Stress tolerance is a key attribute that must be considered when using yeast cells for industrial applications. High temperature is one factor that can cause stress in yeast. High environmental temperature in particular may exert a natural selection pressure to evolve yeasts into thermotolerant strains. In the present study, three yeasts (Saccharomyces cerevisiae, MC4, and Kluyveromyces marxianus, OFF1 and SLP1) isolated from hot environments were exposed to increased temperatures and were then compared with a laboratory yeast strain. Their resistance to high temperature, oxidative stress, and antioxidant response were evaluated, along with the fatty acid composition of their cell membranes. The SLP1 strain showed a higher specific growth rate, biomass yield, and biomass volumetric productivity while also showing lower duplication time, reactive oxygen species (ROS) production, and lipid peroxidation. In addition, the SLP1 strain demonstrated more catalase activity after temperature was increased, and this strain also showed membranes enriched in saturated fatty acids. It is concluded that the SLP1 yeast strain is a thermotolerant yeast with less oxidative stress and a greater antioxidant response. Therefore, this strain could be used for fermentation at high temperatures.
Yeasts are eukaryotic unicellular fungi that are widely distributed in natural environments. They are used in many industrial processes, such as the production of alcoholic beverages, biomass, and metabolic products. Currently, the majority of yeast biotechnology applications are with the species Saccharomyces cerevisiae. However, the limited stress resistance of S. cerevisiae has led to an increased focus on the potential of the non-Saccharomyces spp. yeasts, such as the Pichia spp., Debaryomyces spp., and Kluyveromyces spp. The Kluyveromyces spp. is usually considered to be a thermotolerant yeast, with important commercial relevance to high temperature fermentation during ethanol production. High temperature is one of the most important factors affecting microbial activity, microbial growth rate and biomass yield.1 The capacity to tolerate high temperatures is related to oxidative stress and the antioxidant response. High temperature increases oxidative stress and overexpression of antioxidant enzyme genes in S. cerevisiae.2,3 However, this effect has not been studied in thermotolerant yeasts. Even less is known about yeasts isolated from damaged environments, where several types of stress affect communities, such as osmotic, temperature, pH, and oxidative stress. These conditions could apply a natural selection pressure on yeast to evolve into thermotolerant strains. Arellano-Plaza et al.4 reported that the Saccharomyces cerevisiae (MC4) and Kluyveromyces marxianus (OFF1 and SLP1) yeast strains were able to resist oxidative stress for a long period of time compared with W303-1A (S. cerevisiae reference strain). The MC4, OFF1, and SLP1 yeast strains were isolated from spontaneous mezcal fermentation carried out at handcraft mezcal distilleries in Oaxaca, San Luis Potosí, and Guerrero (all Mexican states). Mezcal production occurs between October and May, when the environmental temperature decreases to −5°C in winter, and can increase to 45°C in spring. Little information is currently available regarding oxidative stress induction and the antioxidant response to increased temperatures in thermotolerant yeasts. The aim of this work was to select a thermotolerant yeast (yeasts that were isolated in regions of Mexico with high-temperature environments) and study its oxidative stress and antioxidant response.
Materials and methodsYeast strainsYeast strains were obtained from the culture collection of the CIATEJ (Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, México)5 and from the ATCC (American Type Culture Collection, Rockville, MD, USA). Two K. marxianus yeast strains, SLP1 and OFF1, were isolated at handcraft mezcal distilleries in the Mexican State of San Luis Potosi and Guerrero, respectively, and one S. cerevisiae, MC4, was isolated at an Oaxaca state distillery. The ATCC yeast, W303-1A, was also used for comparison.
Growth conditionsYeast growth was studied using YPD media containing 1% yeast extract, 2% peptone, and 2% glucose as the carbon source. Cells were grown at 30°C and shaken at 180rpm for 24h.
Cell viabilityYeast strains were grown as mentioned above. The yeasts were then collected and inoculated 1×107cell/mL in fresh YPD medium. The culture was incubated for 24h under the same conditions. The cells were quantified, and yeast suspensions were cultured on YPD agar plates and incubated for 24h at temperatures from 30 to 45°C. After 24h, the colony forming units (CFU) were determined.6 The CFU at room temperature were taken as 100%.
Effect of temperature on specific growth rate, biomass yield, volumetric productivity, and duplication time.
The specific growth rate (μ) was calculated by cell growth, measured by optical density (OD) of the cell suspensions, and estimated using the Lineweaver–Burk equation. For biomass determination yeast cells in broth were harvested, washed with distilled water, and dried in an oven at 80°C until reaching a constant weight. The biomass (Dw) was reported in dry cell mass (g/L). The volumetric productivity of biomass (Qp) was calculated by dividing the biomass yield by the corresponding culture time. The duplication time (Td) was calculated with the equation ln(2)/K, where K is the rate constant.
Temperature increaseAn increase in temperature was applied as described by Kim et al., 7 with some modifications. Yeast strains were grown in a 10mL YPD (2%) medium for 24h at 30°C and 180rpm. A concentration of pre-cultured cells of A600=0.03 was transferred to fresh YPD (2%) media, incubated at 30°C and 180rpm until the stationary phase. Then, the cultured cells were incubated for 2h at 40°C.
