The inhibition of Listeria monocytogenes ATCC 7644 on fresh-cut tomato was investigated using nisin alone, and in combinations with organic salts. Nisin at a concentration of 5000UI/mL was introduced alone or in combination with an organic salt (sodium citrate or sodium acetate each at 3 and 5g/100mL each) on fresh-cut tomato previously inoculated with 108CFU/mL of L. monocytogenes ATCC 7644. Chlorine at 200ppm was used as a control. The inoculated samples were incubated at different temperatures (4, 10 and 25°C) and examined at 0, 24, 48 and 72h. The effects of the antimicrobial treatments on quality parameters of tomato (pH, soluble solids, titratable acidity and vitamin C) were also evaluated, and colour parameters were observed at the lowest storage temperature for 10 days. Both nisin and the organic salts inhibited growth of L. monocytogenes, but the combinations of two compounds were more effective. The nisin–sodium citrate (5%) combination was significantly (p≤0.05) effective, while chlorine was least effective against L. monocytogenes. The quality parameters were substantially retained, especially at 4°C, suggesting good shelf stability at a low temperature. These results substantiate the use of the cheap and eco-friendly approach to reducing this pathogen of health concern in common fresh produce.
Fresh produce is an essential component of human diet. It contains micro-nutrients, vitamins and certain phytochemicals which contribute significantly to human diet.1 Growing demands for exotic fresh produce in recent years have attracted laudable attention due to its health-promoting nature.2 It has been strongly advocated by relevant global agencies, that consumption of approximately 400g of fresh produce per day, which equals to “five servings a day” as a recommended daily intake has a prophylactic capacity to stem the tide of certain maladies in humans such as carcinoma, diabetes and cardiovascular diseases.3,4
Despite its veritable contribution to human diet, the benefits of fresh produce consumption have been greatly challenged by the emergence of disease outbreaks linked to certain pathogens, since a large proportion of fresh produce is often used raw, with little or no antimicrobial treatment. Listeria monocytogenes has been reported as one of such pathogens implicated in the outbreaks.4,5
L. monocytogenes is a tenacious micro-organism that can be found in various locations in the environment, such as soil, water and animal dung, and in a wide variety of fresh and minimally-processed produce.6 This gram-positive opportunistic bacterium is of very high public health concern as the cause of a life-threatening disease called listeriosis. The disease has the capacity to affect the entire vulnerable population. A recent multi-state incidence of this disease outbreak, linked to a commercially produced apple product, has been reported in the United States in 35 cases, of which 34 were hospitalised and eventually left seven patients dead.7 In another related development, Canada public health agencies are also on the alert as two similar cases of contamination with L. monocytogenes of same genetic background have been reported.8 As control measures, various interventions have been endorsed to reduce its contamination of fresh produce along the food chain and during processing. Over time, a chlorine wash has been commonly employed to reduce microbial loads on fresh produce prior to subsequent processing. However, development of resistant mutants, and accumulation of halogen-based residues, which could elicit a noxious risk to both food and consumers have reduced its continuous utilisation.9,10 These outcomes have incited a clamour to the use of natural antimicrobials such as nisin and organic acids or salts for reducing pathogens with a high mortality rate, such as L. monocytogenes, wherein the compounds serve as biocidal agents, while the nutritional integrity of fresh produce is substantially retained.11
Nisin is a bacteriocin produced by the lactic acid bacterium-Lactococcus lactis subsp. lactis with a preservative capacity in the food industry. It has been reported to exhibit bactericidal effects on gram-positive bacteria such as L. monocytogenes.12 This biological antimicrobial agent has been granted the “generally regarded as safe” (GRAS) status by the United States Food and Drug Administration, which has made it commercially available. Organic salts have also been regarded as veritable antimicrobial agents. They basically reduce pH of the cellular environment, disrupt transport of materials and increase permeability of the cytoplasmic membrane. Advantageously, they are inexpensive, less harmful to personnel's health and the processing environment and their GRAS status has also been endorsed for commercial use.
