We aimed to verify the changes in the microbial community during bioremediation of gasoline-contaminated soil. Microbial inoculants were produced from successive additions of gasoline to municipal solid waste compost (MSWC) previously fertilized with nitrogen-phosphorous. To obtain Inoculant A, fertilized MSWC was amended with gasoline every 3 days during 18 days. Inoculant B received the same application, but at every 6 days. Inoculant C included MSWC fertilized with N–P, but no gasoline. The inoculants were applied to gasoline-contaminated soil at 10, 30, or 50g/kg. Mineralization of gasoline hydrocarbons in soil was evaluated by respirometric analysis. The viability of the inoculants was evaluated after 103 days of storage under refrigeration or room temperature. The relative proportions of microbial groups in the inoculants and soil were evaluated by FAME. The dose of 50g/kg of inoculants A and B led to the largest CO2 emission from soil. CO2 emissions in treatments with inoculant C were inversely proportional to the dose of inoculant. Heterotrophic bacterial counts were greater in soil treated with inoculants A and B. The application of inoculants decreased the proportion of actinobacteria and increased of Gram-negative bacteria. Decline in the density of heterotrophic bacteria in inoculants occurred after storage. This reduction was bigger in inoculants stored at room temperature. The application of stored inoculants in gasoline-contaminated soil resulted in a CO2 emission twice bigger than that observed in uninoculated soil. We concluded that MSWC is an effective material for the production of microbial inoculants for the bioremediation of gasoline-contaminated soil.
Accidental spills of gasoline and other petroleum products in the fuel distribution terminals, mainly during truck cleaning and distribution in pations, are a common cause of soil and water systems contamination in Brazil.1 BTEX compounds (benzene, toluene, ethylbenzene, and xylenes) present in gasoline are highly toxic and harmful to human health; among these, benzene is a known carcinogenic substance.2–4 The presence of ethanol in gasoline can increase the solubility of BTEX dissolved in the groundwater and thereby hindering its natural biodegradation by increasing the persistence of these compounds in the environment.5,6 Ethanol can be biodegraded preferably than BTEX, which leads to consumption of oxygen that would be required for the degradation of hydrocarbons. Furthermore, ethanol may be toxic or inhibitory to degrading microorganisms of monoaromatic hydrocarbons.5 Corseuil and Fernandes7 indicated that contamination of water table caused by Brazilian commercial gasoline may be greater than that by conventional gasolines from other countries. This issue stems from the ability of ethanol to increase the solubility of petroleum hydrocarbons in water. The results of these experiments showed that, in the aqueous phase, fractions of 10–30% of ethanol can increase the concentration of benzene, toluene, and xylene (BTX). This effect of co-solvency responsible for the higher concentration of hydrophobic organic compounds in water is greater to xylenes, which are less soluble compounds among all BTX compounds and when ethanol is added, its solubility is enhanced. Thus, it is also likely that higher concentrations of ethanol in water aquifers facilitate greater solubility of polycyclic aromatic hydrocarbons (PAH), which are extremely hydrophobic and harmful to the human health.
Bioremediation has been applied for the recovery of environments contaminated with oil and oil products. Biotechnological processes applied in bioremediation are promising because they offer better cost benefit than physico-chemical techniques, which are more expensive, difficult to implement, and require continuous monitoring to achieve desirable results.8–10 Bioremediation techniques have great developmental potentials in Brazil, as the climatic conditions of this country are favorable for the biodegradation of contaminants.
Bioremediation includes mineralization or transformation of contaminants into less toxic forms by different groups of microorganisms.11–13 This process, which tends to occur naturally, can be accelerated in two ways: (i) by biostimulation, which is based on stimulation of the catabolic activity of indigenous microorganisms by the addition of limiting nutrient minerals, supplying oxygen or other electron acceptors, and by maintaining suitable conditions of temperature, pH, and moisture and (ii) by bioaugmentation, which is based on the inoculation of a population or consortium of effective microorganisms for the degradation of contaminants.9,14–16 The simplest form of bioremediation is natural attenuation, which includes only monitoring of the natural degradation of the contaminant in the contaminated environment without any intervention. The comparison between the three bioremediation strategies for soil contaminated with diesel demonstrated that the results were site-specific, varying with the soil type.17 In this study, we demonstrated the efficiency of applying microbial inoculants enriched from municipal solid waste compost (MSWC) for the bioremediation of soil contaminated with gasoline. Respirometric tests were employed to evaluate the mineralization of gasoline in inoculants and in soil receiving gasoline contamination. Changes in the microbial community were assessed by analysis of the inoculants after storage for 103 days.
