Ammonia-oxidizing bacteria were immobilized by polyvinyl alcohol (PVA) and sodium alginate. The immobilization conditions and ammonia oxidation ability of the immobilized bacteria were investigated. The following immobilization conditions were observed to be optimal: PVA, 12%; sodium alginate, 1.1%; calcium chloride, 1.0%; inoculum concentration, 1.3 immobilized balls/mL of immobilized medium; pH, 10; and temperature, 30°C. The immobilized ammonia-oxidizing bacteria exhibited strong ammonia oxidation ability even after being recycled four times. The ammonia nitrogen removal rate of the immobilized ammonia-oxidizing bacteria reached 90.30% under the optimal immobilization conditions. When compared with ammonia-oxidizing bacteria immobilized by sodium alginate alone, the bacteria immobilized by PVA and sodium alginate were superior with respect to pH resistance, the number of reuses, material cost, heat resistance, and ammonia oxidation ability.
Ammonia-oxidizing bacteria belong to one of the physiological subsets of the nitrifying bacteria family.1 These bacteria play a role in the first rate-limiting step of nitrification2,3 and in the oxidation of amines to nitrites and are widely used in the denitrification of industrial wastewater and soil.4,5 However, because ammonia-oxidizing bacteria are autotrophic, they have a long generation time, slow growth rate, and high sensitivity, and they can easily be eliminated.6 To address these limitations, cell immobilization technology has been developed.7 Immobilized cell technology offers distinct advantages and combines liquid fermentation and immobilized enzymes. Immobilized cells exhibit improved catalytic activity, reduced production time, low production cost, high yield, and extensive application prospects.8,9 Many raw materials and synthetic polymers such as sodium alginate, polyacrylamide, agar, and polyvinyl alcohol (PVA) have been extensively applied in cell immobilization.10–12 In the present study, ammonia-oxidizing bacteria were immobilized by PVA and sodium alginate to improve their characteristics and applications.
Materials and methodsStrainsThe ammonia-oxidizing bacteria used in this study were screened from activated sludge collected from a sewage treatment plant at China University of Mining and Technology (Xuzhou, China) and exhibited 98% homology with Nitrosomonas sp. GH22.
Culture mediaThe simulation sewage medium (pH 8.0–8.2) comprised 4g/L (NH4)2SO4, 1g/L K2HPO4, 0.5g/L MgSO4, 2g/L NaCl, 0.4g/L FeSO4 and 10g/L CaCO3. The immobilized medium (pH 7.0–7.2) contained 0.8g/L (NH4)2SO4, 1g/L K2HPO4, 0.5g/L MgSO4, 0.4g/L FeSO4 and 10g/L CaCO3.13,14
Preparation of immobilized ammonia-oxidizing bacteriaThe ammonia-oxidizing bacteria were cultured in simulated sewage medium. After the culture was allowed to stand for a certain period, the supernatant was discarded. Subsequently, PVA and sodium alginate were added to the culture at a PVA/sodium alginate culture ratio of 3:1 (v/v). The culture mixture was added to calcium chloride using an injector, and the bacteria were immobilized at 4°C for 6h. Subsequently, the immobilized bacteria were washed with deionized water and inoculated into 250–1000mL of immobilized medium at a concentration of 1–2 immobilized balls/mL of immobilized medium at 30°C and 100r/min for 16–20 days.
Determination of nitrate nitrogen and ammonia nitrogen contentsThe nitrite nitrogen content was determined by α-naphthylamine spectrophotometry and phenol disulfonic acid spectrophotometry, whereas the ammonia nitrogen content was determined using phenol disulfonic acid.
ResultsEffect of PVA concentration on the formation of immobilized ballsTo determine the effect of PVA concentration on the formation of immobilized balls, the following conditions were applied: 1% sodium alginate; 2.0% calcium chloride; and 6.0%, 8.0%, 10.0%, 12.0%, and 14.0% PVA.
The results showed that at PVA concentrations less than 10%, the immobilized balls were transparent and soft, whereas at concentrations higher than 14.0%, the immobilized balls were opaque, hard, and trailing. A PVA concentration of 12% produced immobilized balls that showed better transparency and hardness.
Effect of sodium alginate concentration on the formation of immobilized ballsTo determine the effect of sodium alginate concentration on the formation of immobilized balls, the following conditions were employed: 2.0% calcium chloride; 12% PVA; and 0.8%, 0.9%, 1.0%, 1.1%, and 1.2% sodium alginate.
The results showed that at sodium alginate concentrations less than 1.0%, the immobilized balls were transparent and soft, whereas at concentrations higher than 1.2%, the immobilized balls were opaque, hard, and trailing. A sodium alginate concentration of 1.1% produced immobilized balls that showed better transparency and hardness.
Effect of calcium chloride concentration on the formation of immobilized ballsTo determine the effect of calcium chloride concentration on the formation of immobilized balls, the following immobilization conditions were employed: 1.1% sodium alginate, 12% PVA, and 1.0%, 2.0%, 3.0%, 4.0%, and 5.0% calcium chloride.
The results showed that at calcium chloride concentrations higher than 2%, the immobilized balls were opaque, hard, and trailing, whereas at a concentration of 1.0%, the immobilized balls exhibited better transparency and hardness.