Nisin in synergy with salts of organic acids may be considered a promising anti-listerial barrier which could prolong the shelf stability of fresh produce such as tomato. This hurdle approach has been extensively utilised for foods of animal origin with a successful log reduction of the pathogen. For instance, nisin in combination with sodium lactate applied on cold-smoked rainbow trout reduced the L. monocytogenes population from 3.26 to 1.8logCFU/g over two weeks of storage at 8°C, with no deleterious impact on sensory quality.13 Also, Grosulescu14 reported the use of different organic salt concentrations against L. monocytogenes on some meat products, with significant reductions in bacterial counts. This natural hurdle looks promising and may suffice in the control of L. monocytogenes in fresh produce such as tomato. Therefore this research aimed to investigate the synergistic effects of nisin and salts of organic acids on the survival of L. monocytogenes in fresh-cut produce and their impacts on product quality.
Materials and methodsPreparation of fresh produceFresh tomatoes (Lycopersicon esculentum) were obtained from a local grocery supermarket (Woolworth supermarket, Durban, South Africa). Prior to experimental studies, the produce was washed with distilled water, surface disinfected with 70% ethanol and allowed to dry at room temperature before being cut into 10mm thick slices.
Bacterium and preparation of inoculumAn L. monocytogenes: serovar 1/2c (ATCC 7644) frozen stock culture in glycerol (Merck, South Africa) was allowed to thaw in a water bath at 25°C for 3min. The thawed culture was grown in 50mL of tryptone Fraser broth base (Oxoid, Ltd., England) supplemented with 5g/L of yeast extract, 1.35g/L of potassium dihydrogen phosphate and 12g/L of di-sodium hydrogen phosphate at 37±1°C for 20–24h. Bacterial cells were harvested by centrifugation (Eppendorf centrifuge 5810R) at 3000rpm for 15min at 4±1°C and re-suspended in sterile saline peptone (8.5% NaCl and 1% peptone). A 108CFU/mL suspension of the cells was then prepared using a McFarland standard solution (0.05mL of 1.175% BaCl2·2H2O and 9.95mL of 1% H2SO4).
Preparation of antimicrobials and sample inoculationThe procedures were carried out using modified method described by Samelis et al.15 Nisin (N5764. Sigma–Aldrich, St. Louis, MO, USA) was prepared at 0.5g/100mL, which is equal to 5000IU/mL was prepared and stored at the refrigeration temperature, while organic salts – sodium citrate (Associated Chemical Enterprise, Johannesburg, South Africa) and sodium acetate (Sigma–Aldrich) were each prepared at 3 and 5g/100mL. Chlorine (Sodium hypochlorite Merck Chemicals Ltd., Gauteng, South Africa) which serves as control was prepared at 200ppm. Tomato slices were placed on aluminium foil in a biohazard hood and 1mL of inoculum was spread on both sides of each slice using a sterile bent glass rod. The inoculated slices were left to stand separately at 4°C for 15min and then dipped antimicrobial solutions. The solutions were poured aseptically into pre-sterilised stainless steel pots equipped with strainers to facilitate dipping and draining for 1min each. The slices were then stored in a perforated stomacher bag at different temperatures (4, 10 and 25°C) and for different period of time (0, 24, 48 and 72h) to assess log reduction levels of the pathogen.
Bacterial analysisRecovery of the L. monocytogenes ATCC 7644 populations from the fresh-cut tomato was performed after 0, 24, 48 and 72h of storage at 4, 10, and 25°C. The tomato slices from stomacher bag were diluted with 9mL of buffered peptone water and blended in a homogeniser for 120s at a high speed. Aliquots of the mixtures were serially diluted in buffered peptone water, and 100μL of the dilutions were spread on Listeria agar base (Oxoid) with a Listeria selective supplement (Sigma–Aldrich). Colonies were counted after incubation at 37±1°C for 48h using a Doc-it colony counter (Analytik Coy Germany). All treatments were conducted in triplicate.
Quality parametersQuality parameters (pH, soluble solids, titratable acidity, vitamin C and colour values) of the fresh-cut tomato slices were evaluated before and after the experiment. The pH values were determined using a penetration-electrode pH metre (Model Basic 20 Crison Instrument, Barcelona, Spain). Soluble solids (SS) were determined using a refractometer (PR-201+, Atago, Pty., Ltd). Titratable acidity (TA) was determined by diluting 10mL of a homogenised sample with 10mL of distilled water, followed by titration of the mixture with 0.1N NaOH up to pH 8.1. Vitamin C was determined by the method of Mohammed et al.,16 using a UV spectrophotometer (Jenway 7305,Bibby Scientific, Ltd., Stone, Staffordshire, UK). The International Commission on Illumination CIE L* a* and b* values were determined at the refrigerated temperature using a colorimeter (Color Flex EZ, 0840, Hunter Lab, Reston, USA) colorimeter. All parameters were evaluated in triplicate.