Intracellular reactive oxygen speciesIntracellular reactive oxygen species in yeast cultures were determined using fluorescence assays with 2′,7′-dichlorodihydrofluorescein diacetate.8 The cultured cells obtained from a pre-culture were incubated for 2h at 40°C, cells were counted and diluted in YPD medium to a final concentration of 0.5×107cells. A 5-mM stock solution of dichlorofluorescein diacetate was added to each sample and incubated in the dark for 15min at 30°C. Afterwards, cells were harvested by centrifugation, washed, and re-suspended in 1930μL 50mM Tris/HCl buffer (pH 7.5). The cells were permeabilized by adding chloroform and SDS and vortexing at high speed for 20s. The tubes were left to settle for 10min. Cells were pelleted by centrifugation, and the supernatant fluorescence was measured using a Shimadzu RF-5301 fluorometer (excitation, 502; emission, 521 λ).
Lipid peroxidationThe extent of lipid peroxidation was determined through the thiobarbituric acid (TBA) assay.9 Temperature increase was generated as previously described. Cells were re-suspended in Tris–HCl buffer, pH 7.4, containing 10% trichloroacetic acid, and glass beads were added. The samples were broken by agitation on a vortex. After centrifugation at 300g, supernatants were mixed with EDTA 0.1M and 1% (w/v) thiobarbituric acid in NaOH 0.05M. The reaction mixture was heated for 15min in a boiling water bath, and then centrifuged. The absorbance at 532nm was measured in a Perkin–Elmer spectrophotometer. The results were calculated using the molar extinction coefficient for malondialdehyde (1.56×105M−1cm−1).
Lipid extraction and fatty acid analysisLipids were extracted from yeast homogenates using the Bligh and Dyer method.10 For fatty acid analysis, methyl esters were generated by the BF3-methanol assay of Morrison and Smith.11 After extraction with n-hexane, samples of methyl esters were separated by gas chromatography (Perkin Elmer Clarus 500) on a 30m×0.25mm Omega wax capillary column using high-purity N2 as the carrier gas. Fatty acids were identified and quantified by comparison of their retention time with those of authentic standards. The unsaturated/saturated index was determined. The values under 1 indicate that the percentage of saturated fatty acids is higher than the percentage of unsaturated fatty acids.
Determination of catalase activityCatalase activity was quantified by the oxygen production rate using H2O2 (50mM) as substrate; cells were placed in MES-TEA buffer (pH 6.0) in a sealed glass chamber with constant stirring, the oxygen generation rate was quantified with a Clark-type oxygen electrode coupled to a biological oxygen monitor.12