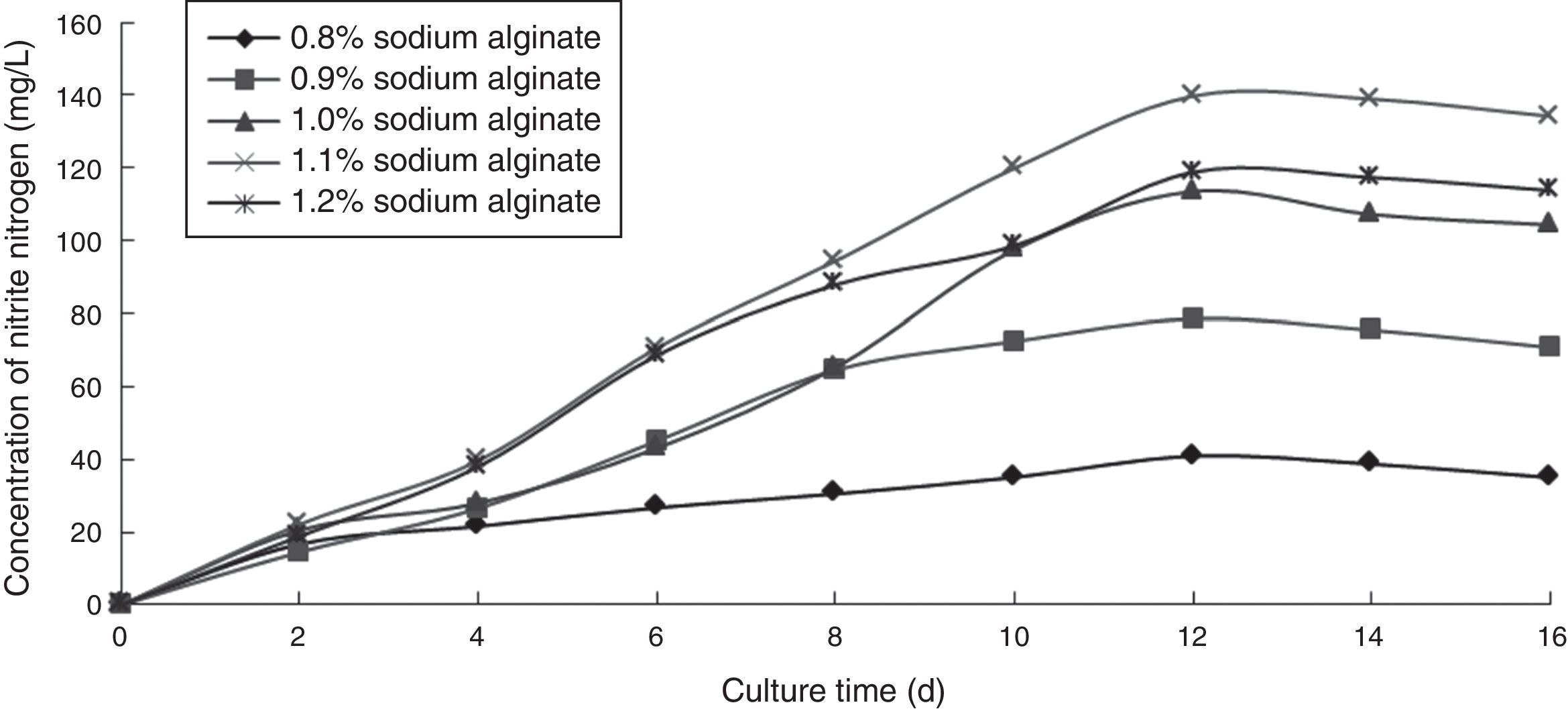
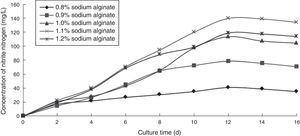
Effect of sodium alginate concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of sodium alginate concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 1.0% calcium chloride; 12% PVA; 0.8%, 0.9%, 1.0%, 1.1%, and 1.2% sodium alginate; and 1.3 immobilized balls/mL of immobilized medium. The reaction was performed in a 250-mL shake flask containing 250mL of immobilized medium of pH 8.0 maintained at 30°C and 120r/min for 16 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is shown in Fig. 1.
The concentration of sodium alginate was observed to affect the hardness of immobilized cells. The higher the concentration of sodium alginate was, the greater the strength of immobilized cells became. However, if the concentration was too high, immobilized cells would not grow, and if the concentration was too low, immobilized balls would break. Furthermore, the nitrite nitrogen concentration was observed to increase with increasing cultivation time. The optimal sodium alginate concentration was 1.1%, which produced the highest nitrite nitrogen concentration.
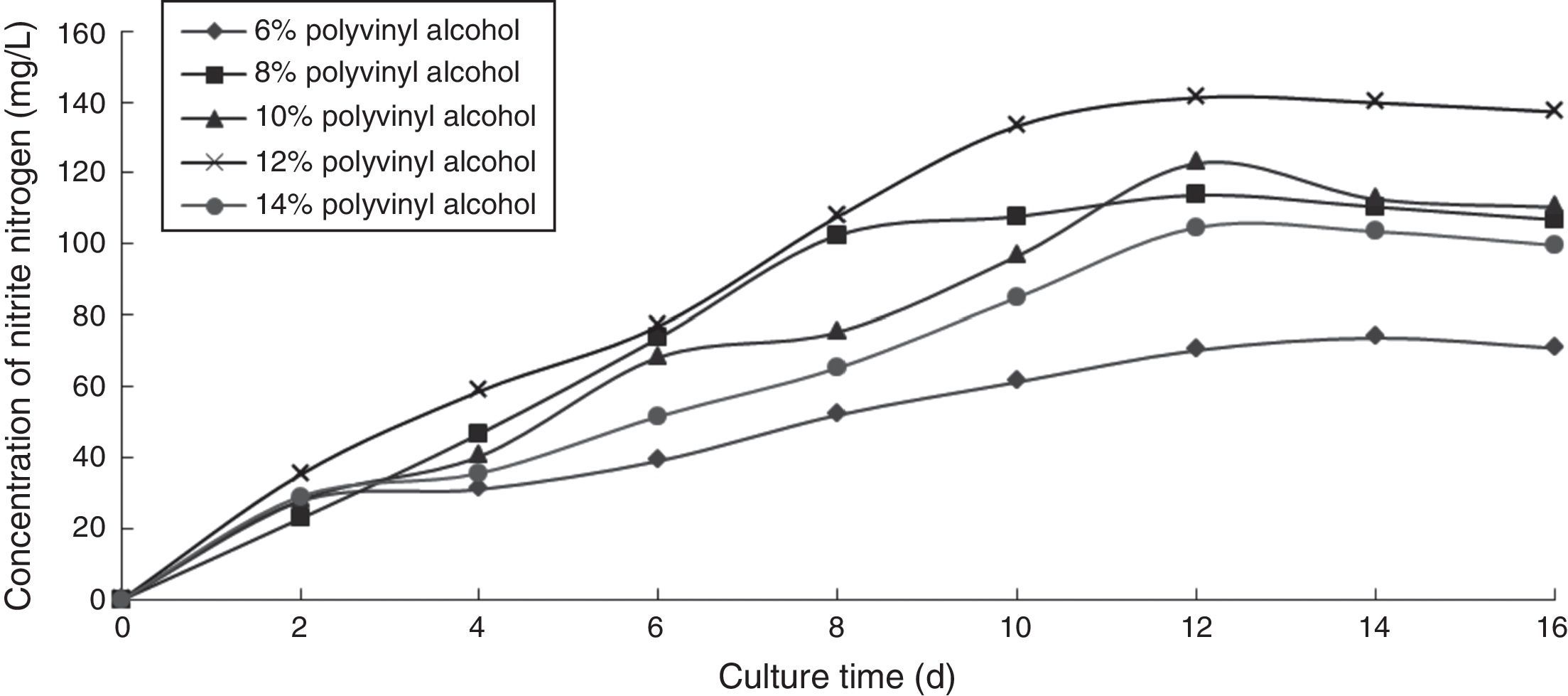
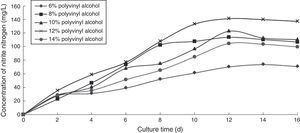