Statistical analysisAll experiments were replicated thrice for each treatment. The data obtained were subjected to analysis of variance, and the means were separated using Duncan's multiple-range test (p≤0.05). Results were analysed using the SPSS software package.
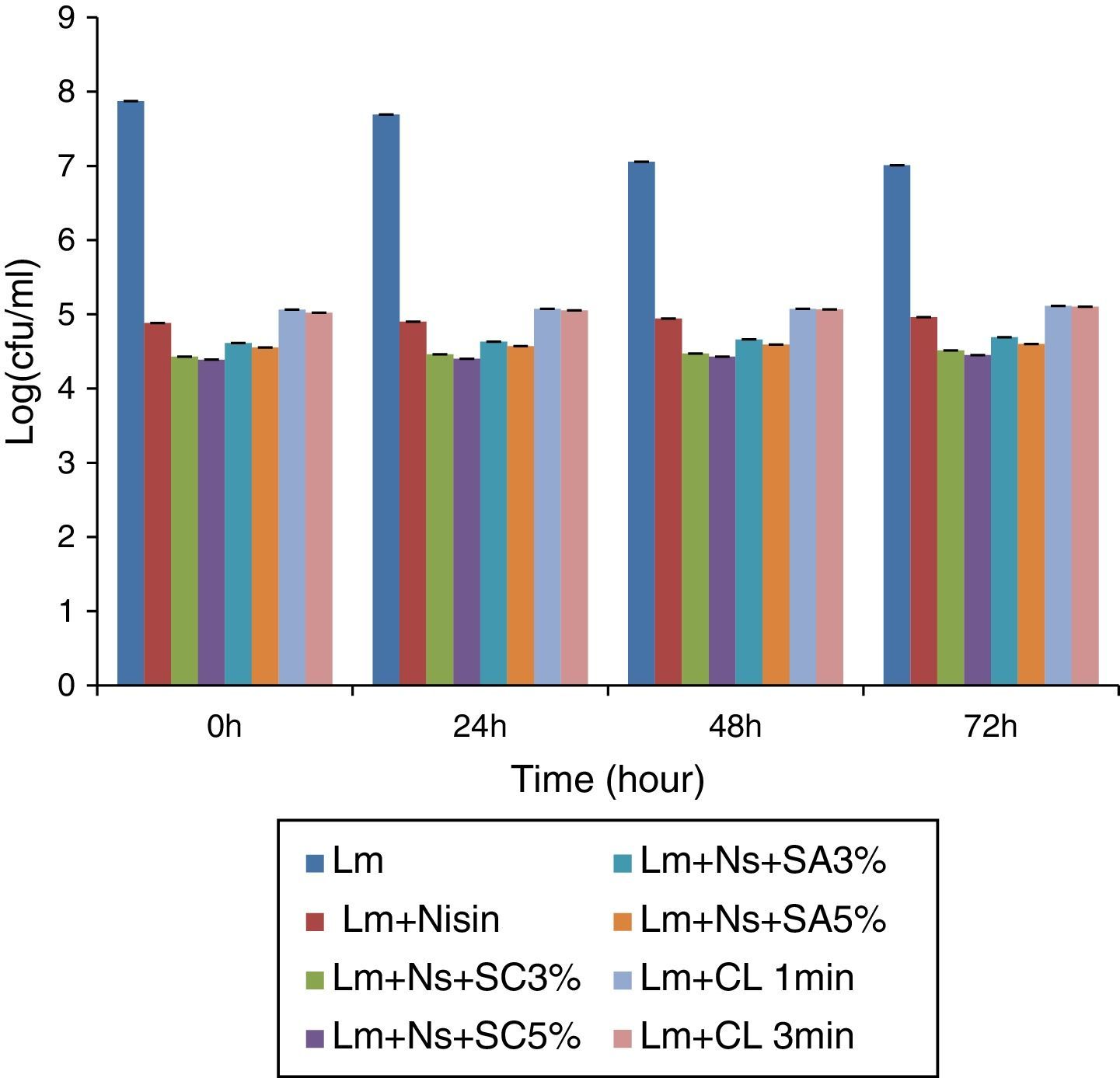
Results and discussionEffect of anti-microbial treatments on Listeria monocytogenes populationsThe results obtained from the antimicrobial dipping treatments showed a significant reduction in the survival of L. monocytogenes at 4°C (Fig. 1). The fresh-cut tomato slices dipped in chlorine for 1 and 3min showed significantly (p≤0.05) smaller log reduction values of 1.90–2.81 and 1.91–2.85CFU/mL, respectively depending on the time of incubation. The nisin-alone showed a log reduction of ∼2.05 to 2.99CFU/mL depending on the time of incubation. However, nisin in the combinations with sodium citrate, especially at 5% concentration of the latter, showed the highest log reduction of 2.56–3.48CFU/mL depending on the time of incubation compared to the other antimicrobial treatments.