Material and methodsSoil contamination and characterizationThe experimental soil was sifted through 5-mm mesh and identified to be extremely clayey by physical and chemical analyses (Table 1). After adjusting the moisture content of the soil to 60% of the WHC and the C:N:P ratio to 100:10:1 (assuming C added as gasoline), the soil was contaminated artificially with 20mL/kg of gasoline containing approximately 22% ethanol.
Physical–chemical characteristics of soil.
| Characteristic | Unit | Value |
|---|---|---|
| Thick sand | % | 12 |
| Fine sand | % | 11 |
| Silt | % | 4 |
| Clay | % | 73 |
| pH (H2O) | – | 4.8 |
| WHC | % | 48.89 |
| Org C | g/kg | 32.0 |
| N total | g/kg | 0.6 |
| P | mg/dm3 | 0.5 |
| K | mg/dm3 | 39 |
| Ca2+ | cmolc/dm3 | 0.33 |
| Mg2+ | cmolc/dm3 | 0.01 |
| Al3+ | cmolc/dm3 | 0.77 |
WHC, water holding capacity; Org C, organic carbon; cmolc, cent mol of charge.
The inoculants were produced by enrichment of MSWC with gasoline (50mL/kg). Inoculant A received gasoline application every 3 days for up to 18 days. The gasoline application interval for inoculant B was 6 days. The C:N:P ratio of the compost was adjusted to 100:10:2, considering the amount of carbon added in the form of gasoline in the first application. During inoculants enrichment, the moisture content of the soil was maintained at 40–60% of the WHC. The Inoculant C consisted of MSWC fertilized with N and P sources, but no gasoline. All treatment soils were stored under the same conditions.
Oil decomposition activity in the contaminated soil as a function of inoculant doseTest treatments were constructed using gasoline contaminated soil (20mL/kg) and inoculants A, B or C that were added in the proportions of 10, 30, or 50g/kg of dry soil mass. In the treatments that used 10 and 30g/kg of inoculants A, B and C, sterile non-contaminated MSWC was used to ensure the presence of the same amount of compounds (50g/kg) in all treatments. MSWC was sterilized with a dose of 25 Mrad γ radiation by a 60 Co source.
Three control treatments were performed. Control treatment 1 was constructed using only gasoline contaminated soil. Control treatment 2 was constructed using gasoline contaminated soil plus sterile MSWC (50g/kg). Sterile MSWC was obtained using a dose of 25 Mrad γ radiation by a 60 Co source. In Control treatment 3, the sterile soil was added with sterile MSWC (50g/kg).
Three replicates for each treatment were performed, totaling 36 plots. Each plot consisted of a respirometric 750mL flask connected to a respirometer equipped with a CO2 infrared detector with intermittent air flow (Sable System, NE, USA). The respirometric analysis was performed for 266h at 22–30°C (Table 2).
Treatments submitted to respirometric assay for determining the appropriate concentration of inoculants for adding to gasoline-contaminated soil.
| Treatment | Inoculum concentrations |
|---|---|
| Inoculant A | 10, 30, or 50g/kg |
| Inoculant B | 10, 30, or 50g/kg |
| Inoculant C | 10, 30, or 50g/kg |
| Soil | 0 |
| Sterilized MSWC+Soil (SC+S) | 50g/kg |
| Sterilized MSWC+Sterilized Soil (SC+SS) | 50g/kg |
The inoculants were stored at the room temperature or under refrigeration (6–8°C). After 103 days of storage, the inoculants were applied to 60g of gasoline-contaminated soil (20mL/kg) at a dose of 30g/kg. The soil was subjected to respirometric analysis under the same conditions as described previously for 685h at 21–32°C. After the respirometric analyses, the soil was enumerated for culturable bacterial populations and microbial community analysis by ester-linked fatty acid methyl ester (EL-FAME).
