The aim of the present study was to investigate the effect of Lactobacillus plantarum adhesion to the surface of olives during storage through studying the interaction between the surfaces of the olives and L. plantarum. The results showed that the total number of adherent L. plantarum increased exponentially from 1.2×106 to 1.3×108cfu/g. Images obtained using environmental scanning electron microscopy (ESEM) after 4 days of storage revealed that the olive surface was covered with a uniform and compact biofilm constituted of L. plantarum and yeast. Physicochemical analysis of surface of L. plantarum revealed that it was hydrophilic (Giwi>0mJ/m2). The surface of the olives also appeared to be hydrophilic (Giwi=3.28mJ/m2). The electron-donor characteristics of the surfaces of L. plantarum and olive were γ−=53.1mJ/m2 and γ−=28.1mJ/m2, respectively. The formation of a protective biofilm of L. plantarum increased the hydrophilicity (from 3.28 to 46.14mJ/m2) and the electron-donor capacity (from 28.1 to 67.2mJ/m2) of the olive surface by 1 day of storage. Analysis of the impact of the biofilm that formed on the surface of the olives during storage showed a reduction in the content of undesirable planktonic microorganisms, such as fungi, which could have occurred due to competition for nutrients and oxygen or modifications in the physicochemical properties of the olives. Thus, coating the surface of olives with a natural material, such as L. plantarum, may be a first step in developing strategies to prevent their microbial colonization.
The adhesion of microorganisms to a surface is a complicated process that is affected by the physicochemical interactions of the microorganismal and contact surfaces. These interactions can be categorized into the three following classes: Lifshitz-Van der Waals interactions, electrostatic interactions,1 and polar or Lewis acid-base interactions (i.e., electron-donor and electron-acceptor).2,3 Studies have shown that parameters such as hydrophobicity,2,4 surface charge,2,5 and electron donor–electron acceptor (acid–base) properties significantly affect microbial adhesion.6 It is important to note that environmental conditions, such as the pH, ionic forces, temperature and exposure time, and the cellular structures and cell density strongly affect the adhesion of microorganisms.7 However, few studies have examined the changes in physicochemical proprieties that occur after bacterial adhesion.8 Furthermore, it is clear that modification of each parameter involved in the adhesion process could increase or decrease the surface adhesion potential. Thus, better understanding of these features is extremely important for the development of effective adhesion-control strategies that will ultimately prevent biofilm formation. The formation of a protective biofilm on the surfaces of devices used in the food industry or medicine could be beneficial because its presence may effectively modify the physicochemical properties of the substrates and reduce the level of adhesion of undesirable planktonic microorganisms.9 Biofilms formed by lactic acid bacteria have received considerable attention due to their potential use in establishing a competitive microbiota,10 and changes in the physicochemical properties of the cell surface enhanced the adhesion of protective biofilms. Furthermore, the growth of undesirable organisms can be inhibited by nutrient competition or the production of antagonistic compounds, such as acids, bacteriocins, or biosurfactants, which consist of proteins, polysaccharides and phosphates, due to their anti-adhesive effect.11,12 LAB have long been considered food-grade bacteria that play major roles in the biopreservation of fermented foods derived from raw agricultural materials, such as milk, meat, vegetables, and cereals, due to their ability to produce a range of antimicrobial compounds. These compounds include organic acids, bacteriocins, fatty acids, hydrogen peroxide, and diacetyl. Lactobacillus plantarum is the most frequently encountered LAB, found in fermented plant materials in which phenolic compounds are abundant and used for food preservation because it does not have detrimental effects on the sensory properties of processed foods, making this species a suitable candidate for the creation of protective biofilms. A previous investigation conducted in our laboratory showed that applying L. plantarum during the olive-oil production process preserved the phenolic compounds, which formed volatile phenols that increased the level of antioxidant activity. This phenomenon was possibly due to the development of a biofilm by the L. plantarum cells, which utilized the oxygen the solution, which is responsible for the auto-oxidation of phenolic compounds.13 Nevertheless, to our knowledge, there are limited or no data concerning characterization of the formation of a L. plantarum biofilm with preservative properties on vegetable surfaces. Therefore, the aims of this work were to characterize the surfaces of olives and L. plantarum, to determine the physicochemical properties of the surface with adherent L. plantarum and to study the effect of bioadhesion on the growth of undesirable planktonic microorganisms during olive storage.
