Abiotic stress is one of the major limiting factors for plant development and productivity, which makes it important to identify microorganisms capable of increasing plant tolerance to stress. Dark septate endophytes can be symbionts of plants. In the present study, we evaluated the ability of dark septate endophytes isolates to reduce the effects of water stress in the rice varieties Nipponbare and Piauí. The experiments were performed under gnotobiotic conditions, and the water stress was induced with PEG. Four dark septate endophytes were isolated from the roots of wild rice (Oryza glumaepatula) collected from the Brazilian Amazon. Plant height as well as shoot and root fresh and dry matter were measured. Leaf protein concentrations and antioxidant enzyme activity were also estimated. The dark septate endophytes were grown in vitro in Petri dishes containing culture medium; they exhibited different levels of tolerance to salinity and water stress. The two rice varieties tested responded differently to inoculation with dark septate endophytes. Endophytes promoted rice plant growth both in the presence and in the absence of a water deficit. Decreased oxidative stress in plants in response to inoculation was observed in nearly all inoculated treatments, as indicated by the decrease in antioxidant enzyme activity. Dark septate endophytes fungi were shown to increase the tolerance of rice plants to stress caused by water deficiency.
Rice is one of the most consumed cereals in the world due to its nutritional value and because it represents an affordable source of protein.1 Rice belongs to the genus Oryza, which comprises two cultivated species (Oryza glaberrima and Oryza sativa L.) and a great diversity of wild species (not cultivated).2
Water deficiency is one of the most important causes of abiotic stress and compromises global food production, including the production of rice. This stress causes irreversible oxidative damage because the activity of the plant's antioxidant system is not sufficient to limit the reactive oxygen species (ROSs) derived from byproducts of aerobic and photosynthetic metabolism to non-toxic levels. ROSs therefore accumulate in the plant tissues,3 where they can cause damage to cell organelles; oxidize important biological molecules such as nucleic acids, lipids and proteins; and compromise the integrity of the cell membrane and decrease photosynthesis.4
Plants respond to these stresses by producing catalase (CAT) and ascorbate peroxidases (APX), the primary enzymes responsible for the maintenance of H2O2 at non-toxic levels in cell compartments.5 However, in a scenario of prolonged stress, the antioxidant system cannot prevent cell damage caused by oxidation, and other mechanisms become relevant. Positive effects of plant associations with beneficial microorganisms under stress conditions have been reported.6–8
Dark septate endophytes (DSEs) are conidial or sterile ascomycetous fungi that colonize living plant roots without causing any apparent negative effects.9,10 They are characterized by intense dark pigmentation and the formation of septate hyphae and occasionally microsclerotia, as well as various arbuscular mycorrhizal fungi (AMF). These fungi can be grown in culture medium and can colonize several plant species. They can be found in plant cortical cells inter- and intracellularly and are present in several environments, even under drought conditions, in the presence of heavy metals and in oligotrophic soils.11–16 The hypotheses presented thus far for the dominance of DSEs in stressed environments and their effects on host plant protection are primarily related to the presence of the pigment melanin at the endophytes’ hyphae and microsclerotia.12 Melanin can act as an antioxidant agent and also bind heavy metal ions, thereby protecting cell structures from the oxidative damage produced under such conditions.17 However, the DSE action mechanisms involved in plant protection have not yet been elucidated,18 but likely involve the presence of extraradical hyphae and extracellular enzymes that can improve soil exploration by roots.13
The goals of the present study were to assess the ability of DSE fungi, originating from tropical soils, to grow under stress conditions and to induce stress tolerance in rice plants (O. sativa L.).
Material and methodsDSE isolates A, B, C and D (ERR 01, ERR 04, ERR 16 and ERR 46, respectively) obtained from the roots of wild rice (Oryza glumaepatula) in the Brazilian Amazon by Ribeiro et al.19 were tested. These isolates are stored at COFMEA (Embrapa Agrobiology Culture Collection of Micorrhizal Fungi), and they were only partially taxonomically defined up to now.19 These isolates were previously characterized through amplification and sequencing of the internal transcribed space (ITS1-5S-ITS2), and it was possible to position the isolates at the order level.19 Following this analysis, A101 is a member of Calosphaeriales, A102 is member of Capnodiales and A103 and A106 are members of Pleosporales. The ITS1-5S-ITS2 sequences are also deposited at the NCBI GenBank, accession numbers KR817246, KR817247, KR817248 and KT780724.19
Nipponbare, an improved variety commonly used in rice studies, and Piauí, a wild variety grown in a dry land system, were used in all assays involving plant–fungus interactions.
