The production of KPC (Klebsiella pneumoniae carbapenemase) is the major mechanism of resistance to carbapenem agents in enterobacterias. In this context, forty KPC-producing Enterobacter spp. clinical isolates were studied. It was evaluated the activity of antimicrobial agents: polymyxin B, tigecycline, ertapenem, imipenem and meropenem, and was performed a comparison of the methodologies used to determine the susceptibility: broth microdilution, Etest® (bioMérieux), Vitek 2® automated system (bioMérieux) and disc diffusion. It was calculated the minimum inhibitory concentration (MIC) for each antimicrobial and polymyxin B showed the lowest concentrations for broth microdilution. Errors also were calculated among the techniques, tigecycline and ertapenem were the antibiotics with the largest and the lower number of discrepancies, respectively. Moreover, Vitek 2® automated system was the method most similar compared to the broth microdilution. Therefore, is important to evaluate the performance of new methods in comparison to the reference method, broth microdilution.
Klebsiella pneumoniae carbapenemase (KPC) – producing bacteria are a group of emerging highly drug-resistant Gram-negative bacilli causing infections associated with significant morbidity and mortality.1,2 These infections also represent a challenge in clinical practice involving hospitalized patients, requiring multidisciplinary efforts for infection prevention. Found primarily in K. pneumoniae, KPC is an enzyme capable of hydrolysing a broad spectrum of β-lactams including the penicillins, cephalosporins, carbapenems and monobactams. In addition, this resistance mechanism has high potential for dissemination due to its plasmid location, which facilitates transfer to the interspecies gene. Therefore, KPC has been identified in several other Enterobacteriaceae and nonfermenting Gram-negative bacilli (Acinetobacter baumannii and Pseudomonas aeruginosa).3,4
Carbapenems are first-line agents for the treatment of serious nosocomial infections caused by multidrug-resistant Enterobacteriaceae. However, the increasing incidence of carbapenemase-producing Enterobacteriaceae (CPE) infections hinders the use of this class of antibiotics.2,5 Therefore, tigecycline and polymyxins are commonly required to treat infections caused by CPE.6
Due the few remaining available treatment options, optimization of dosing regimens and combination therapy may be the most appropriate treatment strategies. The benefits of combination therapy include potential synergistic effects and suppression of emerging resistance.7 In addition, the selection of a dosing regimen depends on the ability to determine the minimum inhibitory concentration (MIC) to an antibiotic.8 Few studies about methods of evaluation of multidrug-resistant Enterobacter spp. have been found. Therefore, the aim of this study was to compare four methods used for detect antimicrobial susceptibility: broth microdilution (BMD), Etest® – plastic strip with gradient of antibiotic concentrations to determine the minimum inhibitory concentration of antibiotics, Vitek 2® automated system for microbial identification and antimicrobial susceptibility testing and disc diffusion (Kirby Bauer) for five antimicrobials agents (polymyxin B, tigecycline, ertapenem, imipenem and meropenem) among KPC-producing Enterobacter spp. clinical isolates.
Materials and methodsBacterial isolatesForty samples of KPC-producing Enterobacter spp. (thirty E. cloacae, nine E. aerogenes and one E. gergoviae), isolated from patients hospitalized in University Hospital of Londrina, between July of 2010 and December of 2013, that showed carbapenem resistance, according to CLSI (Clinical and Laboratory Standards Institute),9 were evaluated. The isolates were stored in TSB (Oxoid-England) glycerin at −20°C until use for the study. The samples were previously identified by the BD Phoenix™ automated system.
Characterization of clinical isolatesThe isolates were subjected to modified Hodge test (MHT), according to Lee et al.10 to phenotypically identify the presence of carbapenemases. Class A carbapenemase production was confirmed by using of boronic acid, as described by Tsakris et al.11 The polymerase chain reaction (PCR) was performed with specifics primers, as described by Bradford et al.12 for research of blaKPC-2 gene, previously identified. The performance of the tests for the detection of carbapenemases was determined using PCR as the reference standard. Sensitivity was calculated from the number of true-positive isolates, whereas specificity was calculated from the number of true-negative isolates.
Antimicrobial susceptibility testingDisc diffusionThe disc diffusion test was performed with Mueller Hinton Agar and discs from Oxoid®-England by Kirby-Bauer technique and according to CLSI document M2-A10.9 The test discs were: tigecycline 15μg, imipenem 10μg, meropenem 10μg, ertapenem 10μg and polymyxin B 300U. There is no standardization, by CLSI, for polymyxin by disc diffusion test, only for broth microdilution. Therefore, standard breakpoints for P. aeruginosa were used for polymyxin B.