Materials and methodsL. plantarum strain, media and culture conditionsThe L. plantarum strain used in the study was isolated from traditionally fermented green olives of the Tunisian variety “Meski”, grown in the laboratory13 and identified using an API 50CHL kit (bioMérieux Inc., Marcy l’Etoile, France) and 16S rDNA sequencing analysis.
L. plantarum cells cultivated in MRS (de Man, Rogosa, and Sharpe) broth (Biokar, Allonne, France) for 18h at 37°C were harvested by centrifugation for 15min at 6000×g. The pellets were then quickly washed using deionized water, and the cells were resuspended in a sterile saline solution (0.9%). These bacteria were used to inoculate the batch of olives used in this study.
Characterization of the surface of the olivesThe “Chetoui” variety of olive used in this study is large, weighing 2g/olive. The olives were cut horizontally into four square pieces (1cm2) to obtain a planar surface (1-mm thick) and stable, so-called “plateau”, contact angles. The contact angles (CAM) were measured at 0 and 1 day of biofilm formation.
Characterization of the surface of L. plantarumMicrobial cells suspended in a sterile KNO3 solution were deposited on cellulose-acetate membrane filter (0.45μm) by filtration using negative pressure. Filters containing the bacteria (108 cell/mm2) were allowed to air dry for 30–60min to obtain stable, so-called “plateau”, contact angles. Three independently grown cultures were used, from each of which three filters were prepared and evaluated.14
L. plantarum adhesion assayThe olive samples were divided into two groups, one of which was inoculated with L. plantarum (2×106cfu/g) and the other of which was not inoculated and was used as the control. The olives were divided into the following portions: (1) non-inoculated olive: non-inoculated olive samples stored at 25°C for 16 days and (2) inoculated olive: inoculated olive samples stored at 25°C for 16 days in contact with the inoculum.
Assessment of bacterial adhesionThe number of bacterial cells that had adhered to the olive samples was determined at 0, 4, 8, 12, and 16 days of storage. For this assay, 10g of olives was taken from each group (inoculated and non-inoculated). The olive samples were transferred to 90ml of dilution medium, which consisted of a 1% aqueous peptone solution, to remove the planktonic cells, after which the adherent cells were removed using standardized sterilized swabs. The swabs were transferred to test tubes containing 10ml of the same dilution medium and were stirred vigorously using vortexing for one minute. At each time point, two replicate samples were analyzed. For the total microorganism counts, aliquots of each dilution were seeded on Plate Count agar (PCA, Biokar, Allonne, France) and the plates were incubated at 30°C for 72h. For the lactic acid bacterial (LAB) counts, the samples were plated on MRS agar (Biokar) and were incubated at 37°C for 48h under anaerobic conditions. The viable yeast and fungal cells were enumerated on Sabouraud agar supplemented with chloramphenicol (500μg/ml) (Biokar) after incubation for 48 to 72hours at 30°C. For the total coliform counts, the samples were plated on deoxycholate agar (Biokar) and incubated at 37°C for 48h.15
The morphological, biochemical, and physiological characteristics and carbohydrate-fermentation profile of the LAB were determined using an API 50CHL instrument (API System, bioMérieux). The yeast cells were characterized using carbohydrate-assimilation analysis (API C Aux, BioMérieux). The characterization was conducted after 4 days of biofilm formation.
Environmental scanning electron microscopy analysisAt 0, 1 and 4 days of contact with the inoculated L. plantarum, the olives were gently rinsed three times using sterile distilled water9,16 to remove the non-adherent cells and were immediately observed using an Environmental Scanning Electron Microscopy (ESEM Quanta 200, FEI, Hillsboro, OR, USA) equipped with a tungsten filament (FEI). The signal was collected using a gaseous secondary electron detector (GSED).