Enumeration of cultivable bacterial populationsThe cultivable heterotrophic bacteria were counted by plating serial dilutions (in sodium pyrophosphate 1g/L on nutrient agar – HIMEDIA®). To inhibit fungal growth, 100mg/L of cycloheximide was added to the medium. The plates were incubated at 30°C until development of colonies.
Microbial diversity by ester-linked fatty acid methyl ester analysisBefore extraction and analysis of EL-FAMEs to describe the microbial communities of the inoculants, we developed a method to eliminate the interference of hydrocarbons in the samples. For this purpose, to each sample, 3mL of sterile distilled water and 1mL of hexane were added. After homogenization, the samples were centrifuged at 544×g for 15min. Immediately after which, the non-polar phase containing the hydrocarbons was removed, followed by removal of water. Water was only used to separate the compost from hexane. The entire process was repeated three times.
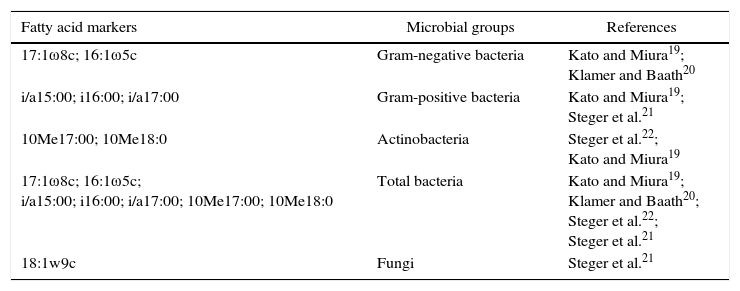
EL-FAMEs were extracted according to the method given by Shutter and Dick18 with some modifications. A reduction of 2/3 of the volume of reagents and samples was performed. In the last step of this method, the solvent containing FAMEs was evaporated under vacuum until complete dryness and the residue was resuspended in 1/3rd of the original volume. The extracts were then analyzed in the Agilent Technologies 7890 gas chromatograph. The identification of the fatty acids was performed by Sherlock Microbial Identification System® (MIDI, Newark, DE, USA), using the reference libraries ITSA 1.0®, IR2A1®, or RTSBA6®. Fatty acids markers of microbial groups are shown in Table 3.
Fatty acids markers used for determining the microbial groups present in inoculants and soil.
| Fatty acid markers | Microbial groups | References |
|---|---|---|
| 17:1ω8c; 16:1ω5c | Gram-negative bacteria | Kato and Miura19; Klamer and Baath20 |
| i/a15:00; i16:00; i/a17:00 | Gram-positive bacteria | Kato and Miura19; Steger et al.21 |
| 10Me17:00; 10Me18:0 | Actinobacteria | Steger et al.22; Kato and Miura19 |
| 17:1ω8c; 16:1ω5c; i/a15:00; i16:00; i/a17:00; 10Me17:00; 10Me18:0 | Total bacteria | Kato and Miura19; Klamer and Baath20; Steger et al.22; Steger et al.21 |
| 18:1w9c | Fungi | Steger et al.21 |
In the respirometric tests, a significant interaction was noted between the concentration and type of inoculants added to gasoline-contaminated soil. The addition of inoculants A and B showed greater CO2 evolution at the application of the largest dose of 50g/kg. For inoculant C, inverse response was observed, that is, the higher the dose, lower was the CO2 emission (Fig. 1).
CO2 emission by gasoline-contaminated soil (20mL/kg). In the soil-treatment (Soil), inoculants were not added. In other treatments, the inoculants A (A), B (B), or C (C) were added. The presented data refers to the treatments with 50g/kg of inoculants. Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations Inoculant C refers to MSWC with no gasoline added.
