrient availability affected biofilm formation by L. monocytogenes strains in polystyrene surfaces, highlighting that nutritionally poor media favored the growth in biofilm state. On the other hand, Zeraik and Nitschke14 demonstrated that L. monocytogenes produced more biofilm in nutrient rich medium. In the present study, BHI broth was used because it has been reported as an effective medium to promote biofilm formation by L. monocytogenes isolates from different origins, at various surfaces.2,15 It was observed 106–108 CFU of L. monocytogenes/cm2 were present in biofilms formed on stainless steel and glass coupons, despite incubation times evaluated. Previous studies on L. monocytogenes biofilms also demonstrated that, after a quick initial adhesion to surfaces, populations did not increase largely.5,6 The present results also indicated after initial colonization of abiotic surfaces, the number of planktonic L. monocytogenes cells remained constant due to dispersal from biofilms, which agrees with the report by Gram et al.5. Oliveira et al.,6 also reported the biotransfer potential of L. monocytogenes from biofilms to the surrounding medium, highlighting the importance of this as a source of contamination in food contact surfaces.
The microscopic observations done for L. monocytogenes mature biofilms showed a honey comb-like structure, that is known to offer fitness advantages due to improvement of mechanical stability of biofilm and absorption of nutrients by bacterial cells.16 Borucki et al.4 reported L. monocytogenes produced a dense 3-D biofilm structure, while Pilchová et al.17 demonstrated also a honeycomb-like, as the present study. The uneven colonization of abiotic surfaces is not uncommon for L. monocytogenes in biofilms,6,18,19 although there is also a literature report on a thin biofilm structure covering most of the abiotic surface.3 In this study, CLSM revealed in biofilms up 192h for the two strains of L. monocytogenes there were hollows of different sizes, suggestive of cell death or dispersal (Fig. 1B). Another structural aspect that should be emphasized is the presence of a “red carpet” surrounding viable cells at later incubation times, which may indicate of the presence of extracellular DNA. Moreover, the overlap of the dyes Syto 9 and PI (Fig. 1B, yellow color) also suggests cell lysis and release of e-DNA.20
In conclusion, L. monocytogenes did not form thick biofilms but they presented a firm and highly organized structure, with cells under different physiological states, which can offer selective advantage for bacterial survival under suboptimal conditions.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors are grateful to Multiuser Laboratory of Confocal Microscopy – FMRP-USP (São Paulo Research Foundation – FAPESP process# 2004/08868-0) for the CLSM analysis, and to the Biochemistry Laboratory, Department of Physics and Chemistry – FCFRP-USP for the use of the Microplate Reader. This research was funded by São Paulo Research Foundation – FAPESP (Processes #2010/10051-3 and 2011/07062-6).