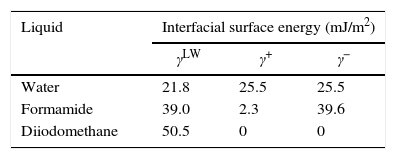
Contact angle measurements and determination of surface tension componentsThe surface energy was inferred from the contact angle, which was measured using the sessile drop technique.17 Three to six contact angle measurements using the three following pure liquids with known energy characteristics (Table 1): distilled water, formamide (purity>99%; Sigma-Aldrich, St. Louis, MO, USA) and diiodomethane (purity>99%; Sigma-Aldrich) were taken on each substratum surface. The contact angle of one 2.0-μl drop of a pure liquid on the surface of the olive samples was measured taken each second for 30s.
Calculation of the hydrophobicityThe cell surface hydrophobicity was evaluated through contact angle measurements and using the approach of Van Oss et al.18 In this approach, the degree of hydrophobicity of a given material (i) is expressed as the free energy of interaction between two entities of that material when immersed in water (w) as a ΔGiwi value. If the interaction between the two entities is stronger than the interaction of each entity with water, the material is considered hydrophobic (Giwi<0) and conversely, ΔGiwi>0 for a hydrophilic material. The ΔGiwi value is calculated using the values of the surface energy components of the interacting entities, according to the following formula:
Calculation of the total free energy of interactionThe values for the Lifshitz-van der Waals (γLW), electron donor (γ−) (or Lewis-base), electron acceptor (or Lewis acid) (γ+) components of the surface energy of bacteria were estimated using the approach proposed by Van Oss.19θD was the contact angle obtained using diiodomethane and s represented the surface area.
The interfacial tension was equal to the sum of the two components (γsLW and γsAB), as follows:
The Lewis acid–base surface energy component was defined as follows:
The Lifshitz-van der Waals surface energy component was defined as follows:
It was possible to estimate the hydrophilicity or hydrophobicity of the surfaces based on the interfacial tension component values. The surfaces were hydrophilic when γLW≤45mJ/m2 and were hydrophobic when γLW≥45mJ/m2.8
The total free energy of interaction among the molecules of the surface(s) immersed in water (w) was determined as the sum of the apolar and polar free energies of interaction, ΔGswsLW and ΔGswsAB, respectively,2 as follows
where ΔGswsLW=−2(γsLW−γwLW°)1/2 and ΔGswsAB=−4((γs+γs−°)1/2+(γw+γw−°)1/2−(γs+γw−°)1/2−(γw+γs−°)1/2)When ΔGswsTOT>0, the surface was considered hydrophilic. Conversely, when ΔGswsTOT<0, the surface was considered hydrophobic.
From the values of the components of the interfacial surface energy, it was possible to determine the total free energy of adhesion (ΔGadhesion) between two surfaces (microbial cells (b) and olive surfaces (s)):
When free energy is related to the interfacial surface energy, then ΔGadhesion can be obtained using the following equation:
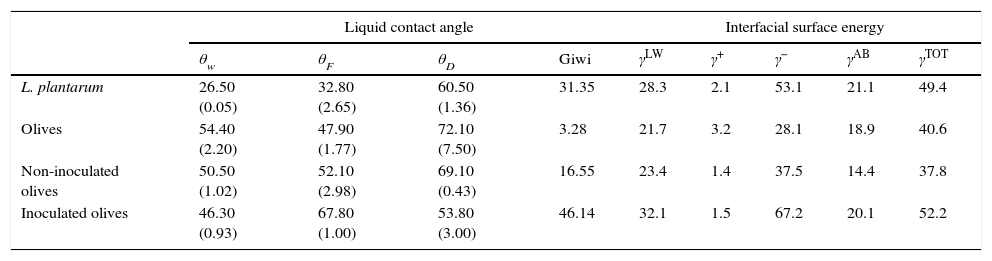
where γbs is the interfacial tension between the bacterial surface and the adhesion surface, γbl is the interfacial tension between the bacterial surface and the liquid, and γsl is the interfacial surface energy between the adhesion surface and the liquid. The ΔGadhesion values allow evaluation of the thermodynamics of the adhesion process; if ΔGadhesion<0, the process is favorable, but if ΔGadhesion>0, the process is unfavorable.8Results and discussionPhysicochemical characterization of the olive surfaceThe water-contact angle can be used as a qualitative indicator of the hydrophobicity of the surface of a material, with higher values indicating a more hydrophobic surface [θw (°)>65]. The water-contact angle of the olive surface was found to be less than 65° [θw (°)=54.4] (Table 2), indicating that it is a hydrophilic surface. The free energy (ΔGiwi=3.28mJ/m2>0) (Table 2) and the Lifshitz-Van der Waals compound (γLW=21.7mJ/m2≤45mJ/m2) values (Table 2) confirmed the hydrophilicity of the olive surface.