The capacity of the DSE isolates to grow under stress conditions was tested in two preliminary tests in Petri dishes. Both were placed in a phytotron, using growth medium with sodium chloride (NaCl) or polyethylene glycol (PEG 6000) for the induction of salt and water stress, respectively. The PEG 6000 is a widely used polymer to simulate the effect of drought in studies involving organisms, primarily because it is chemically inert and non-toxic.20
For salt stress, 0.2, 0.4, 0.7 or 1molL−1 NaCl was added to the culture medium at 26°C to obtain an osmotic potential of −0.49, −0.99, −1.73, and −2.43MPa, respectively. A control treatment was included with no salt addition. The salt concentrations needed to obtain the different osmotic potentials were calculated using the Van’t Hoff equation21:
where, ψos=osmotic potential (atm); R=ideal gas constant (0.082atm. 1mol−1K−1); T=temperature (°K); C=concentration (molL−1).Fungal mycelial discs 7mm in diameter, grown for two weeks in potato-dextrose-agar (PDA) growth medium, were placed in Petri dishes containing 39gL−1 commercial PDA medium (Sigma–Aldrich, St. Louis, MO, USA) with the addition of 0, 0.2, 0.4, 0.7 or 1molL−1 NaCl. Three replicates of each treatment were performed. The dishes were grown for 9 days at 26°C. Colony diameter was measured after 9 days using a caliper and expressed in mm.
Stress due to PEG addition was measured by placing fungal mycelial discs, also 7mm in diameter, in Petri dishes containing Hoagland solution solidified with Phytagel (2.5gL−1), to which PEG-6000 had been added at different concentrations (0, 79.791, 121.139, 180.231 and 264.246gL−1) to obtain 0, −0.1, −0.2, −0.4, and −0.8MPa water resistances, respectively.20 The incubation conditions were the same as the above.
Growth promotion of rice plants by DSE under water deficitThe experiment was maintained in the phytotron in a randomized complete block design in a factorial arrangement 5×2×5 (four inoculated fungi and an uninoculated control), two rice varieties (Nipponbare and Piauí), and 5 PEG concentrations (0, 79.791, 121.139, 180.231 and 264.246gL−1), with four replicates (two plants=one experimental unit). The rice plants were grown in Hoagland solution solidified with 2.5gL−1 Phytagel at a mean temperature of 26°C.
The seeds of the two tested rice varieties were sterilized with 2.5% sodium hypochloride and 70% ethanol for 3min and inoculated with fungal mycelia grown for two weeks in PDA, followed by pre-germination for 5 days in Petri dishes containing Hoagland solution solidified with 1% agar. Fungal mycelial discs 7mm in diameter were placed on the dishes’ surface close to the seedlings. The control treatments consisted of non-inoculated seeds without the addition of fungal mycelial discs to the growth medium with one PDA disc added (7mm in diameter).
Following seed pre-germination, the seedlings were transferred into pots containing sterile half-strength Hoagland solution supplemented with 0.3gL−1 MgSO4 and solidified with 2.5gL−1 Phytagel.22 The pots were kept under a 12h light/12h dark photoperiod at a 380μMmolm−2s−1 photosynthetic photon flux density, 70% mean air relative humidity, and temperature of 28/24°C (day/night). Plant height as well as shoot and root fresh and dry weights were measured at 30 days after sowing.
Protein concentration and antioxidant enzyme activityA completely randomized experimental design was used in a factorial arrangement of 2×2×3 (inoculated fungi, isolate A and uninoculated control), two rice varieties (Nipponbare and Piauí), and three PEG concentrations (0, 35 and 70gL−1) in four replicates (two plants=one experimental unit). The growth conditions have been described in the above experiments.
Protein concentrations and CAT and APX activities were measured in rice plants harvested at 30 days after sowing. Leaves were frozen in liquid N2 and stored at −70°C for subsequent quantification of protein concentrations and enzyme activities according to the following methods.
- (a)
Enzyme extraction and determination of protein concentration
Enzyme extraction was performed by homogenizing 200mg of leaf in liquid N2 and adding 1.5mL of the extraction buffer 100mM potassium phosphate buffer (pH 7.0), with 1mM EDTA, 2mM DTT, 0.8mM PMSF, 1% PVPP and 1mM ascorbic acid (ASC). The extract was centrifuged at 14,000rpm and 4°C for 30min. The supernatant was collected and stored at −70°C during the period of analysis. The supernatants collected were used for all enzyme determinations. Catalase and peroxidase activities were expressed as μM H2O2 and μM ascorbate (AsA) per milligram (mg) protein, respectively. The protein contents were determined according to the method of Bradford23 using a bovine serum albumin (BSA) standard curve.
- (b)
Determination of CAT activity
The CAT activity was determined (according to Havir and Mchale24) by measuring the consumption of H2O2 at 240nm for 1min. The reaction mixture consisted of 20μL enzyme extract and 0.980mL of 50mM potassium phosphate buffer, pH 7.0, with 20mM H2O2 and was incubated at 28°C. The molar absorbance coefficient adopted was 36mM−1cm−1 at 240nm.