Listeria monocytogenes populations (logCFU/mL) on fresh-cut tomato (n=3) stored at 4°C with nisin alone and in combinations with the organic salts as well as in the chlorine control (Lm−, L. monocytogenes; Lm+Nisin, L. monocytogenes and nisin; Lm+Ns+SC3%, L. monocytogenes, nisin and 3% sodium citrate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium citrate; Lm+Ns+SA3%, L. monocytogenes, nisin and 3% sodium acetate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium acetate; at Lm+CL− L. monocytogenes and chlorine).
The antimicrobial treatments against the L. monocytogenes population on fresh-cut tomato incubated at 10°C exhibited similar pattern to those observed at 4°C (Fig. 2). The fresh-cut tomato slices dipped in chlorine for both (1 and 3min) showed significantly (p≤0.05) lower log reduction values of ∼1.32 to 1.48 and 1.38 to 1.50CFU/mL, respectively depending on time of incubation. The treatment with nisin alone resulted in an about 1.88–1.72CFU/mL log reduction, depending on storage temperature. Meanwhile, nisin in the combination with 5% sodium citrate showed higher log reduction values of ∼ 2.27 to 2.28CFU/mL compared with nisin alone.
L. monocytogenes populations (logCFU/mL) on fresh-cut tomato (n=3) stored at 10°C with nisin alone and in combinations with the organic salts as well as in the chlorine control (Lm−, L. monocytogenes; Lm+Nisin, L. monocytogenes and nisin; Lm+Ns+SC3%, L. monocytogenes, nisin and 3% sodium citrate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium citrate; Lm+Ns+SA3%, L. monocytogenes, nisin and 3% sodium acetate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium acetate; at Lm+CL− L. monocytogenes and chlorine).
Similar trend of log reduction of L. monocytogenes was observed at elevated temperature of 25°C (Fig. 3). Chlorine treatment at both contact time significantly (p≤0.05) retained the least log values of ∼0.26 to 0.39 and 0.37 to 0.54CFU/mL respectively. Nisin alone resulted in log reduction of 1.60–1.76CFU/mL. Nisin–sodium citrate combination at 5% concentration retained the highest log reduction values of recovery mean values of 2.44–2.56logCFU/mL.
L. monocytogenes populations (logCFU/mL) on fresh-cut tomato (n=3) stored at 25°C with nisin alone and in combinations with the organic salts as well as in the chlorine control (Lm− L. monocytogenes; Lm+Nisin, L. monocytogenes and nisin; Lm+Ns+SC3%, L. monocytogenes, nisin and 3% sodium citrate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium citrate; Lm+Ns+SA3%, L. monocytogenes, nisin and 3% sodium acetate; Lm+Ns+SC5%, L. monocytogenes, nisin and 5% sodium acetate; at Lm+CL− L. monocytogenes and chlorine).