MIC testingSusceptibility to antibiotics was also determined by broth microdilution (BMD), the reference method, Vitek 2® automated system (bioMérieux) and Etest® (bioMérieux), but the Etest® was performed only to tigecycline. The bacterial suspensions were adjusted according to CLSI recommendations. As described in CLSI, for meropenem, imipenem e ertapenem, P. aeruginosa ATCC 27853 and Enterococcus faecalis ATCC 29212 were used for quality control to broth microdilution test. For tigecycline, the bacteria used were Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 and for polymyxin B, P. aeruginosa ATCC 27853 and E. coli ATCC 25922. The microplates were incubated at 37±2°C for 21 to 24hours and visually evaluated. The MIC is defined as the lowest concentration of antibiotic capable of inhibiting the growth of the microorganism. Therefore, the concentrations required to inhibit 50 and 90% of the strains (MIC50 and MIC90, respectively), were calculated for all tested antibiotics.
MIC breakpointsAccording to the recommendation of the ANVISA (Agência Nacional de Vigilância Sanitária), in Technical Note N° 01/2010,13 the following breakpoints were used to evaluate polymyxin B susceptibility (susceptible≤2μg/mL and resistant≥4μg/mL), tigecycline (susceptible≤1μg/mL, intermediate 2μg/mL and resistant≥4μg/mL), ertapenem (susceptible≤0.5μg/mL, intermediate 1μg/mL and resistant≥2μg/mL), imipenem (susceptible≤1μg/mL, intermediate 2μg/mL and resistant≥4μg/mL) and meropenem (susceptible≤1μg/mL, intermediate 2μg/mL and resistant≥4μg/mL).
Incidence of errorsResults obtained from BMD testing were considered the reference standard to which results from Vitek 2® automated system, Etest® and disc diffusion were compared. Error classification was assessed using interpretive criteria for susceptibility. Very major errors (VMEs) were identified when an isolate was determined to be susceptible to a given agent by Vitek® automated system, Etest® and disc diffusion, but resistant by BMD. Major errors (MEs) were identified when an isolate was determined to be resistant to a given agent by Vitek 2® automated system, Etest® and disc diffusion, but susceptible by BMD. A result was deemed to be a minor error (MiE) when the same for a given agent was intermediate by any of the testing methods studied but was determined to be either susceptible or resistant by the other comparative method, as recommended in CLSI.
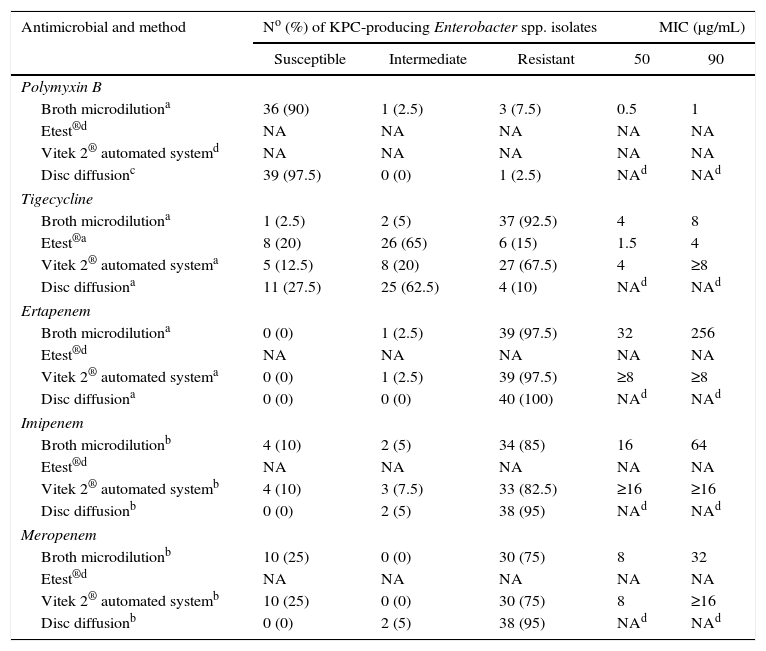
ResultsSensitivity and specificity values for the MHT were of 97.5% and 93.3%, while for boronic acid were of 100% and 63.3%, respectively. The susceptibilities and the MIC50 and MIC90 values for forty clinical isolates of Enterobacter spp. blaKPC positive were determined for the five antimicrobial agents of interest using the four assays, as shown in Table 1.