Effect of PVA concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaThe higher the concentration of polyvinyl alcohol was, the greater the strength of the immobilized cells became. However, increased viscosity made the operation more difficult and detrimental to matrix transfer and the growth of ammonia-oxidizing bacteria. To determine the effect of PVA concentration on the ammonia nitrogen removal ability of immobilized ammonium-oxidizing bacteria, the following immobilization conditions were employed: 1.1% sodium alginate; 1.0% calcium chloride; 6.0%, 8.0%, 10.0%, 12.0%, and 14.0% PVA; and 1.3 immobilized balls/mL of immobilized medium. The reaction was performed in a 250-mL shake flask containing 250mL of immobilized medium of pH 8.0 maintained at 30°C and 120 r/min for 16 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is shown in Fig. 2.
The nitrite nitrogen concentration increased with increasing cultivation time. The optimal PVA concentration was 12.0%, which produced the highest nitrite nitrogen concentration.
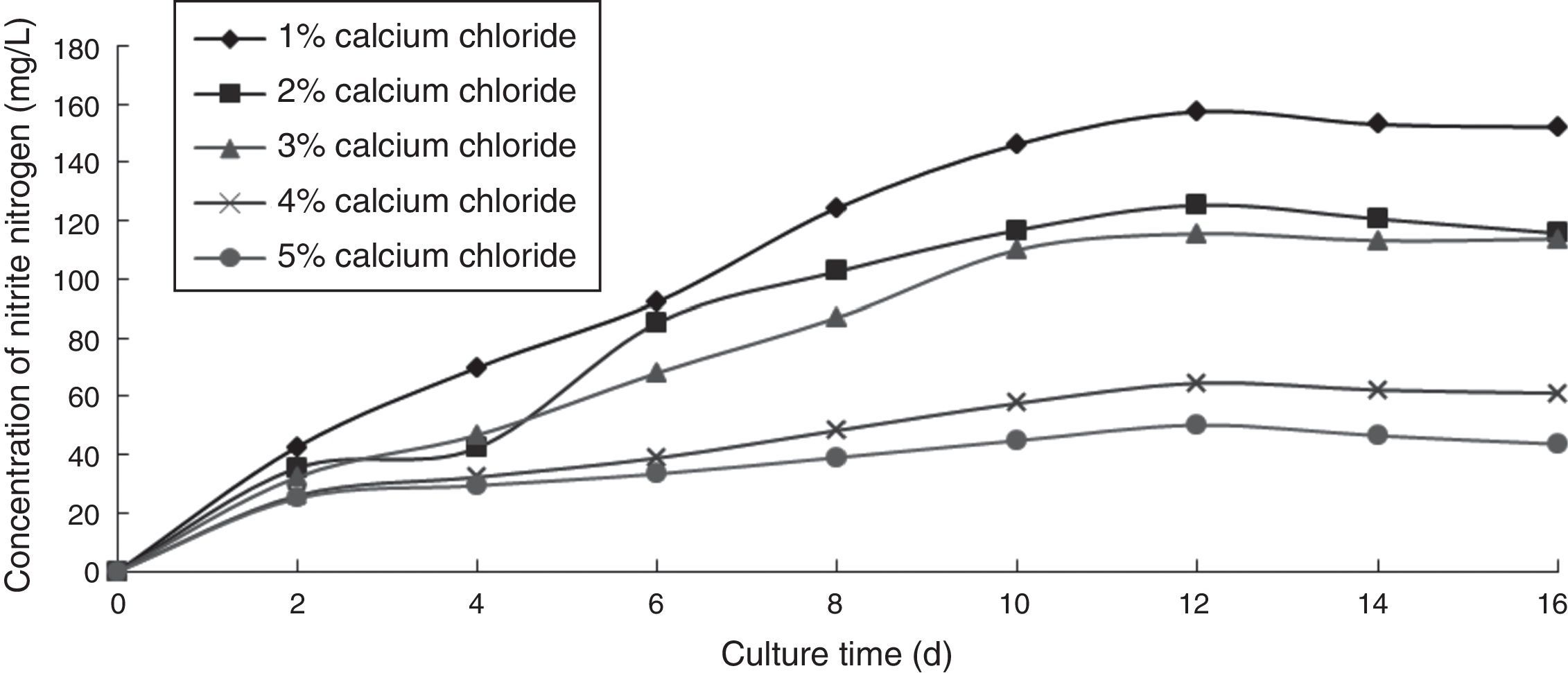
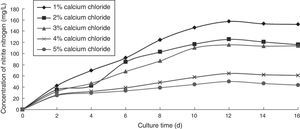
Effect of calcium chloride concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of calcium chloride concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 12% PVA; 1.1% sodium alginate; 1%, 2%, 3%, 4%, and 5% calcium chloride; and 1.3 immobilized balls/mL of immobilized medium. The reaction was performed in a 250-mL shake flask containing 250mL of immobilized medium at pH 8.0, 30°C, and 120r/min for 16 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is presented in Fig. 3.