Statistical analysisAll values are means of three separate experiments. Differences in means were analyzed using Student's t test with independent measures. Differences were considered statistically significant with p<0.05, p<0.01, and p<0.001 as b, c, and d, respectively.
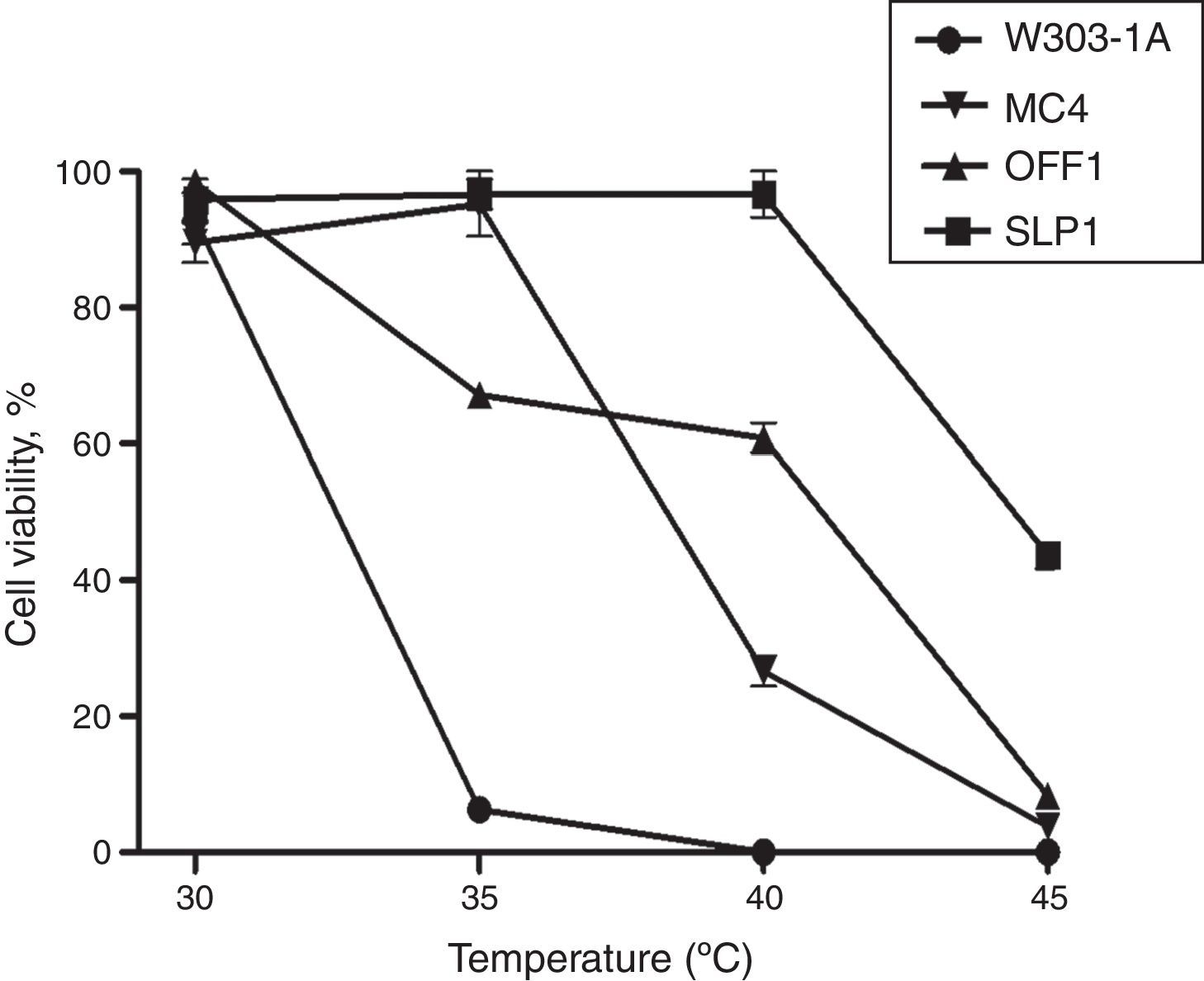
ResultsThe K. marxianus SLP1 strain displayed a normal viability pattern at a high temperature (40°C). Fig. 1 shows cell viability results at different temperatures (30, 35, 40, and 45°C). Cell survival decreased in a temperature-dependent manner from 30 to 40°C in the W303-1A, MC4, and OFF1 strains. The K. marxianus SLP1 strain showed viability at 40°C, identical to that observed at 30°C (Fig. 1). These findings indicate that the SLP1 strain was more tolerant than the other strains at 40°C. At 45°C the four yeast strains showed less than 50% viability.
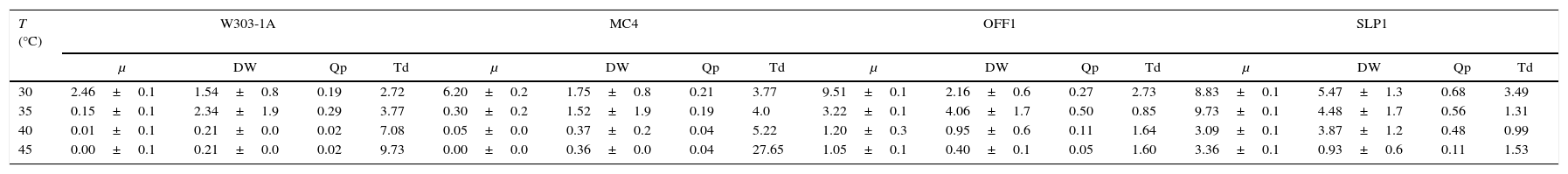
The K. marxianus SLP1 strain showed the best kinetic pattern at temperatures from 35 to 45°C. Temperature markedly affected the kinetic parameters. In the W303-1A and MC4 strains, specific growth rate (μ), biomass (Dw), volumetric productivity of biomass (Qp), and the duplication time (Td) evaluated were reduced when the temperature was raised, showing the best results at 30°C. The SLP1 strain had the best yield in the kinetic parameters evaluated between 35 and 40°C (Table 1).
Effect of temperature on specific growth rate, biomass yield, volumetric productivity, and duplication time.
| T (°C) | W303-1A | MC4 | OFF1 | SLP1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μ | DW | Qp | Td | μ | DW | Qp | Td | μ | DW | Qp | Td | μ | DW | Qp | Td | |
| 30 | 2.46±0.1 | 1.54±0.8 | 0.19 | 2.72 | 6.20±0.2 | 1.75±0.8 | 0.21 | 3.77 | 9.51±0.1 | 2.16±0.6 | 0.27 | 2.73 | 8.83±0.1 | 5.47±1.3 | 0.68 | 3.49 |
| 35 | 0.15±0.1 | 2.34±1.9 | 0.29 | 3.77 | 0.30±0.2 | 1.52±1.9 | 0.19 | 4.0 | 3.22±0.1 | 4.06±1.7 | 0.50 | 0.85 | 9.73±0.1 | 4.48±1.7 | 0.56 | 1.31 |
| 40 | 0.01±0.1 | 0.21±0.0 | 0.02 | 7.08 | 0.05±0.0 | 0.37±0.2 | 0.04 | 5.22 | 1.20±0.3 | 0.95±0.6 | 0.11 | 1.64 | 3.09±0.1 | 3.87±1.2 | 0.48 | 0.99 |
| 45 | 0.00±0.1 | 0.21±0.0 | 0.02 | 9.73 | 0.00±0.0 | 0.36±0.0 | 0.04 | 27.65 | 1.05±0.1 | 0.40±0.1 | 0.05 | 1.60 | 3.36±0.1 | 0.93±0.6 | 0.11 | 1.53 |
μ (h−1); DW (gL−1); Qx (gL−1h−1); (Td). Each value represents the mean±SEM (Standard error of the mean) (n=3).