- (c)
Determination of APX activity
APX activity was determined by monitoring ascorbate oxidation at 290nm for 1min. The reaction mix consisted of 35μL enzyme extract and 0.965mL of 50mM potassium phosphate buffer, pH 7.0, with 1mM H2O2, 0.8mM l-ascorbic acid and distilled water. The molar absorbance coefficient adopted was 2.8mM−1cm−1 at 290nm.25
- (d)
Statistical analysis
The data obtained were analyzed using ASSISTAT 7.6 beta (pt) software to assess the normality, homogeneity and variance of the data using the Lilliefors, Cochran and Bartlett tests, respectively. An analysis of variance (ANOVA) was performed, followed by Tukey's test or the Scott-Knott test for comparison of means at p<0.05. Regression analyses were performed to analyze the effect of different PEG 6000 levels on the growth of DSE isolates and/or rice plants.
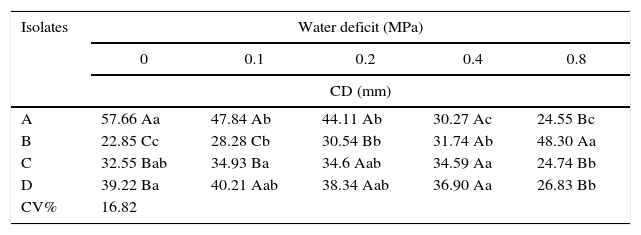
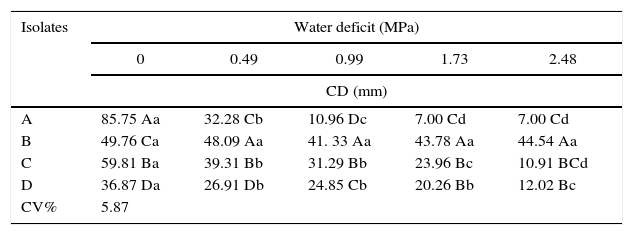
Stress caused by the addition of NaCl and PEG-6000 affected DSE growth (Tables 1 and 2). The tested DSE isolates exhibited different tolerances to both stresses. In general, DSE isolates were more sensitive to the water deficit caused by NaCl than to the addition of PEG-6000 to the growth medium. Both stresses are related to water deficit in the growth medium. The increase in the water resistance of the culture medium favored the growth of isolate B, which exhibited statistically significant differences between different levels of PEG 6000 and NaCl.
Fungal colony diameter (CD) of dark septate endophytes at 9 days of growth in Hoagland solution solidified with Phytagel and with PEG 6000 addition. The values are the means of three replicates.
| Isolates | Water deficit (MPa) | ||||
|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.4 | 0.8 | |
| CD (mm) | |||||
| A | 57.66 Aa | 47.84 Ab | 44.11 Ab | 30.27 Ac | 24.55 Bc |
| B | 22.85 Cc | 28.28 Cb | 30.54 Bb | 31.74 Ab | 48.30 Aa |
| C | 32.55 Bab | 34.93 Ba | 34.6 Aab | 34.59 Aa | 24.74 Bb |
| D | 39.22 Ba | 40.21 Aab | 38.34 Aab | 36.90 Aa | 26.83 Bb |
| CV% | 16.82 | ||||
Values followed by the same upper case letters within the same column or lower case letters within the same row are not significantly different from each other according to Tukey's test at p<0.05.
Fungal colony diameter (CD) of dark septate endophytic isolates at 9 days of growth in PDA medium with NaCl addition. The values are the means of three replicates.
| Isolates | Water deficit (MPa) | ||||
|---|---|---|---|---|---|
| 0 | 0.49 | 0.99 | 1.73 | 2.48 | |
| CD (mm) | |||||
| A | 85.75 Aa | 32.28 Cb | 10.96 Dc | 7.00 Cd | 7.00 Cd |
| B | 49.76 Ca | 48.09 Aa | 41. 33 Aa | 43.78 Aa | 44.54 Aa |
| C | 59.81 Ba | 39.31 Bb | 31.29 Bb | 23.96 Bc | 10.91 BCd |
| D | 36.87 Da | 26.91 Db | 24.85 Cb | 20.26 Bb | 12.02 Bc |
| CV% | 5.87 | ||||
Values followed by the same upper case letters within the same column or lower case letters within the same row are not significantly different from each other according to Tukey's test at p<0.05.
Isolate B was the most tolerant to both stresses tested. The addition of larger amounts of NaCl to the culture medium, corresponding to an osmotic potential of −0.49MPa, resulted in only a 10% decrease in the fungal colony's radial growth. Moreover, the higher water deficit in the culture medium caused by the addition of PEG 6000 (−0.8MPa) increased the growth of this endophyte by approximately 50% (Table 1).
Isolate A exhibited lower tolerance to salinity resulting from NaCl addition, exhibiting no growth at the osmotic potentials −0.99, −1.73 and −2.48MPa and approximately 63% decreased growth at −0.49MPa (Table 2). At the −0.8MPa water deficit level resulting from the addition of PEG 6000, this isolate exhibited a 50% decrease in growth compared with the control treatment. Isolates C and D exhibited similar growth under water deficits due to increasing levels of both NaCl and PEG 6000 in the growth medium. The colony diameter of these two isolates gradually decreased with increasing NaCl or PEG 6000 additions to the culture medium.