When the concentration of calcium chloride was low, the immobilized balls were soft and easily broken. As the calcium chloride concentration was increased, the microbial cell activity decreased due to the high osmotic pressure of salt, which causes cell dehydration and reduces microbial activity. Moreover, the nitrite nitrogen concentration increased with increasing cultivation time. The optimal calcium chloride concentration was 1.0%, which produced the highest nitrite nitrogen concentration.
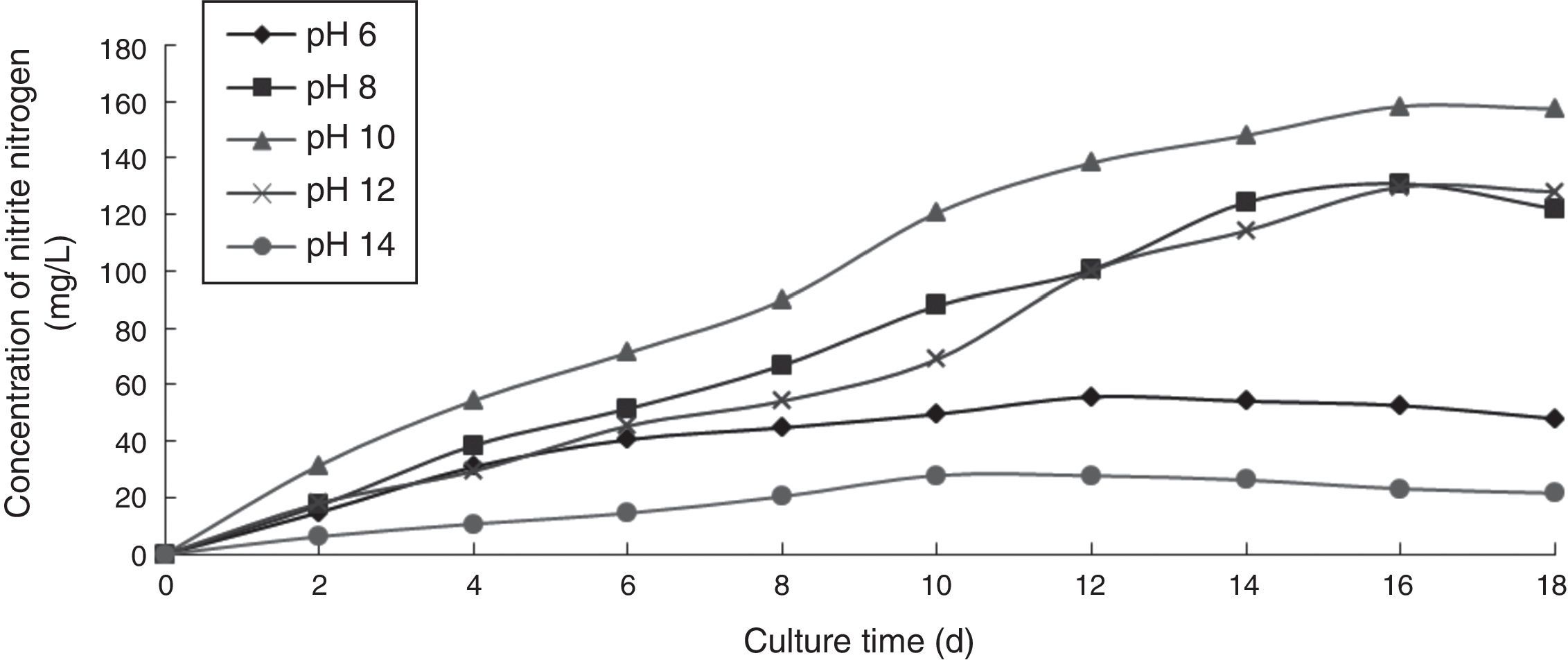
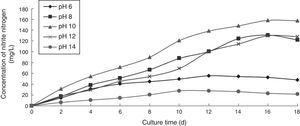
Effect of pH on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of pH on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 12% PVA; 1.1% sodium alginate; 1.0% calcium chloride; and 1.3 immobilized balls/mL of immobilized medium. The reaction was conducted in a 500-mL shake flask containing 500mL of immobilized medium at different pH values, 30°C, and 110r/min for 18 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is presented in Fig. 4.
Strong acidic and strong alkaline conditions destroyed all of the immobilized cells. Hydrogen ions affected the charge balance at the cell surface and the membrane permeability. Strong acidic conditions caused the nutrients to become unavailable to immobilized cells in the culture liquid. Strong alkaline conditions caused nucleic acid and protein denaturation and cell death. Furthermore, the nitrite nitrogen concentration was observed to increase with increasing cultivation time. The optimal pH was 10, at which point the highest nitrite nitrogen concentration was obtained.