In treatments including inoculation of soil, an acceleration of CO2 emission was noted after approximately 90h of incubation. Treatment with uninoculated soil showed a much more prolonged phase adjustment that extended until about 210h (Fig. 1). The results showed that microorganisms present in inoculants added to gasoline-contaminated soil were involved in the biodegradation of hydrocarbons. At the dose of 50g/kg, inoculants differed in their ability to stimulate biodegradation. Most CO2 emissions were noted for treatments with 50g/L dose of inoculant A; lower emission was recorded for inoculant C. Analyzing the curve slope revealed that the maximum emission rates did not differ between inoculants A and B. However, the accelerated phase of CO2 emission began a little earlier in the treatment with inoculant A. The result of these differences was greater for overall CO2 emissions in the treatment with inoculant A. For doses 10 and 30g/L, no difference was noted between the inoculants on the CO2 emissions (excluding the inoculant C applied at the rate of 30g/L, which resulted in CO2 emission lower than that with inoculants A and B).
The addition of inoculants A and B to gasoline-contaminated soil showed greater counts of cultivable bacteria (Fig. 2). In the uninoculated treatment, bacterial count could not be performed owing to the prevalence of fungi in the plate despite the addition of cycloheximide to the culture medium.
Heterotrophic bacterial count after application of inoculants to gasoline-contaminated soil at concentrations 10, 30, or 50g/kg. The numbers in parenthesis indicate the dose of inoculants (g/kg). Inoculant A (A); inoculant B (B); inoculant C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
The fatty acid profiling of gasoline-contaminated soil revealed that in the treatment with no inoculants (Soil) predominated fatty acid markers of Gram-positive bacteria in relationship to the number of fungi and actinobacteria markers. Markers of Gram-negative bacteria were not detected in this treatment (Fig. 3). The MSWC addiction in the soil resulted on the reduction of fatty acid markers of the soil for actinobacteria and enhancement in the numbers of fungi for all the treatments, with growth of Gram-negative bacteria markers in some of treatments (Fig. 3). The latter bacterial group were detected in all treatment groups inoculated with inoculant C and only at a particular dose of inoculants A (10g/kg) and B (50g/kg), with low relative proportion as compared with the markers of other groups (Fig. 3). In inoculated soils, the relative proportion of markers for fungi and Gram-positive bacterial fatty acids was always more abundant.
The proportion of relative markers of fatty acids for the microbial groups in gasoline-contaminated soil after application of inoculants. Gram-positive (G+), Gram-negative (G−), actinobacteria (actino), and fungi. The numbers in parenthesis indicate the dose of inoculants (g/kg). Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
Regarding the profile of total fatty acids in soil, the arrangement of treatments by PCA was influenced by the type of inoculant added to the soil (Fig. 4). In group I, the samples of uninoculated treatment (soil) were grouped, which presented an extremely different profile from those of treatment groups receiving the inoculants. The group II included treatment groups containing sterile MSWC (SC+SS) and the non-sterile soil inoculated with sterile MSWC (SC+S). The treatments with Inoculant C (application of compound without gasoline) were pooled in group III, while the treatments with the addition of inoculants A and B were grouped in group IV.
Principal components analysis of the fatty acid profiles of gasoline-contaminated soil in response to the application of inoculants. SC+S: non-sterile soil inoculated with sterile compound; SC+SS: sterilized soil plus sterilized compost; S: soil; C: soil inoculated with unenriched compound; A: soil inoculated with inoculant A; B: soil inoculated with inoculant B. Group I: soil samples without inoculants; Group II: soil samples with sterile compound; Group III: soil samples with unenriched compound; Group IV: soil samples with inoculants A and B.
Storage resulted in a significant reduction in the density of heterotrophic bacteria in the inoculants (Fig. 5). The cooling temperature was found to be the most suitable for maintaining the viability of the bacterial population of the inoculants. Higher counts of bacteria were obtained for inoculant A, except during storage at room temperature, a condition in which no difference in the results was noted among the three inoculants (Fig. 5). The higher counts for inoculants A and B as compared to that of inoculant C (which did not receive gasoline application) indicate that the application of this fuel favored the growth of hydrocarbonoclastic populations.