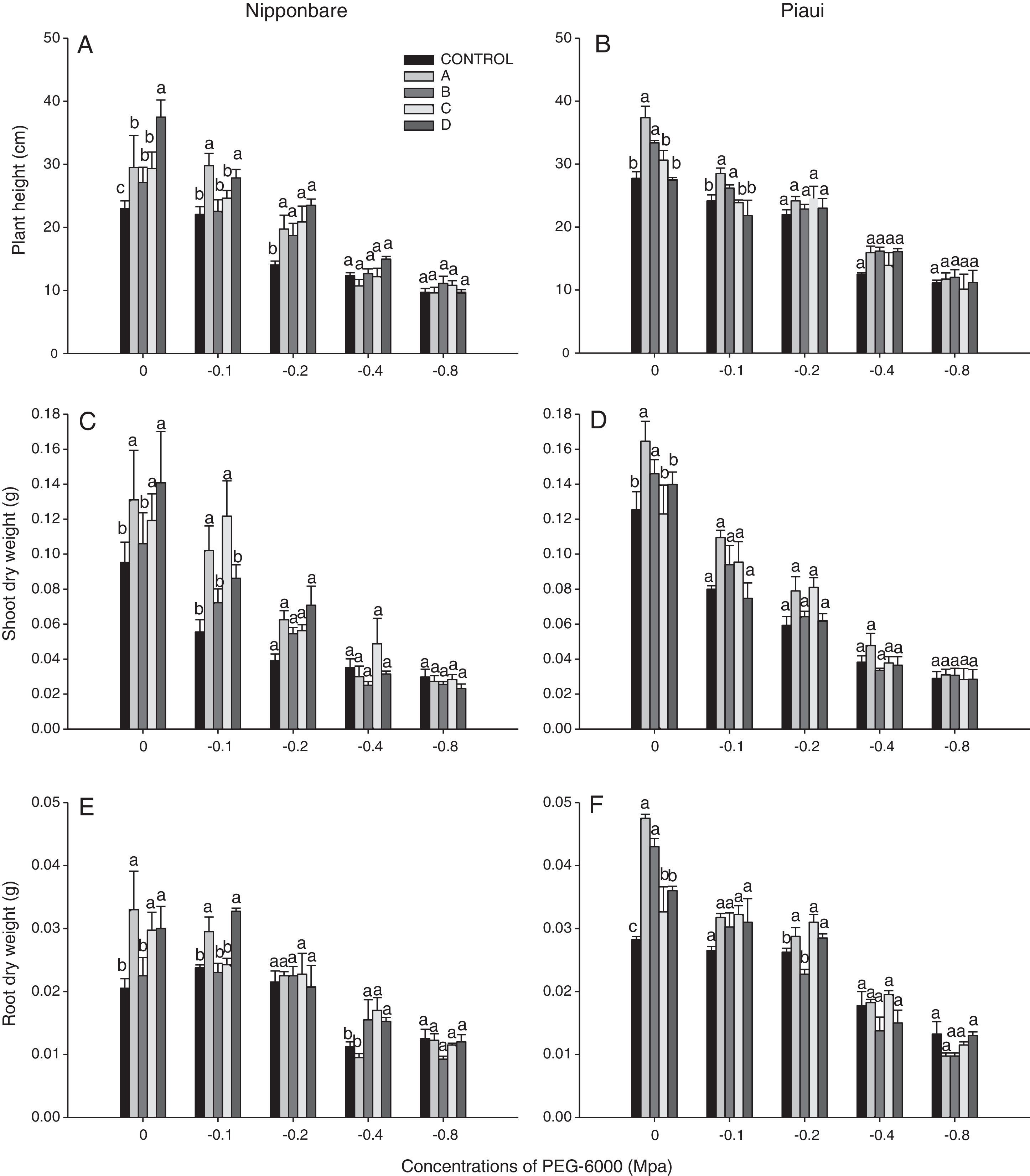
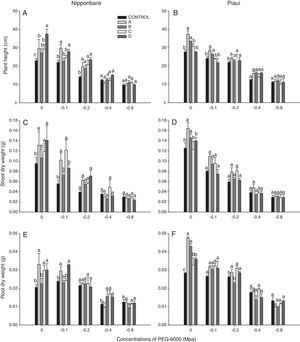
Growth promotion of rice plants by DSE under water deficit conditionsDSEs were observed to promote the growth of rice plants (Fig. 1). In the absence of PEG 6000, Nipponbare plants inoculated with all isolates exhibited a greater plant height than the control treatment, and isolate D was significantly different from other treatments for this variable. Isolates A and B had a stronger beneficial effect on Piauí plants than the remaining treatments. Under conditions of water deficit (−0.2MPa), the Nipponbare plants inoculates with all isolates exhibited a greater plant height than the control treatment.