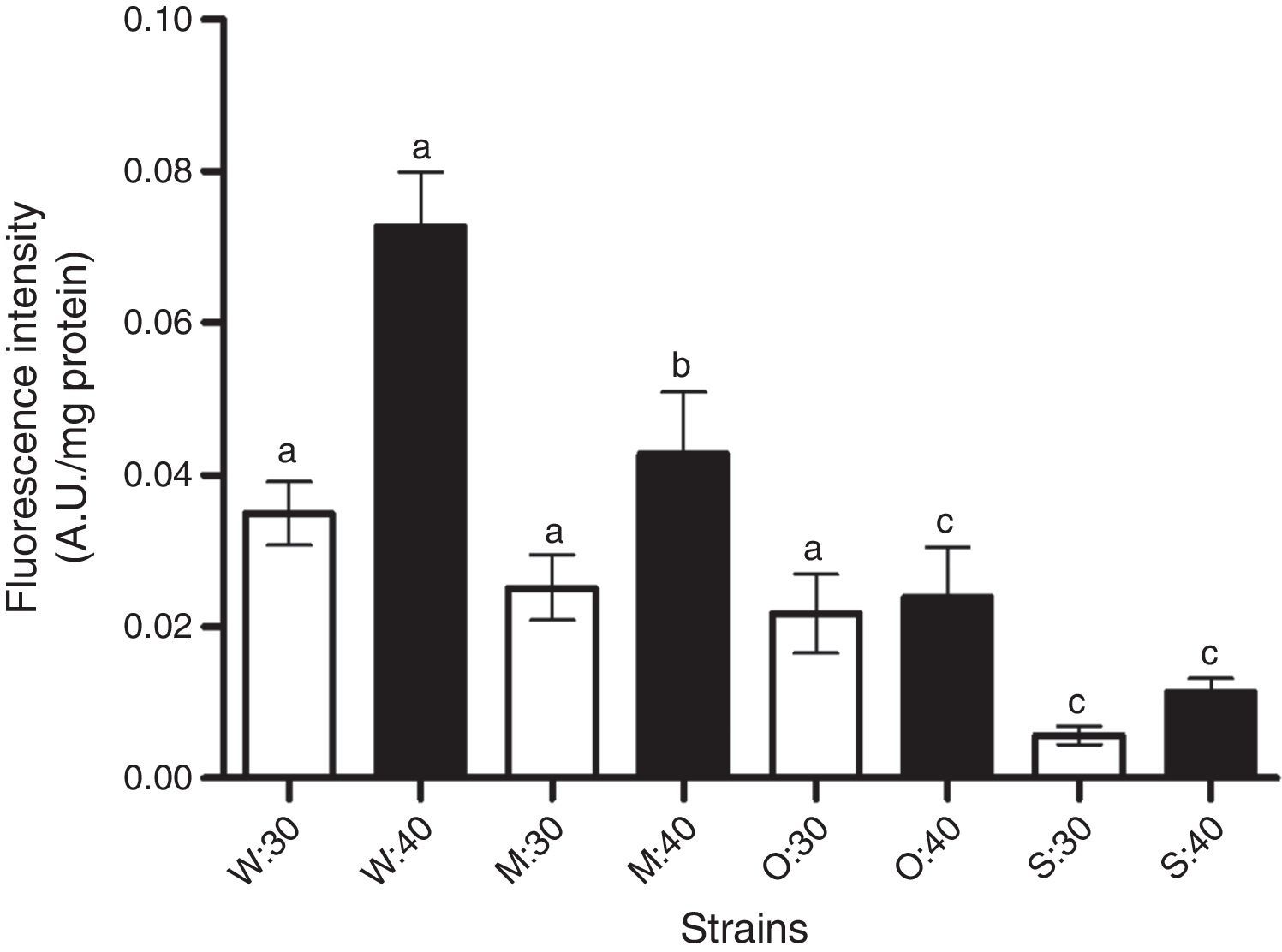
Temperature increase elicits oxidative stress through ROS generation. There was direct molecular evidence of in vivo intracellular oxidation using the oxidant-sensitive 2′,7′-dichlorofluorescein diacetate probe. Measurements of oxidation development were carried out after increased temperature from 30 to 40°C for 2h. At room temperature, only the SLP1 strain showed low ROS production compared with the reference strain W303-1A (Fig. 2). We could see a direct correlation between the increase in temperature and ROS generation. The increase in ROS by temperature was more significant in the reference strain, W303-1A, while the isolated strains showed less ROS generated by the temperature increase. The SLP1 strain had the lowest ROS production under 40°C. To examine whether the increased ROS in the strains was enough to damage cellular components, such as lipids, lipoperoxidation was determined.
Reactive oxygen species (ROS) in yeast strains (W: W303-1A, M: MC4, O: OFF1, and S: SLP1) under normal temperature condition 30 (30°C) and increased temperature 40 (40°C for 2h). Each value represents the mean and SEM (Standard Error of the Mean) values and is indicated as bars (n=3). Other conditions are as described in ‘Materials and methods’ section. Significant differences (bp<0.05, cp<0.01), with respect to W303-1A are indicated.