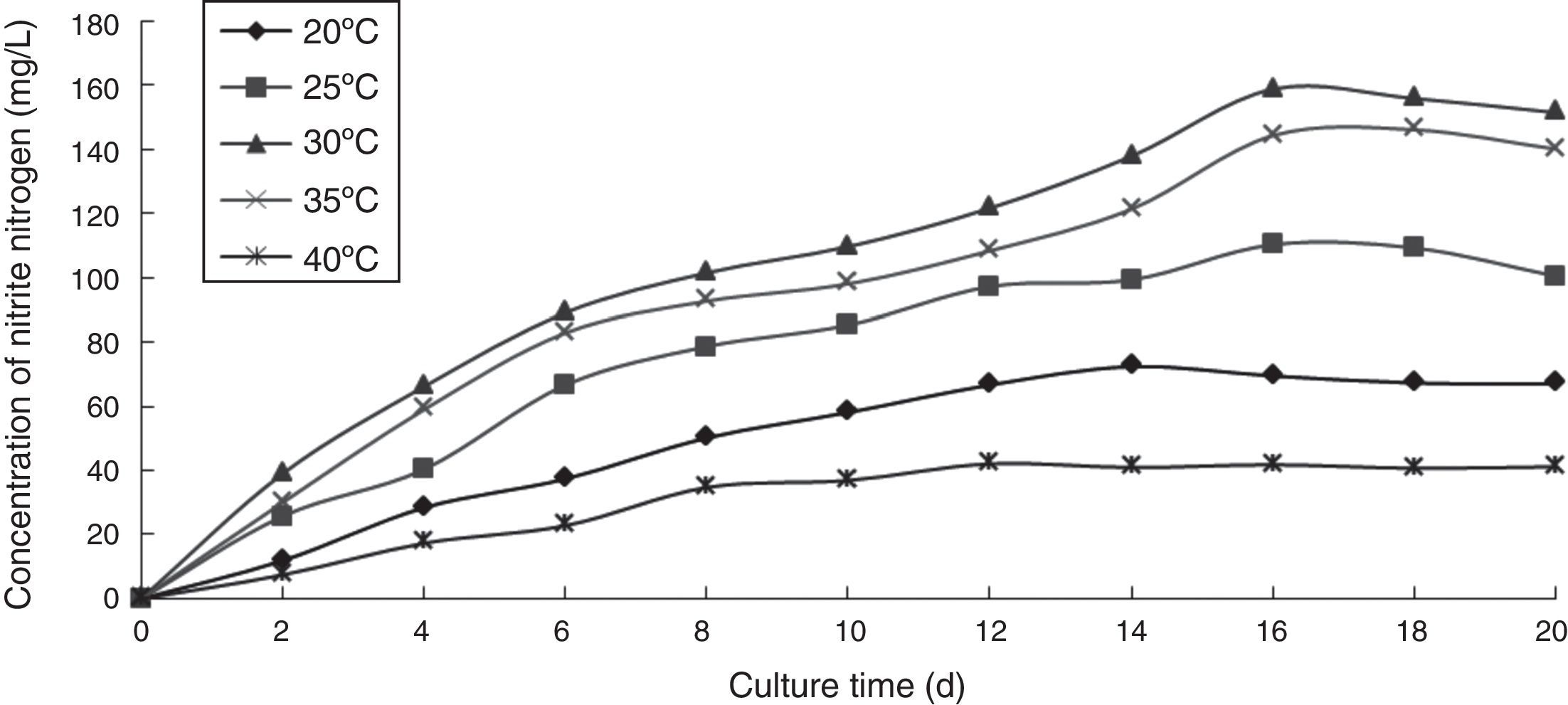
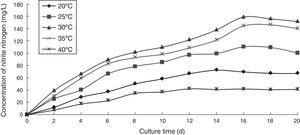
Effect of temperature on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of temperature on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 12% PVA; 1.1% sodium alginate; 1.0% calcium chloride; and 1.3 immobilized balls/mL of immobilized medium. The reaction was performed in a 500-mL shake flask containing 500mL of immobilized medium at pH 10, different temperatures, and 120r/min for 20 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is presented in Fig. 5.
If the temperature was too high or too low, the metabolism of the immobilized ammonia-oxidizing bacteria was slow or was even inactivated. The nitrite nitrogen concentration increased with increasing cultivation time. The growth and metabolism of the immobilized ammonia-oxidizing bacteria were slow at low temperature and increased with increasing temperature and the concentration of nitrite nitrogen. The enzymatic activity of the immobilized ammonia-oxidizing bacteria was disrupted and even nullified at high temperatures. The optimal temperature was 30°C, at which point the highest nitrite nitrogen concentration was obtained. The immobilized ammonia-oxidizing bacteria could grow at high and low temperatures, indicating that immobilization by PVA coupled with sodium alginate improved the temperature resistance of the ammonia-oxidizing bacteria.