Heterotrophic bacterial count of the inoculants after 103 days of storage at different temperatures. The black bar corresponds to heterotrophic bacterial count before storage of inoculants. The lighter gray bar after storage under refrigeration (6–8°C) and dark gray bar after storage at room temperature. Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
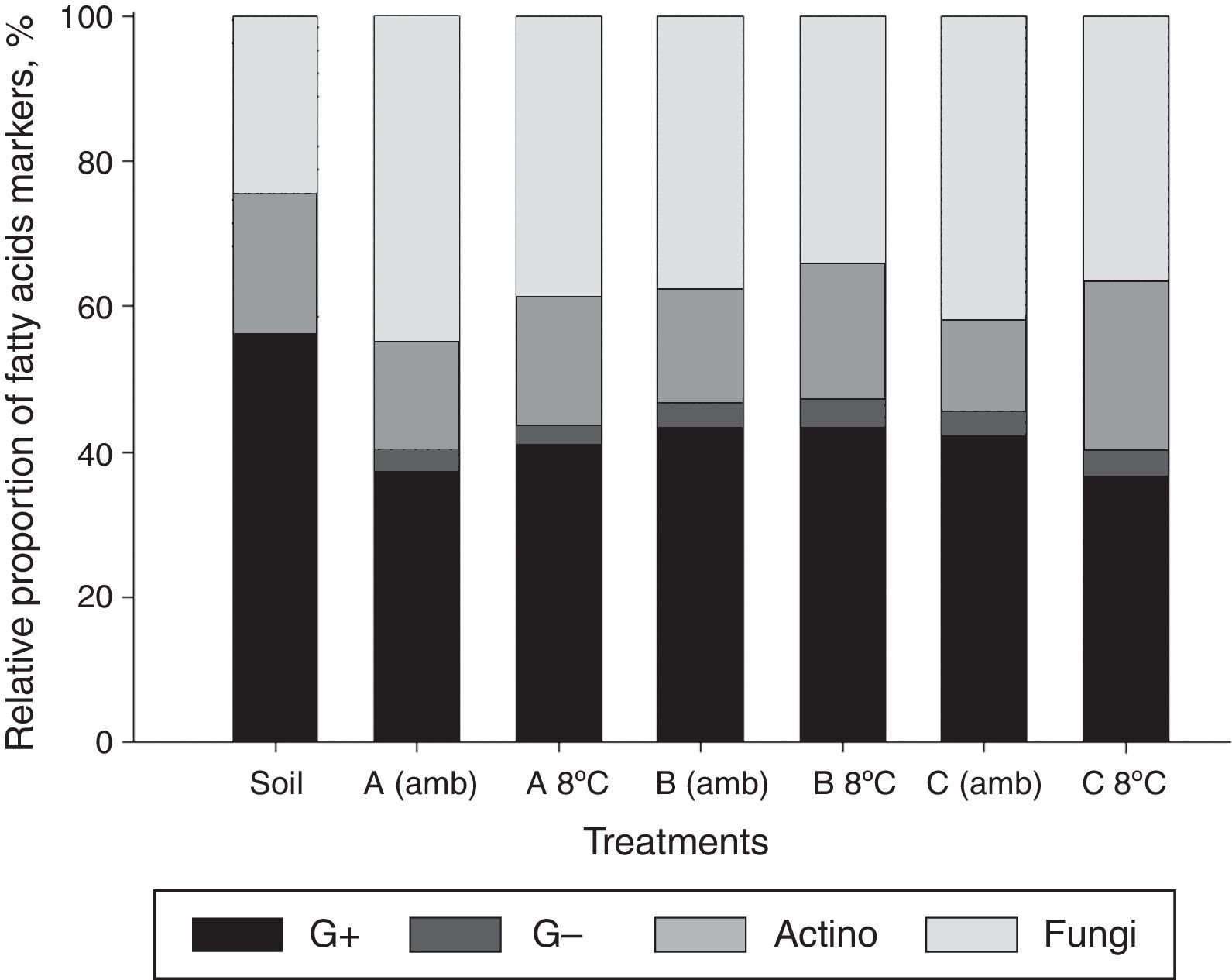
After storage, a reduction was noted in the relative proportion of fatty acids marker from Gram-positive bacteria, while that of markers for fungi and actinobacteria were increased (Fig. 6). The relative proportion of markers for Gram-negative bacteria remained constant for inoculant A, but those of inoculants B and C decreased.