Comparison of interpretative results and MIC50 and MIC90 for antimicrobial agents and susceptibility testing methods.
| Antimicrobial and method | No (%) of KPC-producing Enterobacter spp. isolates | MIC (μg/mL) | |||
|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | 50 | 90 | |
| Polymyxin B | |||||
| Broth microdilutiona | 36 (90) | 1 (2.5) | 3 (7.5) | 0.5 | 1 |
| Etest®d | NA | NA | NA | NA | NA |
| Vitek 2® automated systemd | NA | NA | NA | NA | NA |
| Disc diffusionc | 39 (97.5) | 0 (0) | 1 (2.5) | NAd | NAd |
| Tigecycline | |||||
| Broth microdilutiona | 1 (2.5) | 2 (5) | 37 (92.5) | 4 | 8 |
| Etest®a | 8 (20) | 26 (65) | 6 (15) | 1.5 | 4 |
| Vitek 2® automated systema | 5 (12.5) | 8 (20) | 27 (67.5) | 4 | ≥8 |
| Disc diffusiona | 11 (27.5) | 25 (62.5) | 4 (10) | NAd | NAd |
| Ertapenem | |||||
| Broth microdilutiona | 0 (0) | 1 (2.5) | 39 (97.5) | 32 | 256 |
| Etest®d | NA | NA | NA | NA | NA |
| Vitek 2® automated systema | 0 (0) | 1 (2.5) | 39 (97.5) | ≥8 | ≥8 |
| Disc diffusiona | 0 (0) | 0 (0) | 40 (100) | NAd | NAd |
| Imipenem | |||||
| Broth microdilutionb | 4 (10) | 2 (5) | 34 (85) | 16 | 64 |
| Etest®d | NA | NA | NA | NA | NA |
| Vitek 2® automated systemb | 4 (10) | 3 (7.5) | 33 (82.5) | ≥16 | ≥16 |
| Disc diffusionb | 0 (0) | 2 (5) | 38 (95) | NAd | NAd |
| Meropenem | |||||
| Broth microdilutionb | 10 (25) | 0 (0) | 30 (75) | 8 | 32 |
| Etest®d | NA | NA | NA | NA | NA |
| Vitek 2® automated systemb | 10 (25) | 0 (0) | 30 (75) | 8 | ≥16 |
| Disc diffusionb | 0 (0) | 2 (5) | 38 (95) | NAd | NAd |
Note: For the interpretation of antimicrobial susceptibility testing, was used recommendation of the Agência Nacional de Vigilância Sanitária (ANVISA), in Technical Note N° 01/2010.
The disc diffusion test showed that the strains were more susceptible to tigecycline and polymyxin B than by the other tested methods. In contrast, susceptibility to imipenem and meropenem was more likely with BMD and Vitek 2® automated system, where in both methods showed the same susceptibility values for each of these two carbapenems. In the disc diffusion test was not observed any susceptible strain for these antimicrobials. The strains in study were more resistant to ertapenem than of all antimicrobials studied. In addition, the largest variation of susceptibility between the different methods was noted for tigecycline.
The MIC50 values for tigecycline, imipenem and meropenem were comparable to the BMD and Vitek 2® automated system. Through Etest®, methodology performed only for tigecycline, it was found that the MICs obtained for this test were lower than BMD and Vitek 2® automated system, but the number of susceptible bacterial isolates to the Etest® was similar to the number obtained by disc diffusion. It was also found that strains with the highest MICs (strains more resistant) referred to the ertapenem, whereas with the lowest MICs (strains more susceptible) to the polymyxin B.
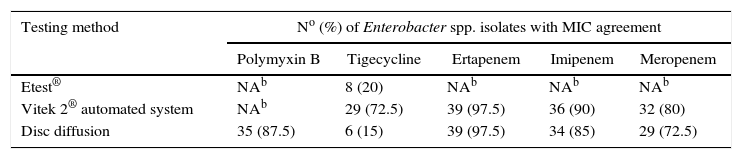
The rate of MIC agreement obtained by the comparison results of Etest®, Vitek 2® automated system and disc diffusion with the reference method, BMD, is shown in Table 2.