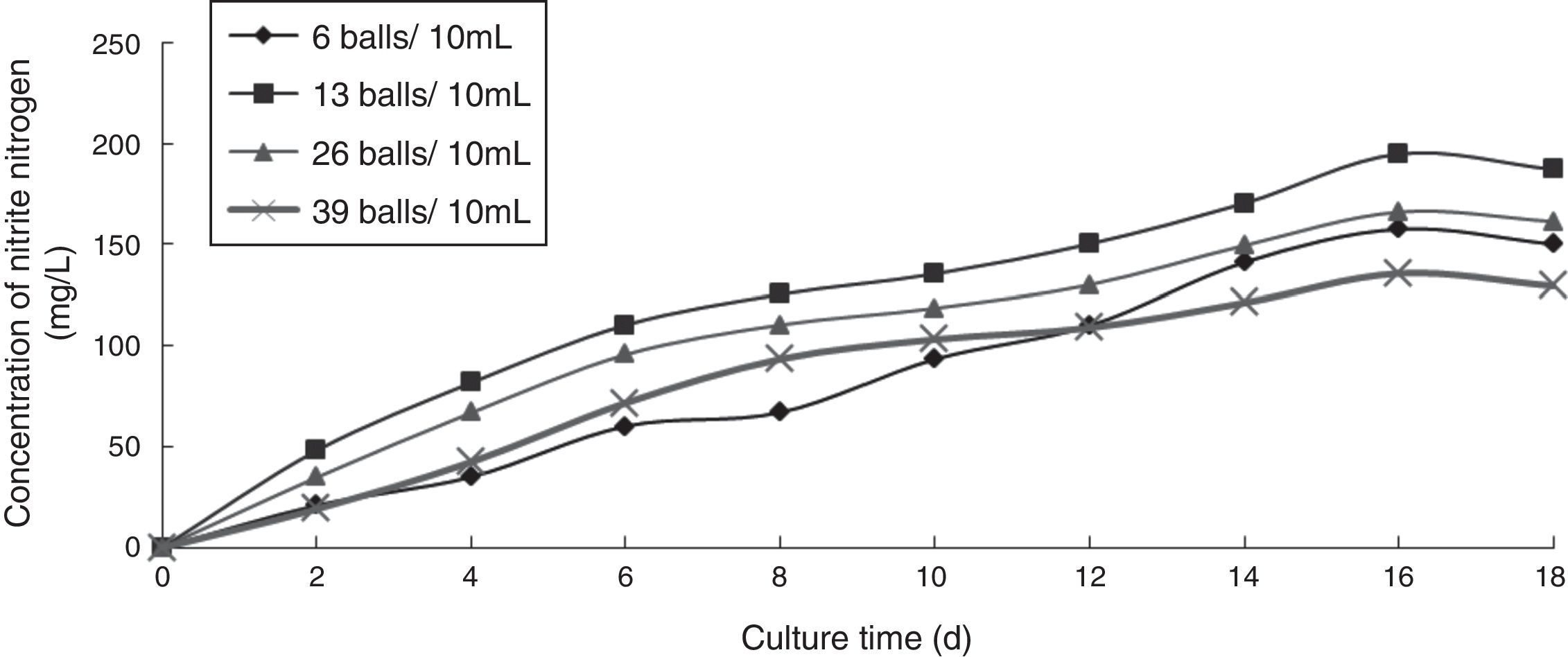
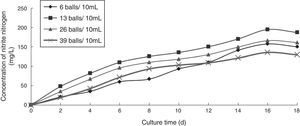
Effect of inoculum concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of inoculum concentration on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 12% PVA; 1.1% sodium alginate; 1.0% calcium chloride; and 0.6, 1.3, 2.6, and 3.9 immobilized balls/mL of immobilized medium. The reaction was conducted in a 500-mL shake flask containing 500mL of immobilized medium at pH 10, 30°C, and 110r/min for 18 days. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the above-mentioned conditions is shown in Fig. 6.
The development of ammonia-oxidizing bacteria is poor when the inoculum concentration is too high because the bacteria will compete for nutrients in the culture medium. Development is also poor when the inoculum concentration is too low because the nutrients in the culture medium are not sufficient for immobilized ammonia-oxidizing bacteria metabolism. Therefore, appropriate inoculum levels are required for immobilized ammonia-oxidizing bacteria. The nitrite nitrogen concentration was observed to increase with increasing cultivation time. When the inoculum concentration was minimal, the growth of the ammonia-oxidizing bacteria was slow, whereas at very high inoculum concentrations, the bacteria competed for nutrients and oxygen. At an inoculum concentration of 1.3 immobilized balls/mL of immobilized medium, the highest nitrite nitrogen concentration of 143.41mg/L was achieved; hence, this inoculum concentration was considered to be optimal.
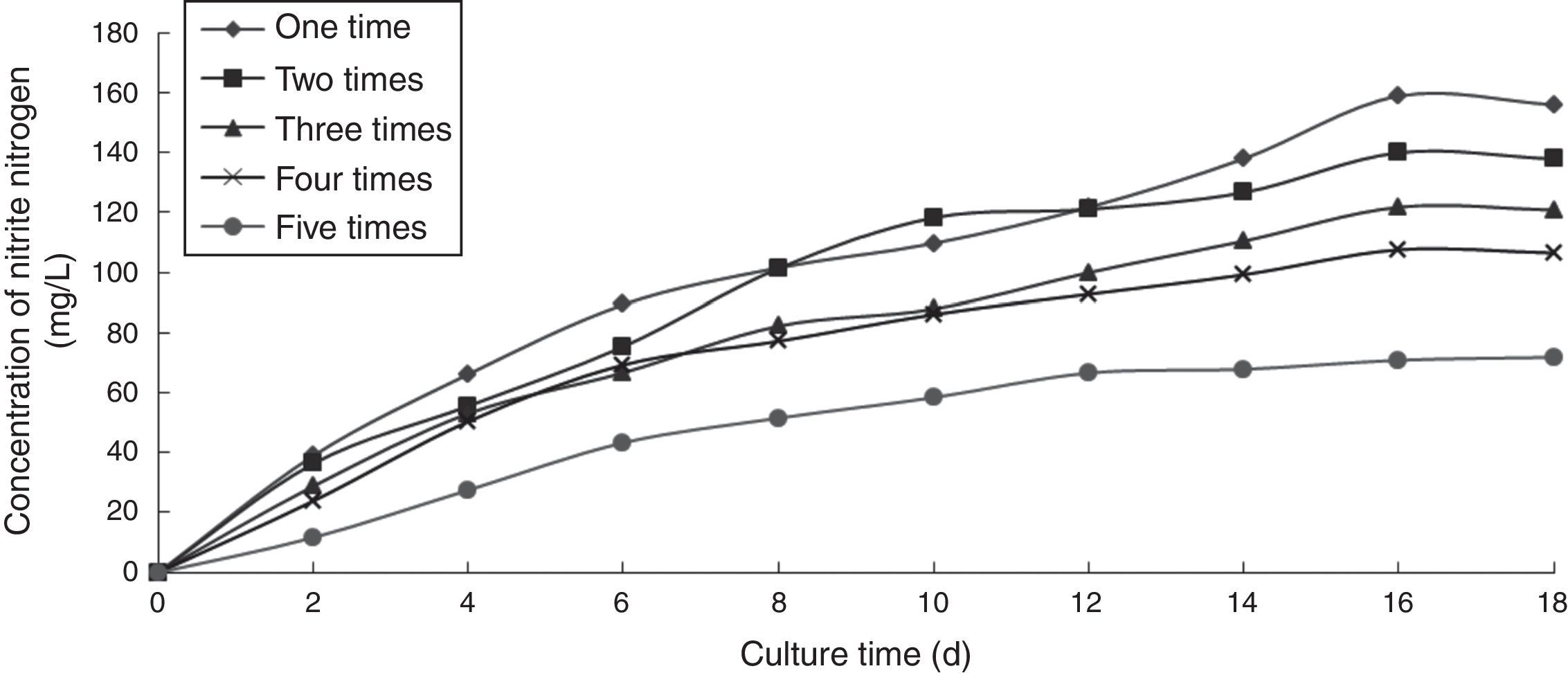
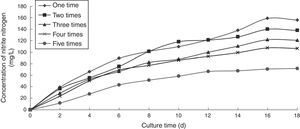
Effect of the number of recycling times on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteriaTo determine the effect of the number of recycling times on the ammonia nitrogen removal ability of immobilized ammonia-oxidizing bacteria, the following immobilization conditions were employed: 12% PVA; 1.1% sodium alginate; 1.0% calcium chloride; and 1.3 immobilized balls/mL of immobilized medium. The reaction was conducted in a 500-mL shake flask containing 500mL of immobilized medium at pH 10, 30°C, and 120r/min. The immobilized medium was replaced with fresh medium periodically. The effects of the number of recycling times on the ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria are presented in Fig. 7.
As the number of recycling times increased, the cells gradually aged, and their metabolisms slowed. When the immobilized cells grew, the calcium alginate walls expanded and were easily broken. In cases in which these protective barriers were lost, immobilized cells died. After the fifth recovery, the denitrifying capacity and activity of the immobilized ammonia-oxidizing bacteria were better preserved, and the volume and