The comparative ineffectiveness of the chlorine control as demonstrated by lower the log reduction values compared to the other antimicrobial treatments can be explained by the development of chlorine resistance by the pathogen. For example, L. monocytogenes is able to form biofilms, which are microbial aggregates in an exo-polysaccharide matrix. Bacterial cells in biofilms resist common sanitisers such as chlorine, causing an immense concern in the fresh produce industry.17 However, Ijabadeniyi,18 reported the inactivation of pathogen biofilms by chlorine sanitisers. Nisin acts as a growth inhibitor via pore formation in the cytoplasmic membrane of gram-positive bacteria such as L. monocytogenes, which enhances the bacterial inactivation on fresh-cut produce. Similar findings by Hoelzer19 indicated 1.33 and 2.64log10CFU reductions of the pathogen by nisin on broccoli and cabbage, respectively. The higher rates of reductions of the pathogen on fresh-cut tomato by nisin in combinations with the salts of organic acids on the fresh-cut tomato can be associated primarily with the reduction of pH and disruption of cellular materials. The relative efficacy of sodium citrate can be linked to its stronger biocidal and metal-chelating capabilities compared to monocarboxylic salts such as acetates.20,21 Increased in storage temperatures tends to favour L. monocytogenes survival on fresh produce as reflected by the log reduction rates.10
Effect of anti-microbial treatment on quality parameter of the fresh-cut tomatoThe results of the quality parameter assays (pH, TA, SS and vitamin C) for fresh-cut tomato are represented in Table 1. Nisin in the combination with sodium citrate at a 3% concentration gave the lowest pH value (p≤0.05) at 4 and 10°C, while nisin–sodium acetate at a 5% concentration showed the highest pH values at both temperatures. At 25°C, however, nisin–sodium acetate (5%) showed the lowest pH value, while that in control was significantly (p≤0.05) higher compared with the other anti-microbial treatments. Anti-microbial treatments showed higher (p≤0.05) TA values than those in control samples at 4°C and lower values than those in control sample at 10°C except nisin–sodium acetate (5%). However, at 25°C, all the antimicrobial treatments were lower than that of the control sample. The amount of soluble solids in the nisin–sodium acetate at (5%) were significantly (p≤0.05) higher than those in the control and other antimicrobial treatments at 4 and 10°C, however, a reverse trend was observed at 25°C. The vitamin C value in the nisin–sodium acetate (5%) treatment of fresh-cut produce was higher than the values in the control and other anti microbial treatments at all temperature regimes, while the treatment with nisin alone showed the lowest vitamin C value. Nisin in the combination with the organic salts resulted in further pH lowering, which agrees with their basic antimicrobial mode of action. Similar findings reported by22 corroborate the above conclusion. Lower pH represents a good keeping quality in fresh produce such as tomato since most spoilage bacteria find it difficult to thrive in such a medium. The decrease in TA with the increasing storage temperature, increases can be linked to a loss of citric acid.23 TA in fresh produce represents the index of maturity. Soluble solids also decreased with increasing storage temperature, which correlates with the sugar level, and dry matter content in tomato and is also inversely proportional to the produce size. Beckles24 discussed in his review previous studies showing that an increased temperature enhance the rates rate of evaporation and transpiration which ultimately affects sugar levels in tomato. The increase in the vitamin C content could be due to the organic compounds of these antimicrobials acting as precursors. Ayala-Zavala25 reported an increase in the ascorbic acid content of fresh – cut tomatoes induced by some natural antimicrobials. However, the vitamin C values decreased as the storage temperature increased which is quite expected.26,27
Some quality parameters: pH, titratable acidity, soluble solid in obrix, and Vitamin C of fresh-cut tomato slices dipped in nisin alone and in combination with other antimicrobials.