MIC agreement among selected testing methods and BMD.a
The highest rates of MIC agreement were obtained with Vitek 2® automated system and disc diffusion for ertapenem (both 97.5%). A relatively high rate of agreement occurred with imipenem MICs determined by Vitek 2® automated system (90%) and disc diffusion (85%), for polymyxin B by disc diffusion (87.5%), for meropenem by Vitek 2® automated system (80%) and disc diffusion (72.5%) and for tigecycline by Vitek 2® automated system (72.5%). In contrast, the lowest rate of MIC agreement according to the results of the tested methods against the reference method occurred for tigecycline by disc diffusion (15%) and Etest® (20%).
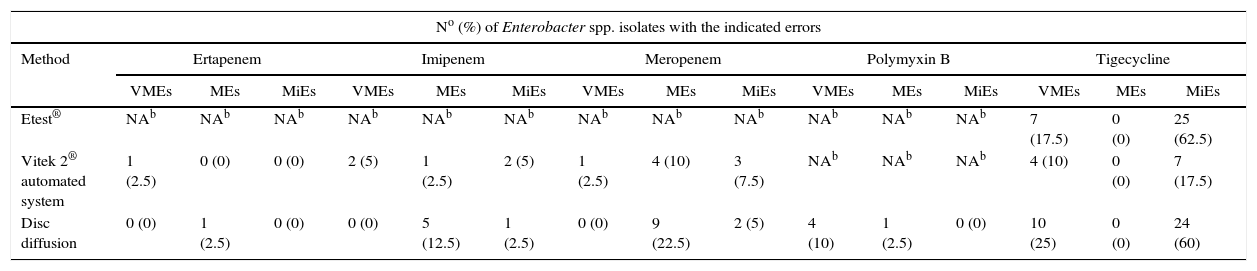
In Table 3 are showed the incidence of error rates: Very Major (VMEs), Major (MEs) and Minor (MiEs) errors to Etest®, Vitek 2® automated system and disc diffusion compared to BMD results.
Incidence of errors for selected testing methods.a
| No (%) of Enterobacter spp. isolates with the indicated errors | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Ertapenem | Imipenem | Meropenem | Polymyxin B | Tigecycline | ||||||||||
| VMEs | MEs | MiEs | VMEs | MEs | MiEs | VMEs | MEs | MiEs | VMEs | MEs | MiEs | VMEs | MEs | MiEs | |
| Etest® | NAb | NAb | NAb | NAb | NAb | NAb | NAb | NAb | NAb | NAb | NAb | NAb | 7 (17.5) | 0 (0) | 25 (62.5) |
| Vitek 2® automated system | 1 (2.5) | 0 (0) | 0 (0) | 2 (5) | 1 (2.5) | 2 (5) | 1 (2.5) | 4 (10) | 3 (7.5) | NAb | NAb | NAb | 4 (10) | 0 (0) | 7 (17.5) |
| Disc diffusion | 0 (0) | 1 (2.5) | 0 (0) | 0 (0) | 5 (12.5) | 1 (2.5) | 0 (0) | 9 (22.5) | 2 (5) | 4 (10) | 1 (2.5) | 0 (0) | 10 (25) | 0 (0) | 24 (60) |
Disc diffusion results yielded 4 VMEs (10%) and 1 ME (2.5%) for polymyxin B. None comparison method had interpretation discrepancies that yielded MEs for tigecycline, but there was a discrepancy in VMEs values for the different methods: 17.5%, 10% and 25% for Etest®, Vitek 2® automated system and disc diffusion, respectively. Regarding MiEs values, it was observed that the rates were similar to the following methods: Etest® (62.5%) and disc diffusion (60%), however Vitek 2® automated system (17.5%) showed a rate lower for this type of error. Vitek 2® automated system and disc diffusion showed similar error rates for ertapenem, but in the case of imipenem and meropenem disc diffusion generated the highest rates of MEs, while Vitek 2® automated system had higher rates of VMEs and MiEs.
DiscussionGram-negative bacteria are increasingly the cause of acquired infections. It is important that reliable tests of susceptibility for these isolates are available. The treatment of these infections is often dependent of the results of antimicrobial susceptibility testing. The MIC determination is not always necessary, but assists in the selection of antimicrobial therapy, as also guides the most appropriate dosing regimen.14
In a comparative study among isolated KPC producers, Anderson et al.15 compared the effectiveness of the three carbapenems (meropenem, imipenem and ertapenem) to identify the resistance mediated by KPC and it found that ertapenem was a more sensitive indicator for KPC mediated resistance, regardless of the method used. In agreement with these authors, in our work, ertapenem was the carbapenem that showed less variation in susceptibility among the different methods.