Contact angles (°), free energy of interaction [Giwi(mJ/m2)] values and values of the interfacial surface energy components [γLW, γ−, γ+, γAB and γTOT (mJ/m2)]of L. plantarum and olives, and of non-inoculated and L. plantarum-inoculated olives after 1 day of storage.
| Liquid contact angle | Interfacial surface energy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| θw | θF | θD | Giwi | γLW | γ+ | γ− | γAB | γTOT | |
| L. plantarum | 26.50 (0.05) | 32.80 (2.65) | 60.50 (1.36) | 31.35 | 28.3 | 2.1 | 53.1 | 21.1 | 49.4 |
| Olives | 54.40 (2.20) | 47.90 (1.77) | 72.10 (7.50) | 3.28 | 21.7 | 3.2 | 28.1 | 18.9 | 40.6 |
| Non-inoculated olives | 50.50 (1.02) | 52.10 (2.98) | 69.10 (0.43) | 16.55 | 23.4 | 1.4 | 37.5 | 14.4 | 37.8 |
| Inoculated olives | 46.30 (0.93) | 67.80 (1.00) | 53.80 (3.00) | 46.14 | 32.1 | 1.5 | 67.2 | 20.1 | 52.2 |
Note: The standard deviation values are presented in parentheses.
Moreover, the component γ− can also be a semi-quantitative indicator of hydrophobicity; γ− values of ≤25.5mJ/m2 indicate a hydrophobic surface regardless of the value of the apolar component.8γ− values of between 25mJ/m2 and 35mJ/m2 suggest that the hydrophobicity depends upon the apolar component value. Furthermore, the olive surface was considered hydrophilic because its γ− value was ≤25.5mJ/m2.
Additionally, the olive surface was found to be an electron donor because the γ− value (28.1mJ/m2) was greater than the γ+ value (3.2mJ/m2) (Table 2).
Physicochemical characterization of the L. plantarum surfaceThe surface of L. plantarum was considered to be hydrophilic because its θw value [θw (°)=26.5] was less than 65°. Additionally, the L. plantarum surface had positive free energy value of ΔGiwi=31.35mJ/m2 and therefore, could be considered to be hydrophilic (Table 2). The components of interfacial tension (γLW=28.3mJ/m2 and γ−=53.1mJ/m2) of the surface of L. plantarum confirmed the hydrophilic characteristics of this strain. The physicochemical properties of the surfaces of lactic acid bacteria have been shown to depend on their molecular compositions.20–22 The LAB surface was shown to be composed mainly of proteins and polysaccharides and to have a hydrophilic nature.
The surface-energy component values obtained showed that the L. plantarum surface was predominantly an electron donor, with a higher value for the electron-donor parameter (γ−=53.1mJ/m2) than for the electron-acceptor parameter (γ+=2.1mJ/m2) (Table 2). In fact, it was observed that all cell surfaces were predominantly electron donors (higher values of γ−), with low electron acceptor parameters (γ+). This polar characteristic might be due to the presence of residual water of hydration or of polar groups.23
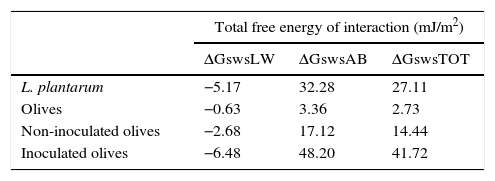
Based on the quantitative data, the surfaces of the L. plantarum strain and the olive samples were both hydrophilic because the total free energy of interaction ΔGswsTOT values of both surfaces were >0 (Table 3).