| Treatment | Temperature | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4°C | 10°C | 25°C | ||||||||||
| pH | TA (g citric acid/10g) | SS (° Brix) | Vit C (mg/10g) | pH | TA (g citric acid/10g) | SS (° Brix) | Vit C (mg/10g) | pH | TA (g citric acid/10g) | SS (° Brix) | Vit C (mg/10g) | |
| Control | 4.40c±0.01 | 6.00a±0.20 | 6.10b±0.20 | 1.18a±0.02 | 4.42d±0.02 | 6.00c±0.20 | 5.90d±0.20 | 0.88a±0.02 | 4.43e±0.02 | 5.70c±0.20 | 5.40c±0.20 | 0.43a±0.01 |
| Nisin | 3.67b±0.02 | 6.20ab±0.10 | 5.80ab±0.20 | 1.17a±0.01 | 3.79c±0.02 | 5.70bc±0.20 | 5.40c±0.20 | 0.83a±0.02 | 3.82d±0.01 | 5.00b±0.50 | 4.90c±0.20 | 0.45a±0.00 |
| Ns+SC3% | 3.46a±0.02 | 6.30ab±0.20 | 5.80ab±0.20 | 1.19a±0.02 | 3.51a±0.02 | 5.60b±0.20 | 4.70ab±0.20 | 0.93a±0.02 | 3.70c±0.02 | 4.70ab±0.20 | 4.30ab±0.10 | 0.49b±0.02 |
| Ns+SC5% | 3.47ab±0.01 | 6.40b±0.10 | 5.60a±0.20 | 1.23b±0.02 | 3.62b ±0.01 | 5.20a±0.20 | 4.93b±0.15 | 0.97a±0.01 | 3.58a±0.02 | 4.83b±0.25 | 4.37ab±0.06 | 0.50b±0.02 |
| Ns+SA3% | 3.48a±0.04 | 6.20ab±0.10 | 5.50a±0.20 | 1.24b±0.01 | 3.56ab±0.01 | 5.70bc±0.10 | 4.60a±0.20 | 0.96a±0.02 | 3.64b±0.01 | 4.30a±0.10 | 4.50b±0.10 | 0.63c±0.02 |
| Ns+SA5% | 3.51d±0.01 | 7.10c±0.20 | 6.60c±0.20 | 1.30c±0.01 | 6.30e±0.10 | 6.30d±0.10 | 6.23e±0.06 | 1.04b±0.20 | 3.67bc±0.02 | 4.70ab±0.20 | 4.20a±0.10 | 0.70d±0.02 |
Values are means±standard deviations of three replicates. Mean values a, b, c, d and e in the same column with same superscript are not significantly different (p≤0.05).
TA, titratable acidity in g citric acid/10g; SS, soluble solid inobrix; Vit C, vitamin C; SC, sodium citrate; SA, sodium acetate.
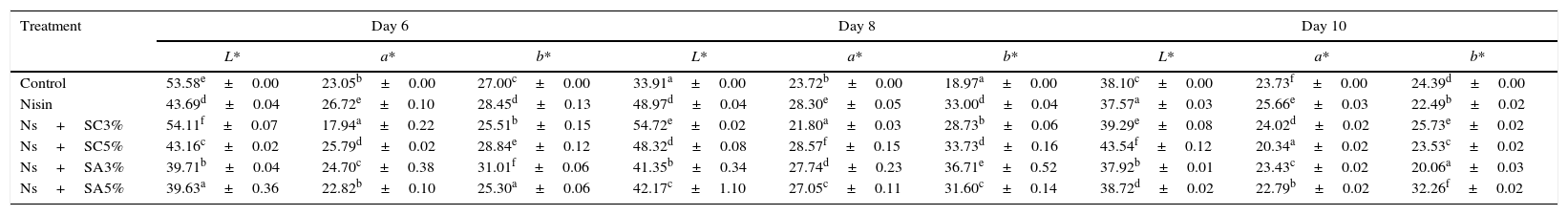
The results obtained for the colour parameters L* (brightness), a* (red), and b* (yellow) of the fresh-cut tomato (Tables 2a and 2b) showed that nisin alone increased the values of redness (p≤0.05) from day 2 to day 10 compared with the control. The degree of redness increased with nisin–sodium citrate (3%) compared to the control from day 0 to day 8. Nisin–sodium citrate (5%) increased the redness values throughout the 10 days, except day 0, compared to the control. Nisin–sodium acetate (3%) did not increase redness on most storage days, except day 8, while the same combination at a 5% concentration of sodium acetate did, except on day 10. The increase in the degree of redness can be linked to the increase in ripening via ethylene biosynthesis and chlorophyll degradation. A previous work by Yanuriati28 showed an inhibitory effect on tomato colour of some natural antimicrobials. Redness of tomato is an important quality attribute for consumer appeal and acceptability.