In study by Lat et al.16 none of 48 isolates of KPC-producing K. pneumoniae evaluated showed susceptibility to meropenem by BMD and Etest®, while in Vitek 2® automated system 27% of isolates were classified as sensitive to the same antimicrobial agent (false susceptibility). In another study conducted by Bulik et al.17 24% of the 46 isolates of KPC-producing K. pneumoniae were falsely sensitive by Vitek 2® automated system. However, we found that the susceptibility for meropenem by BMD and Vitek 2® automated system was the same (25%) in the 40 isolates of KPC-producing Enterobacter spp. This being the case was not observed this false susceptibility related with automated method.
In addition, must be careful when interpreting the results obtained in the cases that the heteroresistant has been described, according Lo-Ten-Foe et al.18 The main disadvantage of Vitek 2® automated system based on these researchers was its low sensitivity to detect subpopulations of resistant strains of Enterobacter cloacae, whereas BMD was able to detect them. In our study, the results obtained by Vitek 2® automated system showed good rates of agreement MIC when compared to the BMD as in the study by Castro et al.19
In a study conducted by Lat et al.,16 it was observed that the MICs of tigecycline measured by Etest® tended to be slightly higher than those measured by BMD, contrasting with our results. The study of these researchers also reported that the susceptibility of Vitek 2® automated system compared to the Etest® resulted in a lower rate according to BMD and a slightly higher rate of MEs. In contrast, in our study there was greater agreement of Vitek 2® automated system than Etest® in comparison with BMD and none of two methods showed MEs, but the incidence of VMEs and MiEs were higher for Etest®.
The VMEs detection rate (false-susceptible) should be less than 1.5% and the MEs (false-resistant) less than 3% of the isolates as recommended in CLSI document M23-A2.20 In our study was observed unacceptable error rates. However, an unacceptably high rate also was reported in previous studies.21,22 In the study of Lat et al.,16 for example, a VMEs rate of 67% was observed for Vitek 2® automated system in comparison the BMD, for cefepime.
VMEs (1.2%) and MEs (11.5%) rates to polymyxin B comparing the disc diffusion method with BMD were observed in the study performed by Heidjen et al.20 However, our results showed VMEs rates of 10% and 2.5% for MEs. Tan and Ng22 and, Gales et al.23 also observed false-susceptible results for isolates from Acinetobacter spp. obtained by the disc diffusion technique in comparison with BMD, the reference method. According Tan and Ng22 the accuracy of the disc diffusion test is not satisfactory for polymyxin, because they can hardly diffuse into the agar and thus the results of a diffusion test must be confirmed by a dilution method. The increasing use of this antibiotic for the treatment of multidrug-resistant Gram-negative bacteria shows that more studies are needed to clarify the effective susceptibility methods for this compound.
The choice of methodology to be used in laboratories is usually based on financial resources, labour and in the volume of tests performed. The variables considered in methodological choice include: ease of performance of the technique, costs of equipment, contractual arrangements and supplemental materials, flexibility in selection of drugs for tests, the use of automated or semi-automated devices to facilitate tests and the perception of precision of methodology.24 Several modern technologies have been proposed as being possible future alternatives to today's technologies in the clinical microbiology laboratory, such as MALDI-TOF MS (matrix-assisted laser desorption ionization-time of flight mass spectrometry), flow cytometry, isothermal microcalorimetry, magnetic bead rotation and test in microdroplets.25
In conclusion, as multidrug-resistant Gram-negative pathogens are increasingly the cause of health care-acquired infections, it is imperative that reliable antimicrobial susceptibility testing for these isolates be available. In this study, the Vitek 2® automated system was the method most similar compared to the BMD. Disc diffusion method is one of the most frequently used techniques in microbiology laboratories. However, the high rates of errors with some antimicrobial agents of this study demonstrate that this method isn’t reliable compared to the broth microdilution method, once this last one is considered the gold standard by CLSI. It suggests that laboratories consider supplemental use of reference BMD or Vitek 2® automated system, mainly for tigecycline, for KPC-producing Enterobacter spp. susceptibility testing, as disc diffusion and Etest® did not provide reliable results for this agent. Each susceptibility test has inherent advantages and limitations, therefore, as new methods becomes commercially available, it is important compare their performance with the reference method.
Conflicts of interestThe authors declare no conflicts of interest.