mechanical strength of the immobilized balls were steady. However, although the immobilized balls did not break, they were soft and approximately 1.5 times larger than the original balls and showed reduced mechanical strength. Thus, we concluded that the immobilization process using a combination of PVA and sodium alginate exerted a protective effect on the ammonia-oxidizing bacteria and presented advantages such as the ability to recycle and save the raw materials used and the bacteria. The immobilized ammonia-oxidizing bacteria retained their ammonia oxidation capacity even after the fifth recovery.
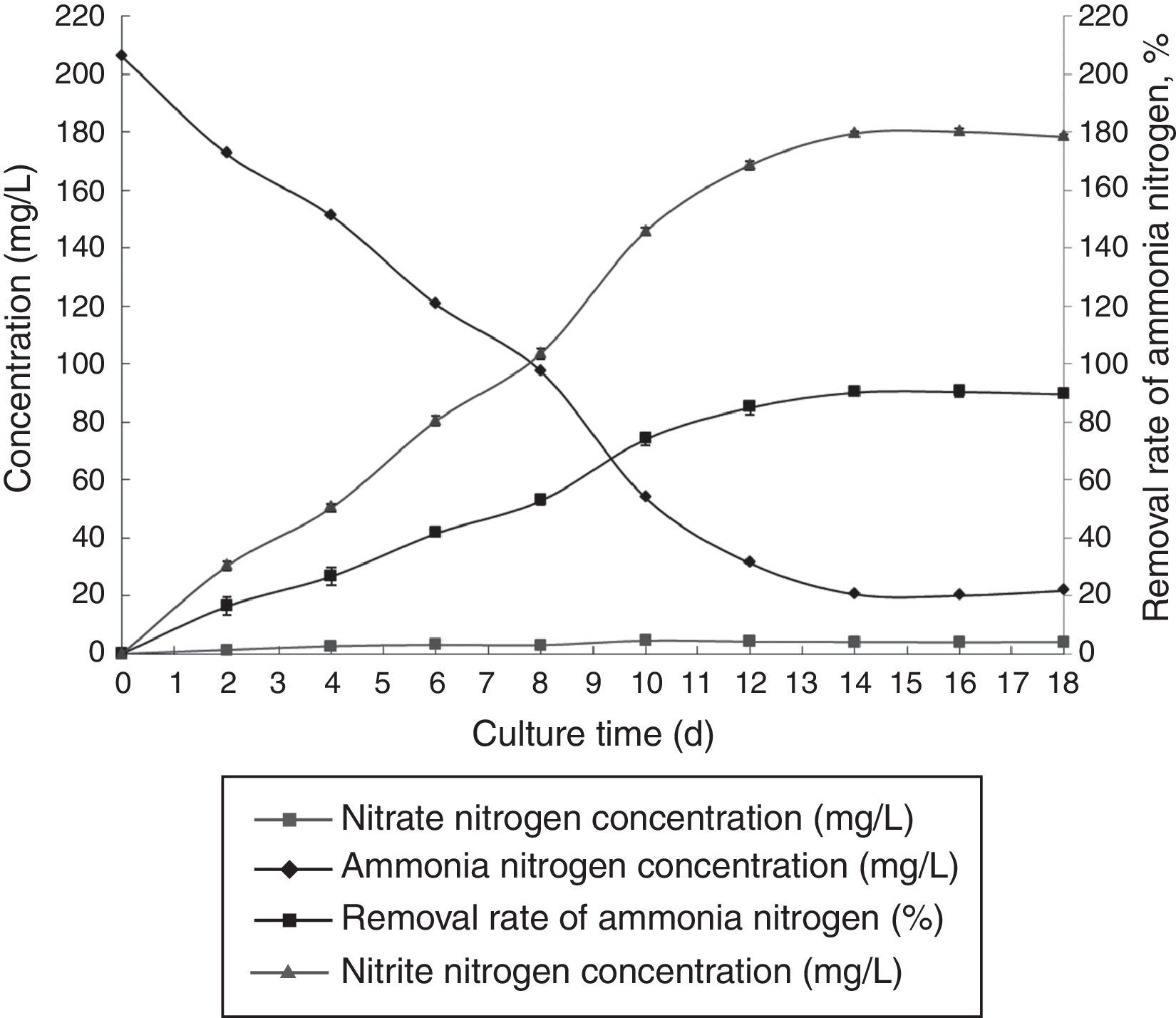
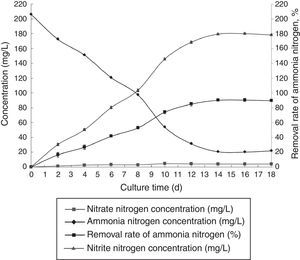
Optimal immobilization conditionsThe optimal immobilization conditions were as follows: 12% PVA, 1.1% sodium alginate, 1.0% calcium chloride, and 1.3 immobilized balls/mL of immobilized medium at pH 10 and 30°C. The reaction was performed in a 1000-mL shake flask containing 1000mL of immobilized medium at 120r/min. The ammonia nitrogen removal ability of the immobilized ammonia-oxidizing bacteria under the optimal conditions is presented in Fig. 8.
Clearly, the ammonia-oxidizing bacteria that were immobilized under optimal conditions exhibited better ammonia nitrogen removal than did the non-immobilized bacteria. The ammonia nitrogen removal rate of the immobilized ammonia-oxidizing bacteria reached 90.30%.
DiscussionCharacteristics of the process of immobilization of ammonia-oxidizing bacteria by sodium alginate and PVAThe experimental results reported above demonstrate that immobilized ammonia-oxidizing bacteria have a strong resistance to adverse environments, are insensitive to changes in temperature and pH, and exhibit increased stability, reusability, short reaction times, low cost and an improved ammonia nitrogen removal ability. The ammonia-oxidizing bacteria were immobilized in sodium alginate and PVA as follows. First, for the PVA preparation, the PVA was swelled in cold water and then dissolved in boiling water. Complete swelling of PVA made the immobilized balls firmer, and the PVA did not dissolve in water after crosslinking itself. Second, agglomeration of the immobilized carrier was improved by adding sodium alginate to defend the carrier from boric acid toxicity. The PVA content (12%) was much higher than the sodium alginate content (1.1%) to ensure the stability of the immobilized balls. Third, although the optimum pH for the immobilized ammonia-oxidizing bacteria was 10, experiments revealed that a pH of 8 was optimal for non-immobilized ammonia-oxidizing bacteria, indicating that the immobilized carrier protected the ammonia-oxidizing bacteria from alkaline conditions. Lastly, high and low temperatures affected the immobilized ammonia-oxidizing bacteria, with the optimum temperature being 30°C.