Under conditions of water deficit, significant differences in SDW were observed with −0.1MPa for variety Nipponbare and were significantly higher with isolates A and C than the remaining treatments. The addition of 79.791gL−1 PEG 6000 (−0.1MPa) to the growth medium resulted in a 43% decrease in SDW compared with the control treatment, whereas the decrease observed for plants inoculated with isolate A was approximately 23%. Isolates A and B exhibited significantly higher SDW under the same conditions for variety Piauí.
Inoculation with isolates A, C and D resulted in a significant difference in RDW between isolate B and the control treatment for variety Piauí in the culture medium without PEG addition 121.139gL−1 (−0.2MPa). In the absence of PEG 6000, the Piauí plants inoculated with all isolates exhibited a greater RDW than the control treatment, and isolates A and B were significantly different between treatments C and D for this variable.
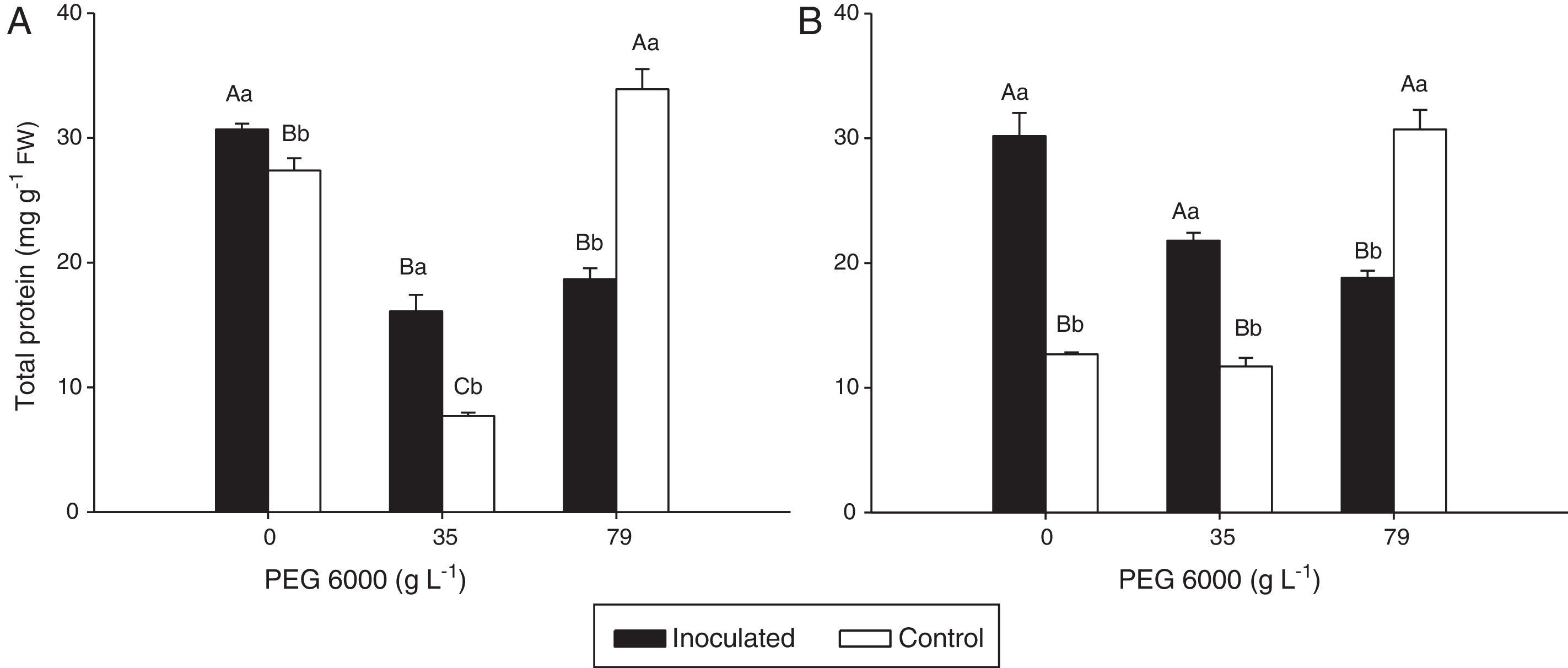
Total protein concentration and CAT and APX activities in the leaves of rice plantsFor inoculated plants of both rice varieties, the leaf total protein concentrations were significantly lower with 79gL−1 PEG 6000 and higher in the absence of PEG 6000 than with 35gL−1 PEG 6000. Protein concentrations were approximately 50% higher in the treatments with 35gL−1 PEG 6000 of inoculated Nipponbare plants and more than 50% higher in variety Piauí in the treatments with 0 and 35gL−1 PEG 6000 than in the treatment control (Fig. 2).
Protein concentrations per fresh weight (FW) of leaves of Nipponbare (a) and Piauí (b) rice plants inoculated with DSE isolate A (Err01) or not inoculated (control) and under different PEG 6000 levels; different lower case letters within the same PEG 6000 level or upper case letters between different PEG levels indicate significant differences according to the Scott-Knott test at p<0.05.