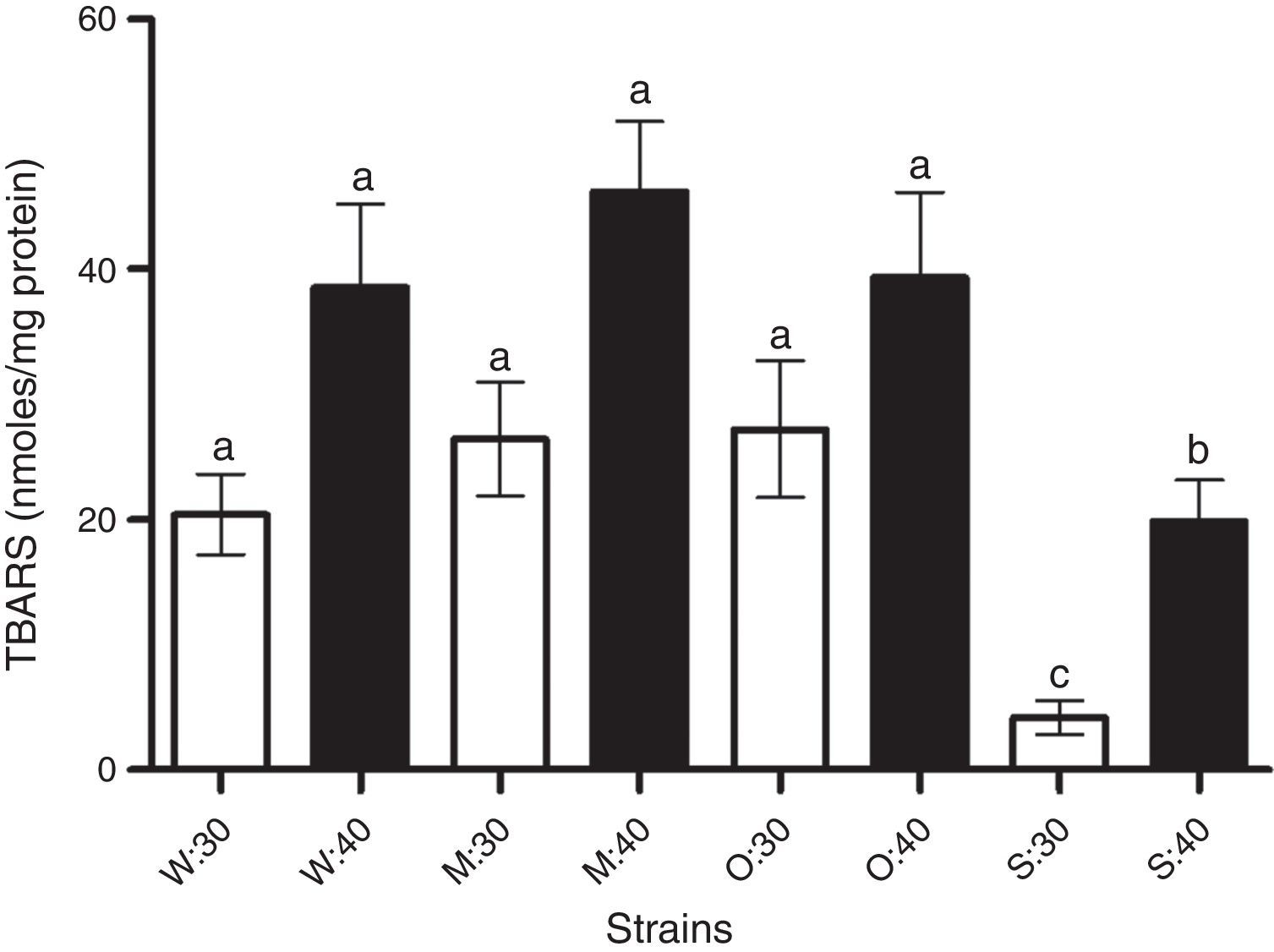
There was a lower degree of lipid peroxidation in the SLP1 strain following increase in temperature. The method based on the reaction of thiobarbituric acid with reactive species derived from lipid peroxidation, particularly malondialdehyde (MDA), was used to determine lipid peroxidation generated by the exposure to increased temperature. No differences in the degree of lipid peroxidation were observed in the MC4 and OFF1 strains at temperatures of 30 or 40°C, with respect to the reference yeast strain (W303-1A). On the other hand, the SLP1 strain showed less lipid peroxidation at normal temperatures and increased temperature (Fig. 3). The degree of lipid peroxidation was directly correlated with the lower ROS production observed (Figs. 3 and 2, respectively). The lower degree of lipid peroxidation in the SLP1 strain could be due to the types of lipids present in their cell membrane or could be a result of more antioxidant system activity; both possibilities were evaluated.
Lipid peroxidation in yeast strains (W: W303-1A, M: MC4, O: OFF1, and S: SLP1) under normal temperature condition 30 (30°C) and increased temperature 40 (40°C for 2h). Other conditions are as described in ‘Materials and methods’ section. Each value represents the mean±SEM (Standard Error of the Mean) (n=3). Significant differences (bp<0.05 and cp<0.01), with respect to W303-1A.