Values of the apolar (ΔGswsLW) and polar (ΔGswsAB) components of the total free energy of interaction (ΔGswsTOT) of L. plantarum and olives, and of non-inoculated and L. plantarum-inoculated olives after 1 day of storage.
| Total free energy of interaction (mJ/m2) | |||
|---|---|---|---|
| ΔGswsLW | ΔGswsAB | ΔGswsTOT | |
| L. plantarum | −5.17 | 32.28 | 27.11 |
| Olives | −0.63 | 3.36 | 2.73 |
| Non-inoculated olives | −2.68 | 17.12 | 14.44 |
| Inoculated olives | −6.48 | 48.20 | 41.72 |
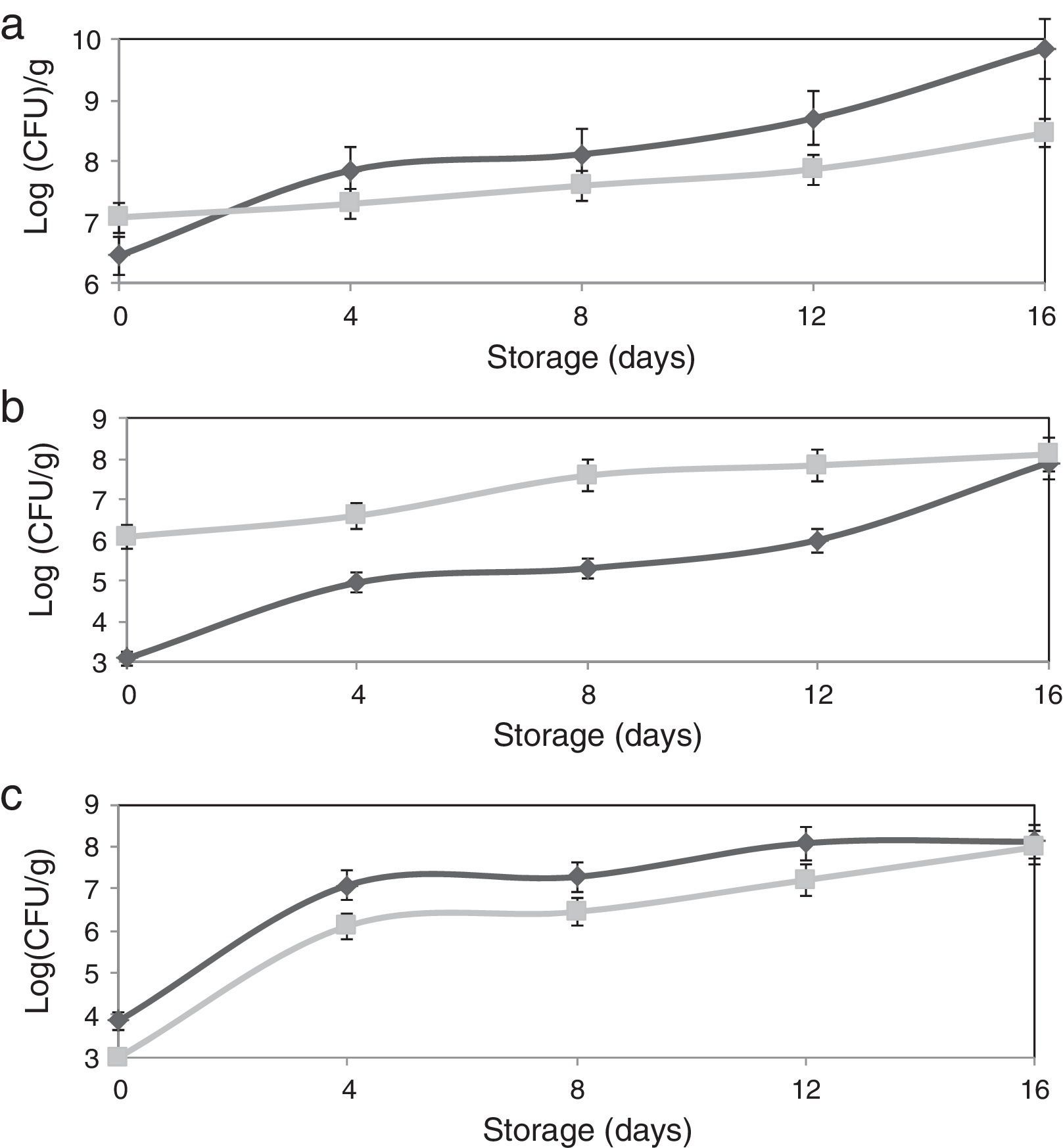
The adhesion of L. plantarum to the olive surface was investigated by counting and identifying the viable cells and ESEM microscopic examination. The number of L. plantarum cells that had adhered to the surface of the olive sample after different contact periods increased exponentially to more 1.3×108cfu/g (Fig. 1b). In addition, after inoculation, the concentration of total microorganisms increased to 1.2×107cfu/g, whereas the non-inoculated olive samples contained 2.8×106cfu/g (Fig. 1a). The yeast and fungi levels of olive samples inoculated with L. plantarum decreased over the test period. The increase in the number of total microorganisms and decrease in the number of yeast and fungi confirmed the adhesion of L. plantarum to the olive surface. The natural microbiota of olives consists of Gram-negative bacteria, particularly coliform species, LAB, and yeasts and molds.24 Evaluation of the viable cells in the samples showed the absence of coliform species when the olives were inoculated with L. plantarum. The ESEM images demonstrated adherent L. plantarum on the olive surface at 1 day of storage (Fig. 2b). Moreover, ESEM evaluation conducted at 4 days of storage showed that the olive surface was covered with a uniform and compact biofilm (Fig. 2c). Microbiological identification of the components of this biofilm demonstrated that it was composed of two types of organisms, bacilli and cocci, which corresponded, respectively, to L. plantarum and yeast [the latter including Candida famata (32%), Candida ciferrii (10%), Rhodotorula mucilaginosa (21%), Crytococcus laurentii (16%) and Pichiaguillier mondii (16%)]. The adhesion of L. plantarum to the samples may be due to the correlation