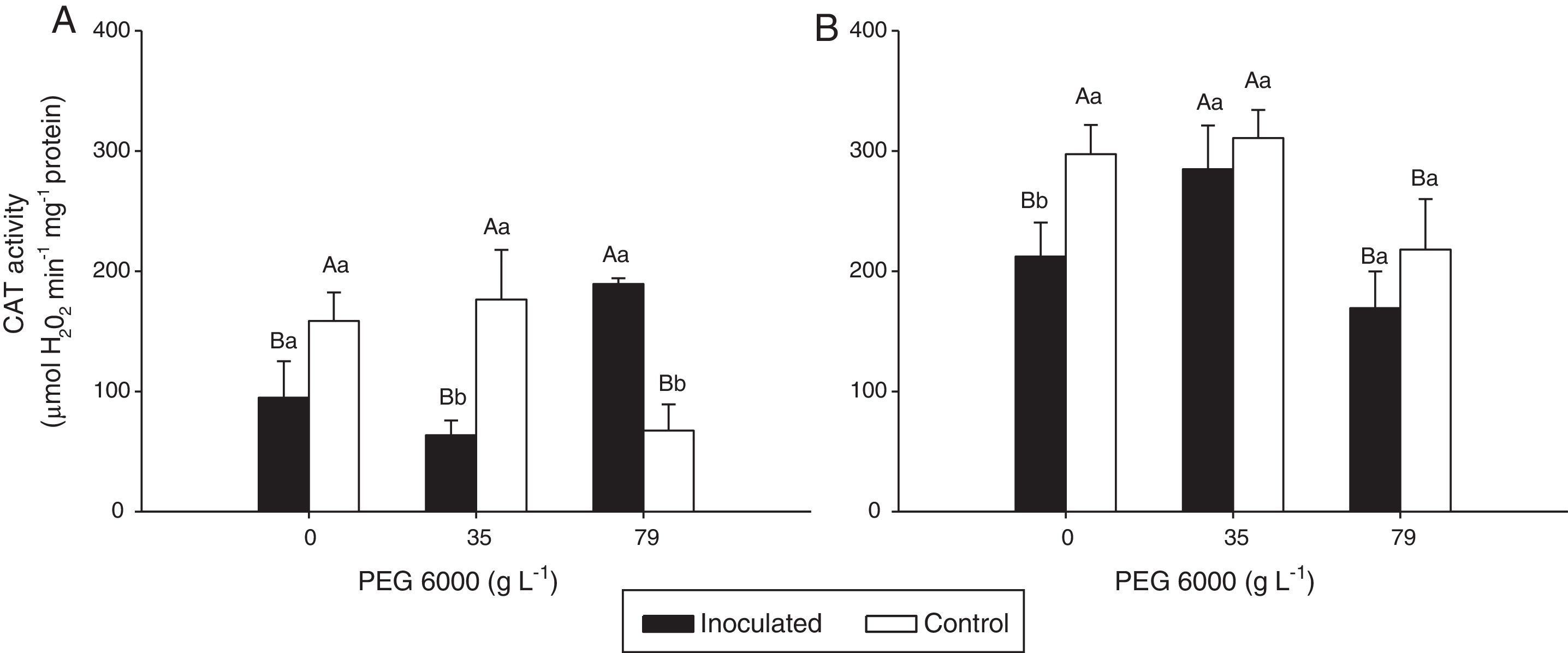
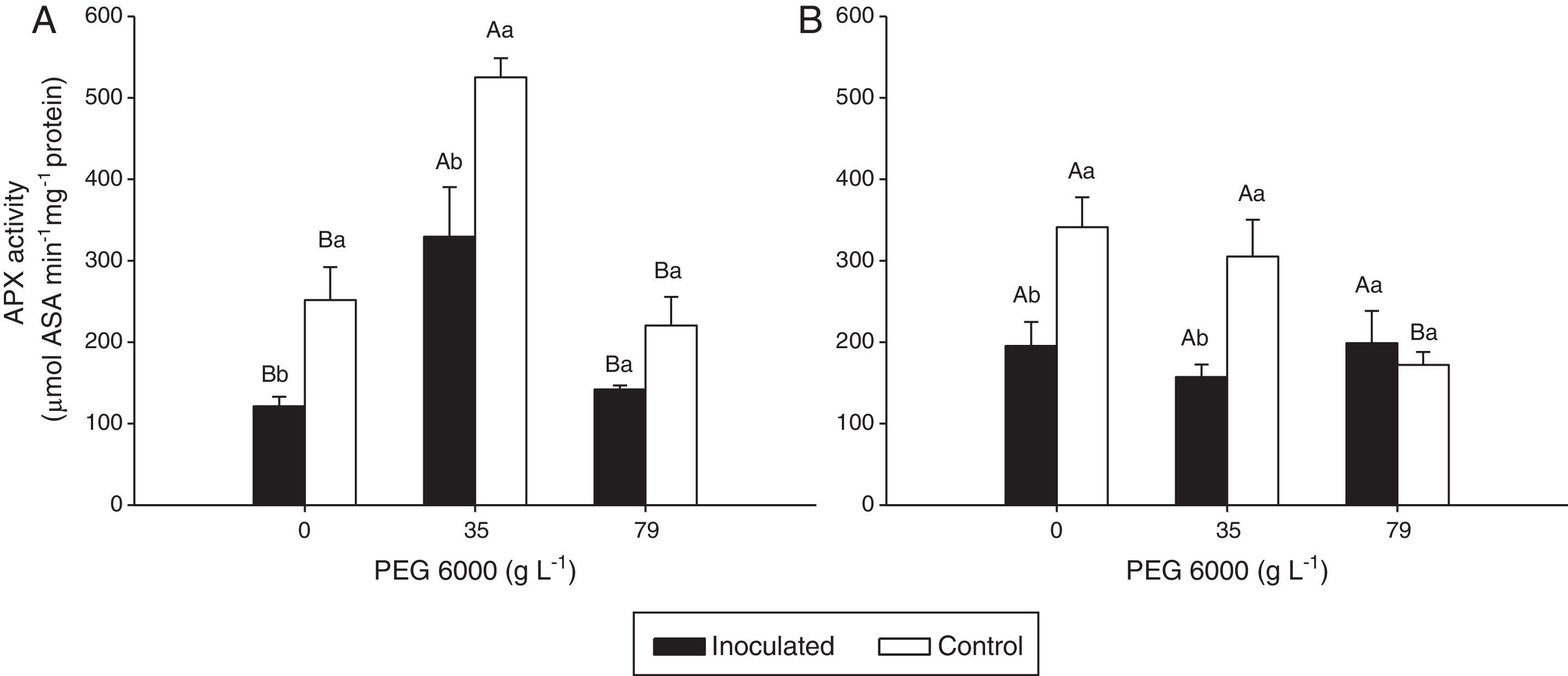
Enzyme activities were differently affected by water deficit and DSE inoculation (Figs. 3 and 4).
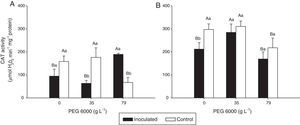
Catalase (CAT) activity in the leaves of Nipponbare (a) and Piauí (b) rice plants inoculated with DSE isolate A (Err01) or not inoculated (control) at different levels of PEG 6000; the values are the means±standard error (n=4); different lowercase letters within the same PEG 6000 level or uppercase letters between different PEG 6000 levels indicate significant differences according to Tukey's test at p<0.05.
Ascorbate peroxidase (APX) activity in the leaves of Nipponbare (a) and Piauí (b) rice plants inoculated with DSE isolate A (Err01) or not inoculated (control) with different additions of PEG 6000; the values are the means±standard error (n=4); different lowercase letters within the same PEG 6000 level or uppercase letters between different PEG 6000 levels indicate significant differences according to Tukey's test at p<0.05.