The most temperature-tolerant yeast strain (SLP1) showed the highest concentration of saturated fatty acids under increased temperature. The fatty acid membranes were identified by comparison with authentic standards. The fatty acid composition percentage of phospholipids is presented in Table 2. The yeast strains showed a saturated fatty acid composition of less than 50%. The percentage of the sum of saturated fatty acids was 45, 48, and 47% for the W303-1A, MC4, and OFF1 strains, respectively (Table 2). The saturated fatty acids found in the yeast strains were myristic, palmitic, stearic, and arachidic. The strain with more viability at higher temperature (SLP1) showed the highest concentration of arachidic fatty acid (Table 2). When the temperature was increased, all of the unsaturated fatty acids increased their concentration. Palmitoleic acid was the unsaturated fatty acid with the highest concentration at 40°C in the W303-1A and OFF1 strains, whereas in the MC4 and SLP1 yeast, the oleic acid had the highest concentration.
Fatty acid composition of lipid membranes of the yeast strains, under normal temperature condition, and increased temperature.
| Myristic (C14:0) | Palmitic (C16:0) | Palmitoleic (C16:1) | Stearic (C18:0) | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3) | Arachidic (C20:0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| W | 2±0.3a | 2±0.4a | 39±0.4a | 31±2.0c | 18±1.3a | 29±0.2d | 15±0.4a | 10±0.5a | 20±0.5a | 23±1.8a | 2±0.4a | 2±0.0a | 1±1.2a | N.D. | N.D. | N.D. |
| M | 7±4.0a | 1±0.7a | 35±15.0a | 26±5.0a | 3±6.0a | 1±1.0a | 19±12.0a | 20±2.0a | 11±10.0a | 37±2.0b | 7±6.0a | 13±2.0a | 6±4.0a | 2±2.0a | 13±5.0a | 1±0.8b |
| O | 4±0.7a | 4±0.6a | 32±2.0a | 33±4.0a | 23±0.7a | 24±1.0a | 9±0.5a | 9±0.5a | 20±1.0a | 20±0.9a | 10±0.7a | 8±6.0a | 2±0.2a | 2±0.4a | N.D. | N.D. |
| S | 5±0.5a | 4±0.2a | 32±9.0a | 28±1.0a | 13±3.0a | 14±0.3a | 14±0.9a | 14±0.6a | 17±12.0a | 24±0.2a | 3±0.7a | 3±0.0a | N.D. | 0±0.2a | 16±2.0a | 13±0.5b |
W303-1A (W), MC4 (M), OFF1 (O), and SLP1 (S); (30°C (3); (40°C for 2h (4). No determinate (N.D.). Other conditions are as described in ‘Materials and Methods’ section. Each value represents the mean±SEM (Standard error of the mean) (n=3). Significant differences (anot significant, bp<0.05, cp<0.01 and dp<0.001), with respect to every strain without increased temperature are indicated.
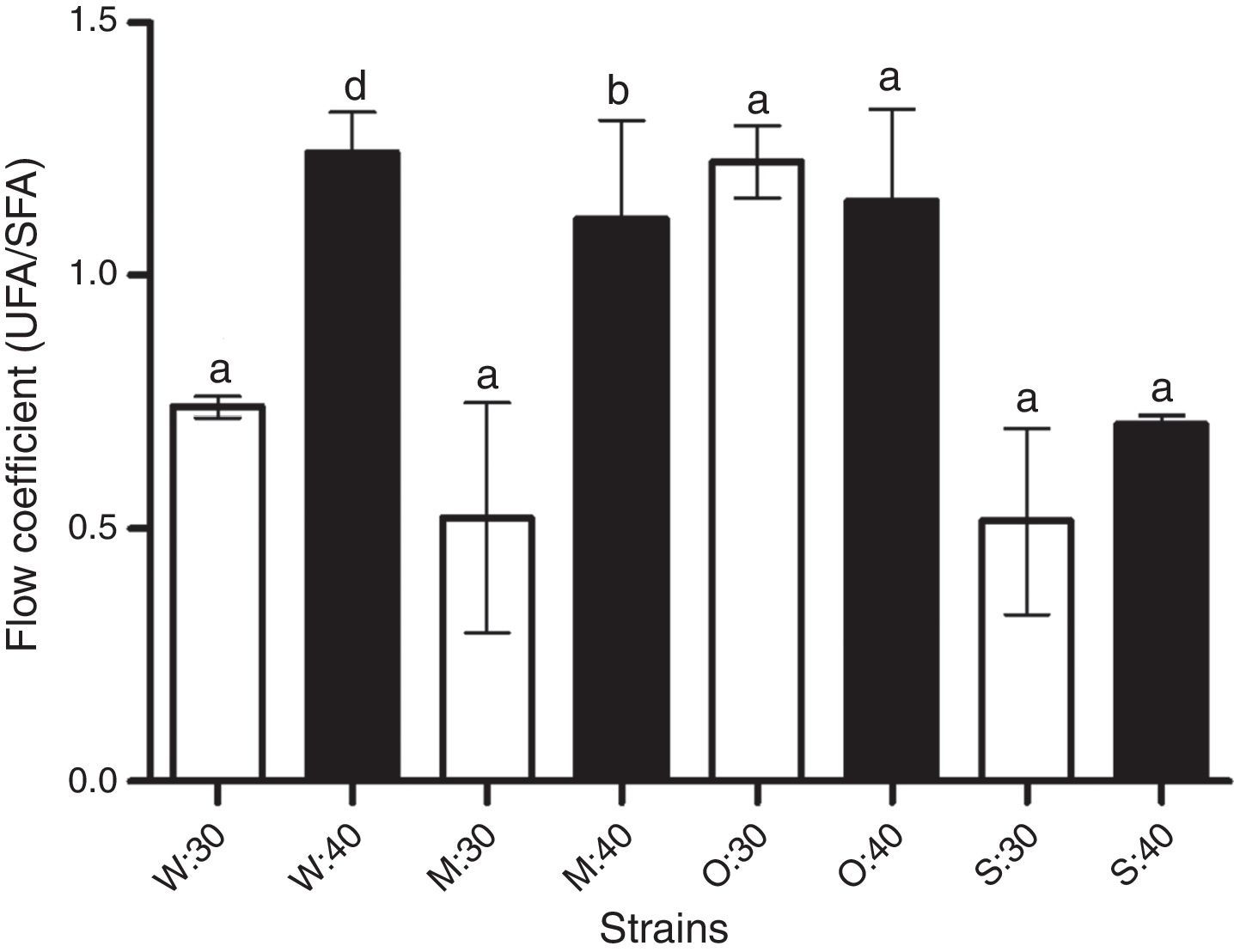
When cells were exposed to 40°C, the unsaturated/saturated index changed for the W303-1A and MC4 strains that showed less resistance to heat. The coefficient of the OFF1 and SLP1 yeast strains remained unaffected. The most heat-tolerant yeast (SLP1) showed a fluidity coefficient without changes under 1, meaning that its saturated fatty acid concentration was higher than the unsaturated fatty acid concentration under both conditions (30 and 40°C) (Fig. 4).
Flow coefficient from the lipid membranes of the yeast strains (W: W303-1A, M: MC4, O: OFF1, and S: SLP1) under normal temperature condition 30 (30°C) and increased temperature 40 (40°C for 2h). UFA, unsaturated fatty acids; SFA, saturated fatty acids. Other conditions are as described in ‘Materials and methods’ section. Each value represents the mean±SEM (Standard Error of the Mean) (n=3). Significant differences (bp<0.05 and dp<0.001), with respect to every strain without increased temperature, are indicated.
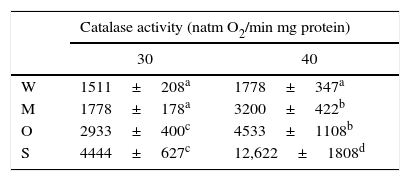