found between the hydrophilicity of the surface of the bacterial cells and their ability to adhere to the hydrophilic olive surface. In fact, according to the physicochemical approach, hydrophobic cells tend to attach to a hydrophobic substrate and hydrophilic cells tend to attach to a hydrophilic substrate. Moreover, during the adhesion process, the cell-surface hydrophobicity has been described as one of the most important properties governing adhesion.25,26 The physicochemical approach appears to be insufficient to explain our results because many hydrophilic cells adhere better to hydrophobic than to hydrophilic substrata. Moreover, these conflicting results were supported by the results of several studies6,8,27 in which the adhesion of B. cereus to stainless steel, wood, and silicone surfaces was observed, despite the difference in the hydrophobicity of the cells and substrata.
Furthermore, various studies have demonstrated that bioadhesion depends mainly on a combination of surface physicochemical properties (such as the Lewis acid-base force, the capacity to undergo favorable van der Waals interactions, and the global surface charge) of both the cells and the solid substratum.17,28 However, it is important to note that cellular structures, such as flagella, fimbriae, and pili, play important roles in the adhesion process, as do extracellular polysaccharides produced by the cells. In addition, environmental conditions, such as the pH, ionic forces, temperature, exposure period, and the density of the microorganisms strongly affect the adhesion process.6
In our study, the apolar free energy of interaction of L. plantarum was negative (ΔGswsLW=−5.17 mJ/m2) (Table 3), indicating that the Lifshitz van der Waals force was predominantly attractive. In contrast, the polar free energy of interaction ΔGswsAB appeared to be positive, indicating a repulsive force. Bernardes et al.8 showed that only surfaces that were considered hydrophilic had ΔGswsAB values of >0, demonstrating that hydrophobicity is predominantly determined by polar forces of attraction. The component ΔGswsAB represents the hydration degree of surfaces, which means that high ΔGswsAB values are correlated with a low surface hydrophobicity.