Non-inoculated plants (control treatment) of both rice varieties exhibited significantly lower CAT activity at 79gL−1 PEG 6000 than at 0 and 35gL−1 (p<0.05). For DSE-inoculated plants, CAT activity was statistically higher at 79gL−1 PEG 6000 for variety Nipponbare and at 35gL−1 for variety Piauí than for the remaining tested PEG 6000 levels.
For Nipponbare plants, inoculation with DSE resulted in approximately 60% lower CAT activity at 35gL−1 PEG 6000 and significantly (approximately 100%) higher CAT activity at 79gL−1 PEG than for the non-inoculated plants. For the Piauí plants, inoculation decreased CAT activity in all treatments (28.66, 8.36 and 22.34% at 0, 35 and 79gL−1 PEG 6000 levels, respectively); however, this difference was statistically significant for only the treatment without the PEG 6000 addition. Non-inoculated (control) plants exhibited higher APX activity at 35gL−1 PEG (p<0.05) than at the remaining tested PEG 6000 levels for the Nipponbare variety and higher APX activity both without and with 35gL−1 PEG 6000 than with 79gL−1 PEG 6000 level for the Piauí variety.
Inoculated Nipponbare plants exhibited the same behavior as non-inoculated control plants, with higher APX activity observed at 35gL−1 PEG 6000. PEG 6000 addition had no effect on the APX activity of inoculated Piauí plants.