The relative proportion of fatty acids markers for microbial inoculants groups before and after storage for 103 days at the room temperature (amb) and under refrigeration (6–8°C). Gram-positive (G+), Gram-negative (G−), actinobacteria (actino), and fungi. Inoculant A (A); B (B); and C (C). Inoculant C refers to MSWC receiving nutrients and water as well as inoculants A and B, but no gasoline application for the enrichment of hydrocarbonoclastic populations.
The storage at both the temperatures resulting in changes on the total fatty acids profiles of inoculants (Fig. 7). PCA analysis revealed that the fatty acid profiles of the samples after storage at room temperature or under cooling was mostly similar to each other than with the samples before storage (Fig. 7). Some discrepancies were noticed in the profiles of fatty acids of the same sample stored at different temperatures (Fig. 7), demonstrating the effect of this variable on the composition of the microbial community of inoculants.
Principal component analysis (PCA) of the fatty acid profiles of gasoline inoculants before (bs) and after storage at room temperature (amb) and under refrigeration (8°C). A, B, and C: samples collected immediately after the incubation period MSWC with different doses of gasoline. Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
Changes in the composition and abundance of fatty acids of inoculants were observed after storage. The total number of fatty acids reduced; this decrease was more accentuated for inoculant C, 41–29 under refrigeration and 30 at room temperature. The inoculants A and B, which received fuel application even before storage showed a smaller range of total fatty acids than inoculant C without fuel addition (35 fatty acids for both inoculants). This observation can be attributed mainly to the reduction in the number of chain fatty acids between 10 and 16 carbons, probably because of the effect of adding gasoline to MSWC. This effect can be attributed to the toxicity of light hydrocarbons as the enrichment of a smaller number of populations. In both the cases, the final result was a reduction in the microbial diversity. Under refrigeration condition, the reduced diversity of total fatty acids was lesser than that at room temperature.
The main effects of storage, regardless of the temperature used on total fatty acids, were decreased number and amount of chain fatty acids (10–16 carbons) and increased abundance of chain fatty acids (17–20 carbons) (data not shown).
The storage effect of inoculants in their efficiency to stimulate biodegradation of gasoline, impact on cultivable bacterial populations, and soil microbial diversityThe storage temperature effect of inoculants on the emission of CO2 from soil contaminated with gasolineThe respirometric assays with gasoline-contaminated soil inoculated with 30g/kg of inoculants revealed interaction between the type of inoculant added to the soil and the storage temperature. Maximum CO2 emission was obtained for treatments with inoculants stored under refrigeration (Fig. 8). However, even when stored at the room temperature, the inoculants stimulated CO2 emission from the contaminated soil as compared to uninoculated one.
The total CO2 production (μmol CO2g−1) from gasoline-contaminated soil after application of the inoculants stored at different temperatures. The treatment means followed by the same letter do not differ at 5% probability by Tukey's test. Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
The treatments with inoculants showed that the cultivable bacterial count was significantly higher than that of uninoculated soil (Fig. 9), regardless of the storage temperature. The bacterial count in the soil with inoculant A stored at room temperature was greater than that of soil treated with the same inoculant stored under refrigeration. The opposite was observed with the treatments receiving application of inoculant C. No effect of inoculant B under storage condition was noticed on the count of cultivable heterotrophic bacteria in the soil after the biodegradation experiment.