Colour parameters of fresh-cut dipped in nisin and in combination with other antimicrobials at 4°C for ten days.
| Treatment | Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | |||||||
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| Control | 52.20f±0.02 | 23.72d±0.00 | 26.71c±0.02 | 54.02e±0.01 | 23.37c±0.01 | 27.36e±0.02 | 52.30f±0.02 | 23.04b±0.02 | 26.90e±0.20 |
| Nisin | 41.41c±0.02 | 22.87c±0.02 | 22.43b±0.02 | 42.08c±0.02 | 25.54c±0.02 | 23.78c±0.00 | 39.24c±0.02 | 24.97c±0.02 | 23.44b±0.01 |
| Ns+SC3% | 44.85d±0.01 | 26.43e±0.01 | 26.74f±0.01 | 44.86d±0.01 | 27.87e±0.02 | 28.77f±0.02 | 43.36e±0.02 | 27.42e±0.01 | 29.08f±0.02 |
| Ns+SC5% | 37.03a±0.02 | 20.46a±0.02 | 17.99a±0.02 | 38.59a±0.00 | 30.21f±0.00 | 22.46a±0.00 | 35.72a±0.02 | 30.63f±0.02 | 23.85c±0.02 |
| Ns+SA3% | 51.13e±0.02 | 21.44b±0.02 | 24.84d±0.00 | 45.29e±0.02 | 22.65a±0.02 | 24.97d±0.02 | 41.34d±0.02 | 22.72a±0.02 | 25.11d±0.00 |
| Ns+SA5% | 40.42b±0.01 | 28.32d±0.02 | 23.41c±0.00 | 43.67c±0.01 | 26.88d±0.02 | 22.91a±0.01 | 38.23b±0.02 | 26.43d±0.02 | 22.58a±0.02 |
Values are means±standard deviations of three replicates.
Mean values a, b, c, d, e and f in the same column with the same superscript are not significantly different (p≤0.05).
L*, lightness; a*, redness; b*, greenness; Ns, nisin; SC, sodium citrate; SA, sodium acetate.
Colour parameters of tomato slices dipped in nisin, in combination with other antimicrobials at 4°C for ten days.
| Treatment | Day 6 | Day 8 | Day 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| Control | 53.58e±0.00 | 23.05b±0.00 | 27.00c±0.00 | 33.91a±0.00 | 23.72b±0.00 | 18.97a±0.00 | 38.10c±0.00 | 23.73f±0.00 | 24.39d±0.00 |
| Nisin | 43.69d±0.04 | 26.72e±0.10 | 28.45d±0.13 | 48.97d±0.04 | 28.30e±0.05 | 33.00d±0.04 | 37.57a±0.03 | 25.66e±0.03 | 22.49b±0.02 |
| Ns+SC3% | 54.11f±0.07 | 17.94a±0.22 | 25.51b±0.15 | 54.72e±0.02 | 21.80a±0.03 | 28.73b±0.06 | 39.29e±0.08 | 24.02d±0.02 | 25.73e±0.02 |
| Ns+SC5% | 43.16c±0.02 | 25.79d±0.02 | 28.84e±0.12 | 48.32d±0.08 | 28.57f±0.15 | 33.73d±0.16 | 43.54f±0.12 | 20.34a±0.02 | 23.53c±0.02 |
| Ns+SA3% | 39.71b±0.04 | 24.70c±0.38 | 31.01f±0.06 | 41.35b±0.34 | 27.74d±0.23 | 36.71e±0.52 | 37.92b±0.01 | 23.43c±0.02 | 20.06a±0.03 |
| Ns+SA5% | 39.63a±0.36 | 22.82b±0.10 | 25.30a±0.06 | 42.17c±1.10 | 27.05c±0.11 | 31.60c±0.14 | 38.72d±0.02 | 22.79b±0.02 | 32.26f±0.02 |
Values are means±standard deviations of three replicates.
Mean values a, b, c, d, e and f in the same column with the same superscript are not significantly different (p≤0.05).
Ns, nisin; SC, sodium citrate; SA, sodium acetate.
The use of natural antimicrobials such as nisin and salts of organic acids remains an economical means of addressing microbial contamination, especially with pathogens of public health concern. Nisin and sodium citrate at a 5% concentration can be adopted to significantly reduce L. monocytogenes contamination in fresh produce without compromising its quality. Further research to evaluate this synergy using a wider range number of fresh produce and challenging pathogens will be a welcome development.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank specially to Centre for Research and Development of Durban University of Technology for funding this research work, and to the staff of the Food Technology Research Group for their technical support.