According to the thermodynamic theory of adhesion, if the attractive forces are stronger than the repulsive forces, short-range interactions play an important role in bacterial adhesion to surfaces. Polar and apolar interactions both contribute to these forces. Bacterial adhesion is favorable if the interactions lead to a decrease in the free energy of adhesion (ΔGadhesion<0).7 In our study, the free energy of adhesion between the olive and L. plantarum surfaces was positive (ΔGadhesion>0) (Table 4), which was thermodynamically unfavorable. This finding was similar to those of Teixeira et al.29 and Bernardes et al.,8 who observed that the free energy of adhesion of strains that adhered to surfaces was positive.
Physicochemical characterization of surface to which L. plantarum adheredThe surface properties of non-inoculated and inoculated olives were evaluated after 1 day of storage to determine whether the adhesion of L. plantarum modified the physicochemical properties of the olive surfaces and to characterize the modified surface to confirm if the modifications exclusively involved L. plantarum or other microbial species that adhered to the olive surface.
In our study, despite the non-uniformity of the biofilm at 1 day of storage, as revealed using ESEM, contact angle measurements were taken. The modified surface was not uniform, but had surface characteristics specific to the biofilm tested (specificity related to the bacterium tested and the age of biofilm).
Differences between the qualitative and quantitative characteristics of the surfaces of the non-inoculated and inoculated olives were observed after 1 day of storage (Table 2). The free energy values of these samples increased from 3.28 to 16.55 and 46.14mJ/m2, respectively, and the electron-donor property (γ−) increased, from 28.1 to 37.5 and 67.2mJ/m2, respectively. These results can be explained by the difference in the number of L. plantarum cells that adhered to the olive samples, which reached 1.2×103cfu/g for the non-inoculated olives and 1.2×106cfu/g for the inoculated olives (Fig. 1b). Several previous reports noted that the interaction of microorganisms and surfaces depended on the inoculated bacterium.6
The increase in the number of L. plantarum cells adhered to the olive surface favored an increase in the hydrophilic and electron-donor levels of the olive surface after 1 day of storage (Table 2). As the γLW value increases, the apolarity of a surface increases, which results in that surface having a lower affinity for polar liquids. A high γAB component value means that there is more water of hydration on the surface and that it has high hydrophilicity. According to these criteria, the surface of olives with adherent L. plantarum would be more hydrophilic than the surface of uninoculated olives because the former's γAB value was higher. Moreover, the polar free energy of interaction of the surface of the inoculated olives increased during storage (ΔGswsAB=48.2 mJ/m2) (Table 3), indicating an increase in the hydrophilicity of the surface. The extent of the changes in the physicochemical properties of the olive surface might be reduced by changes in the adhesive capability of the microorganisms. Furthermore, it is clear that modification of each parameter involved in the adhesion process could result in an increase or decrease of the surface-adhesion potential. Thus, better understanding of these features is extremely important for the development of effective adhesion-control mechanisms that will ultimately prevent biofilm formation. Currently, a protective biofilm on the surface of devices used by the food industry might be beneficial because its presence might effectively modify the physicochemical properties of the substrates to inhibit the adhesion of undesirable planktonic microorganisms.9
Impact of L. plantarum adhesion on the microbial population of stored olivesIn the current study, the effect of the compact L. plantarum biofilm on the microbial population of stored olives was assessed throughout 16 days of storage to determine the limit of the bio-preservative period. The results revealed a reduction in the microbial population of the olive biofilm during storage. L. plantarum cells were able to adhere to the olive surface, and the biofilm that they produced significantly reduced the yeast and mold populations (by 86.3% after the initial adhesion and 76.6% after 4 days of storage) (Fig. 1c). Olives can be contaminated by a wide variety of mold species that occur naturally on the fresh and processed products. Several authors reported that olives supported mycotoxin production when they were stored for weeks under conditions that promote fungal growth. Therefore, toxinogenesis can occur on olives and might lead to the accumulation of mycotoxins in the olives and their possible transfer to olive oil.