DiscussionThe symbiotic efficiency of DSE for rice plants has been demonstrated with different isolates and its specificity under stress conditions. As with other symbionts, such as nitrogen-fixing bacteria and mycorrhizae, not all isolates were observed to positively influence the plant.26 Thus, our results indicate that some DSE stimulate improvements in plants under stress whereas others do not. For example, D induced Nipponbare growth under both normal and stress conditions; however, for Piauí variety, isolate A was more prominent. Furthermore, isolate C promoted increases in leaf dry matter for Nipponbare only when subjected to stress. For Piauí, isolated C also promoted increases. These results suggest that DSE isolates respond differently depending on stress conditions and the plant–fungus genotypes.27
The effects of inoculation with endophytic microorganisms such as DSE on plant growth have been observed to vary and can be positive, negative or null. The outcome of the association depends, among other factors, on the specificity between symbionts, the microorganisms’ ability to establish associations, and the interaction between the endophyte and host plant at levels from the molecular signaling involved in the colonization process up to the host plant's physiological response.28 In this study, only positive and null effects of DSE inoculation were observed on the measured parameters, and no negative effects were observed. Null effects were observed at the highest levels of water deficit although the two rice varieties responded differently to inoculation. According to Redman et al.,28 a single fungal isolate from a specific geographical location can behave as a pathogen, mutualist or commensalist depending on the physiology of the host plant and genetic differences between cultivars, which can alter the outcome of the symbiosis.29 studied the effect of DSE inoculation on growth promotion of the grasses Psathyrostachys juncea, Agropyron cristatum and Bouteloua gracillis under water stress conditions and observed positive effects on A. cristatum and P. juncea but negative effects on B. gracillis. The author considered the different responses to be related to the fact that B. gracillis is a C4 plant, whereas A. cristatum and P. juncea are C3 plants. C4 plants are better adapted to dry environments because they can maintain the efficiency of the photosynthetic apparatus when the stomata close during long dry periods, thus avoiding water loss under conditions of high temperature and low water availability.
Similarly to the present study, increases in rice (O. sativa L.) dry weight were observed by Yuan et al.10 in response to inoculation with the DSE Harpophora oryzae sp. in in vitro experiments performed in China, with the inoculated plants exhibiting 57% higher dry weight than non-inoculated plants. The DSE promoted both shoot and root growth in inoculated plants. However, larger increases were observed in root than in shoot biomass.30 In this study, a lack of response to DSE inoculation was observed for all measured parameters with the exception of RDW in both tested rice varieties under conditions of higher stress. This lack of response may be associated with the lower fungal growth observed under these conditions. With the exception of isolate B, the remaining isolates exhibited significantly lower growth at this level. The only responses observed at the highest level of water deficit (−0.4MPa) were for RDW; this may be related to a higher investment in root growth by plants exposed to these conditions in an attempt to increase water uptake.31
The presence of DSE fungi enveloping root cells inter- and intracellularly may be related to higher protection against the oxidative damage resulting from the −0.4MPa water deficit and the fact that the only effects observed at this level of stress were on RDW. The production of melanized hyphae may be a survival strategy of DSE fungi in stressed environments and may protect plants from free radicals.32 The melanin produced by DSE also has high antioxidant power and may be important for plant survival in stress environments.33
Several genes and proteins respond to stresses caused by different environmental stimuli, such as salinity, low temperatures and drought.34 Under these conditions, the overexpression of cell protection-related proteins, such as antioxidant enzymes, may occur.35,36 Additionally, plant protein concentrations can be influenced by several factors including increases in the stress intensity and/or duration and the efficiency of the plant's antioxidant system.37
CAT and APXs are the primary enzymes involved in the antioxidant system of plants and have similar roles in the elimination of ROS because both act by reducing H2O2 into O2 and H2O. However, APX uses ascorbate as the reducing agent38 and has a higher substrate affinity than CAT.3 Therefore, even low H2O2 concentrations are sufficient to induce APX activity in the process of cellular detoxification.39,40 There are few reports of changes in the activity of antioxidant enzymes as a result of inoculation with endophytic fungi under stress conditions, such as those presented in this work. We observed stress reductions in plants inoculated with isolate A, as indicated by the decrease in CAT and APX activity at nearly all stress levels tested.
Another mechanism by which DSE may have contributed to higher growth of inoculated plants under water stress conditions in the present study is the production of a fungal network extending beyond the root depletion zone.12,13,41
The antioxidant power of the melanin in the fungal structures may also have functioned as an antioxidant agent, controlling the free radicals formed due to oxidative stress and preventing cell damage, thereby contributing to higher plant growth under these conditions.12,18,42–44
Our results suggest that endophytic microorganisms, such as DSEs, are capable of promoting host plant growth through mechanisms other than those related to plant nutrition or nutrient solubilization.45,46 Some DSE isolates obtained from tropical environments exhibit different levels of stress tolerance in vitro. Moreover, some isolate can colonize and promote the growth of the two rice varieties both with and without the presence of a stressor.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES) for the scholarship granted to the author. The Brazilian Agricultural Research Corporation (Embrapa, Carbioma project no. 02.11.05.001), Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ-E – 26/103.242/2011) the National Council for Scientific and Technological Development (CNPq – 562.601/2010-4; 563304/2010-3 and 562955/2010-0), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG CRA – APQ-00001-11), and the Inter-American Institute for Global Change Research (IAI-CRN II-021) are acknowledged for supporting the research activities.