Catalase activity had a direct correlation with lower ROS production and lipid peroxidation produced in the yeast SLP1 by increased temperature. The pattern of catalase activity was directly correlated with the lower level of damage caused in the yeasts by increased temperature. Under normal conditions (30°C) the OFF1 and SLP1 yeasts showed more catalase activity than the reference yeast, W303-1A (p<0.01). Table 3 shows a significant (p<0.001) difference in catalase activity between strains exposed and not exposed to increased temperature, particularly in the SLP1 strain.
Catalase activity in the yeast strains under normal temperature condition and the increased temperature condition.
| Catalase activity (natm O2/min mg protein) | ||
|---|---|---|
| 30 | 40 | |
| W | 1511±208a | 1778±347a |
| M | 1778±178a | 3200±422b |
| O | 2933±400c | 4533±1108b |
| S | 4444±627c | 12,622±1808d |
(W, W303-1A; M, MC4; O, OFF1; S, SLP1); 30 (30°C); 40 (40°C for 2h). Other conditions are as described in ‘Materials and Methods’ section. Each value represents the mean±SEM (Standard error of the mean) (n=3). Significant differences (anot significant, bp<0.05, cp<0.01 and dp<0.001), with respect to W303-1A are indicated.
Thermotolerance is a desirable characteristic for yeast in industrial applications,13 and high-temperature environments could exert a natural selection pressure, evolving yeasts into thermotolerant strains. The effect of high temperature on the viability and kinetic growth parameters of wild yeast isolated from mezcal processes was evaluated and compared with a lab yeast strain (W303-1A). This yeast was used as a control because the stress resistance of this strain is well characterized. Nevertheless, yeast strains with a higher stress resistance, such as the K. marxianus species, may be more useful in terms of their industrial applications. The two K. marxianus strains (OFF1 and SLP1) were able to grow at 40°C, whereas the S. cerevisiae MC4 and W303-1A strains showed low viability (<40%). Specific growth rate (μ) and biomass yield (DW) are physiological features of major importance for a “cell factory” organism to reach high volumetric productivity. The highest viability and DW at 40°C by the SLP1 strain showed the resistance and ability of this yeast to convert substrate into biomass at a high temperature (40°C) for mesophilic yeast. The SLP1 yeast showed a lower duplication time (Table 1), indicating it has a better physiological state. The μ and DW decreased in all of the strains when the temperature was raised (45°C), suggesting that cell reproduction at this temperature generates metabolic cost, affecting the biomass yield. This study showed that the K. marxianus SLP1 strain has 100% viability and the best performance in terms of growth rate, biomass yield, volumetric productivity, and duplication time at 40°C. These results indicate that the K. marxianus SLP1 is a thermotolerant yeast. Arellano-Plaza et al.4 reported that MC4 showed the higher viability when the same yeast strains were exposed to oxidative stress. These results suggest that stress resistance differs in the same yeast strain depending on the stressor. The SLP1 yeast strain has shown that it has the ability to carry out simultaneous saccharification and fermentation of Agave tequilana fructans14 at 30°C. This quality, along with its thermotolerance, could be used as a principal advantage in simultaneous saccharification-fermentation at a high temperature, improving the process.