The ability of L. plantarum to produce biofilms most likely affected the final population of the biofilm on the olive surface due to competitive removal. Fungal proliferation might have been inhibited by competition for nutrients and oxygen and by antagonistic compounds synthesized by the bacteria. Kaizu et al.30 demonstrated that several lactobacilli exhibit antioxidative activity and could decrease the risk of accumulating reactive oxygen species through food ingestion. Furthermore, LAB frequently exhibit antioxidative properties.28 A previous study conducted in our laboratory showed that applying L. plantarum to olive fruits increased the level of antioxidant activity and the content of total phenolic compounds during their storage.13 This phenomenon may be due to the ability of L. plantarum to utilize the oxygen in the solution, which is responsible for the auto-oxidation of phenolic compounds. Viable L. plantarum cells have been show to possess antioxidant activity, and the level of this antioxidant activity increased with the concentration of L. plantarum cells. These results indicated that the adhesion of L. plantarum cells could result in an insufficient supply of oxygen at the surface of the olive, causing the loss of the biofilm. Indeed, Mahdavi et al.31 reported that an air–liquid interface appeared to be very important in promoting the attachment of bacteria to a surface and found that biofilm development was sensitive to the availability of oxygen and nutrients. These findings are also consistent with the results of Habimana et al.,21 who showed that the growth of undesirable microorganisms was inhibited by the competition for nutrients and by antagonistic compounds produced by bacteria, such as acids, bacteriocins, or surfactants, which possess good anti-adhesive properties. Indeed, Rodriguez et al.10 determined the factors that significantly enhanced the adhesive and antimicrobial activity of Lactococcus lactis, including coating a silicone surface with a biosurfactant produced by L. lactis to modify the surface properties and make it anti-adhesive and provide it with antimicrobial activity. Thus, coating the surface of olives with a natural material, such as L. plantarum, may be a first step in developing strategies to prevent their microbial colonization.
ConclusionThis study demonstrated that L. plantarum cells could adhere to the surface of olives, forming a protective biofilm. The results showed that the hydrophobicity and the components of the interfacial surface energy, particularly the electron donor, acid-base and Van der Waals components, were strongly involved in the adhesion of L. plantarum adhesion to the olive surface. The adhesion of L. plantarum to the olive surface could be considered beneficial because its presence appeared to effectively inhibit the adhesion of undesirable planktonic microorganisms during storage.
Conflict of interestThe authors have no conflict of interest to declare.
We gratefully acknowledge the financial support provided by the Tunisian Ministry of High Education and Scientific Research. We wish to thank members of the University Center of Regional Interface (CURI, Fès, Morocco) for conducting the environmental scanning electron microscopy.






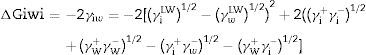
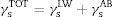
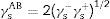
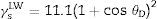
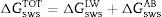
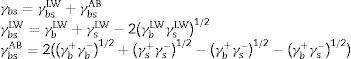
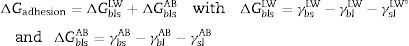
![Effect of L. plantarum adhesion on the biofilm populations of olives [(▴) non-inoculated olives, (■) inoculated olives]: (a) total microbial counts, (b) LAB counts, (c) yeast and mold counts. Effect of L. plantarum adhesion on the biofilm populations of olives [(▴) non-inoculated olives, (■) inoculated olives]: (a) total microbial counts, (b) LAB counts, (c) yeast and mold counts.](https://static.elsevier.es/multimedia/15178382/0000004700000001/v1_201602040959/S1517838215000313/v1_201602040959/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