Comparison of the two immobilization methodsThe ammonia-oxidizing bacteria immobilized by sodium alginate and PVA were superior to those immobilized by sodium alginate alone15 for the following reasons. First, the ammonia-oxidizing bacteria immobilized by sodium alginate and PVA were tolerant to a wider range of pH levels, whereas those immobilized by sodium alginate alone were easily damaged by highly alkaline conditions. Second, although the immobilized balls obtained using a combination of sodium alginate and PVA exhibited a slight change in diameter and color after the fifth recovery, they did not break. In contrast, the immobilized balls obtained using sodium alginate alone had a larger diameter, were more transparent, and were severely broken after the fifth recovery. This finding suggests that a higher concentration of sodium alginate was needed, which could increase the amount of raw materials required and the cost. Finally, the ammonia-oxidizing bacteria immobilized by sodium alginate and PVA exhibited a better ammonia oxidation ability under optimum conditions than that of the bacteria immobilized by sodium alginate alone.
Outlook of future researchImmobilized carriers and methods of immobilized ammonia-oxidizing bacteria must be further researched. For example, to determine the best immobilized carriers and methods, PVA or microcapsules could be evaluated as carriers, and immobilized cells could be prepared using covalent or crosslinking methods. Quick preparation methods for immobilized cells should also be developed for continuous and industrialized operation. Moreover, immobilized ammonia-oxidizing bacteria applications should be extended to small industrial scales.
ConclusionsThe following conclusions were drawn based on the findings of the present study:
- 1.
The optimal conditions for the immobilization of ammonia-oxidizing bacteria were as follows: PVA, 12%; sodium alginate, 1.1%; calcium chloride, 1.0%; inoculum concentration, 1.3 immobilized balls/mL of immobilized medium; pH, 10; and temperature, 30°C. The immobilized ammonia-oxidizing bacteria exhibited strong ammonia oxidation abilities even after being recycled four times. The ammonia nitrogen removal rate of the immobilized ammonia-oxidizing bacteria reached 90.30% under the optimal conditions.
- 2.
Compared with the ammonia-oxidizing bacteria immobilized by sodium alginate alone, those immobilized by PVA and sodium alginate were superior with respect to pH resistance, the number of reuses, material cost, heat resistance, and ammonia oxidation ability.
The authors declare no conflicts of interest.
This research was supported by grants from the Collegiate Natural Science Found of Jiangsu Province (14KJD180003).