There is increasing evidence pointing toward oxygen-derived free radicals as one of the causes for thermal stress-associated damage to microorganisms. High temperature produces ROS,2 and this causes damage to proteins, lipids, and nucleic acids and thereby compromises cell viability. In contrast, overexpression of antioxidant enzyme genes increases thermotolerance in S. cerevisiae. The intracellular oxidant level measured by the oxidation of 2′,7′-dichlorofluorescein was found to be increased following an increase in temperature, a result that correlates with findings reported by Davidson and Schiestl.2Fig. 2 shows the correlation between lower ROS production and resistance to increased temperature. Our results support the possibility that oxidative stress plays a major role in the lethal effect of heat on yeast strains. An electron leakage from the respiratory chain in the mitochondria, the major production site for superoxide, could initiate the cascade of ROS production.15 It has also been reported that reduction in respiratory activity produced by the deletion of one of the respiratory enzymes strongly increases the thermotolerance of S. cerevisiae.3 Thus, further studies analyzing the state of SLP1 yeast mitochondria and the electron transport chain are important. One of the best-described effects of oxidative stress-generated ROS on cells is the oxidation of membrane lipids. Membrane lipids participate in the interaction of proteins with the cell barrier.16 Lipid peroxidation is a free-radical-mediated chain of reactions that, once initiated, results in an oxidative deterioration of poly-unsaturated lipids. In general, cells subjected to increased temperature (40°C for 2h) showed an increase in the level of thiobarbituric acid reactive substances (TBARS) (Fig. 3). Cell survival is inversely correlated with TBARS levels. Thus, the yeast with less viability (Fig. 1) showed a higher TBARS level (Fig. 3), whereas the SLP1 yeast strain that had higher viability showed less lipid peroxidation at 40°C. The susceptibility of lipids to oxidation is determined by the lipid class composition and degree of unsaturation.17 Under heat shock, the extent of cellular damage has been correlated positively with increasing unsaturation of the phospholipid fatty acyl component.18 Sudden changes in environmental conditions cause alterations in the organization and dynamic structure of membrane lipids16 and alter the function of many cellular activities. To assess whether the differences observed in yeast lipid peroxidation could be related to the quantitative and qualitative lipid composition of the cell, these were analyzed. The extent of cellular damage correlated positively with the increased polyunsaturation of the phospholipid fatty acyl component (Table 2). In the SLP1 strain, a higher concentration of arachidic (C20:0) fatty acid was observed. Arthur and Watson19 found that the thermophilic strains had an unusual phospholipid composition, such as that of the SLP1 strain that presented 20% arachidic acid. Our result concurred with the report of Steels et al.20 They reported that the most stress-resistant yeast had membranes enriched in saturated fatty acids. Membrane fluidity is determined by the ratio of saturated versus unsaturated fatty acids,21 and maintenance of membrane fluidity is essential for optimal functioning of membrane proteins.22 Changes in the composition of the cell lipid fraction can influence the activity of many membrane-associated proteins and transporters, thus potentially leading to growth arrest and cell death.15Fig. 4 show that W303-1A and MC4 increased their fluidity coefficient by the gain of unsaturated fatty acids. The fluidity coefficient of the OFF1 and SLP1 strains remained unchanged, being lower in the SLP1 strain, owing to its higher saturated fatty acid percentage. A significant alteration of the membrane lipids such as in the W303-1A and MC4 yeasts strains, could affect the function of proteins, preventing their adaptation and reducing their viability. Kim et al.,7 reported oxidative stress as a result of heat-shock in the Saccharomyces spp. KNU5377 strain, and this stress induced antioxidant enzyme stimulation. Catalase is one of the central components of the detoxification pathways that prevent the formation of the highly reactive hydroxyl radical by catalyzing the decomposition of H2O2 into water and oxygen through two electron transfers.23 Our results support those of Davidson et al.2 who reported that yeast mutants deficient in the antioxidant enzyme, catalase, are sensitive to heat exposure at a temperature of 50°C, whereas overexpression of catalase provides protection from lethal heat-shock. The strains evaluated in this work showed higher catalase activity with respect to the reference strain, with and without the increased temperature (Table 3). The significant increase in the activity of this enzyme in the SLP1 yeast could reduce H2O2, preventing the production of another more toxic ROS, such as OH., and therefore also preventing, together with the higher index of saturated fatty acids, lipid peroxidation in this strain. This effect could explain why this strain has more viability under increased temperature. This work is the first to compare the physiological state of wild yeast isolated from mezcal processes when exposed to increased temperature. The K. marxianus SLP1 is a thermotolerant yeast strain, with less oxidative stress, higher antioxidant response, and a higher percentage of saturated fatty acids in its cell membranes. This thermotolerant SLP1 yeast could be used for fermentation at high temperatures in ethanol production.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge the partial economic support received from the Consejo Nacional de Ciencia y Tecnología (169093, to ASM) and Coordinación de la Investigación Científica, UMSNH (2.16, to ASM) grants.