The count of heterotrophic bacteria in gasoline-contaminated soil after application of inoculants stored at room temperature (amb) and refrigeration (8°C). Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
In the treatment group with no inoculation (Soil), predominated markers of fatty acids of Gram-positive bacteria were found (Fig. 10). No Gram-negative bacteria markers were detected in this treatment, but this group was detected in all treatments with inoculation. In these treatments, a predominance of markers of Gram-positive bacteria and fungi were observed. The relative proportion of fatty acid markers for actinobacteria was higher in treatments with the application of inoculants stored under refrigeration, concurrently with the decrease in fungal markers. The relative proportion of Gram-negative bacteria remained the same according to storage temperature.
The proportion of fatty acid markers on the microbial groups of gasoline-contaminated soil after application of inoculants stored at room temperature (amb) and under refrigeration (8°C). Gram-positive (G+), Gram-negative (G−), actinobacteria (actino), and fungi. Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
According to the PCA analysis for the total fatty acids in soil, the corresponding treatment of uninoculated soil revealed the most distinctive fatty acid profile, showing the effect of inoculation of soil on the profile of fatty acids on the same (Fig. 11). The samples corresponding to the inoculated treatments formed a relatively homogeneous group, with the exception of the treatment receiving application of inoculant C stored at room temperature.
Principal component analysis (PCA) of the soil fatty acid profiles after application of inoculants stored at room temperature (amb) and under refrigeration (8°C). Soil refers to uninoculated control. Inoculant A (A); B (B); and C (C). Inoculants A and B received nutrients plus water and gasoline in distinct times for the enrichment of hydrocarbonoclastic populations. Inoculant C refers to MSWC with no gasoline added.
In this work, we demonstrated that the addition of MSWC, enriched or not with microbial hydrocarbonoclastic populations, promoted the biodegradation of hydrocarbons from gasoline-contaminated soils. Chen et al.10 showed that, among the techniques for soil decontamination by petroleum hydrocarbons, the use of MSWC was efficient and inexpensive, as it increases the content of organic matter as well as the fertility of the soil, causing an increase in the density and diversity of microorganisms in the compound.
The dose effect of the inoculants was examined by the respirometric assays for soil contaminated with gasoline (Fig. 1), as well as by counting the numbers of cultivable soil bacteria (Fig. 2). We observed a direct relationship between the frequency of adding gasoline to MSWC during the preparation of inoculants (and therefore final hydrocarbon concentration) and the population density of heterotrophic bacteria in the gasoline-contaminated soil receiving inoculants application. This relationship was consistent with the further degradation of hydrocarbons from gasoline (measured by CO2 emission) in the soil applied with the highest dose of inoculants A and B. After inoculation in the soil in the approximate range of 100h to 175h (Fig. 1), a sharp increase in CO2 emissions were noted. The result indicated that, in this period, the microbial community adapted/selected utilized more labile hydrocarbon from gasoline, as compared with that after the 175th hours, in which the CO2 emission rates were reduced. The fact of all inoculated samples presents this shift simultaneously, despite having different accumulated CO2 (indicating that, in some treatments, labile fractions probably remain available), may indicate the loss of hydrocarbons by volatilization. The microcosms were mounted on respirometric 750mL flasks coupled to automatic respirometer. Each microcosm was swept with a stream of 500mL/min air for approximately 5min at every 4.2h. These recurring airflows could have caused loss of much hydrocarbons by evaporation, explaining how the difference in the accumulated CO2 values (and hence with different overall degradation) reduced the CO2 emission intensity at the same time. This observation was confirmed by the corresponding treatment, in which a lag phase that lasted until a later time was noted with the decline in CO2 emissions by the inoculated samples. The exponential growth phase of CO2 emission was extremely short. At the end of the incubation period, the shape of CO2 emission curves approaching of the inoculated treatments. This observation proves that the hydrocarbon exhaust in this treatment in terms of biodegradation (CO2 emissions) was extremely low (compared to that in other treatments). Therefore, substrate limitation in this treatment may have been caused by the frequent injection of air in the microcosm.
The higher count of cultivable bacteria in soil applied with the inoculants A and B as compared to inoculant C demonstrated that the procedure of adding fuel to the MSWC favored the enrichment of hydrocarbonoclastic bacterial populations adapted to the toxic effect of this fuel. The increase of the fungal markers after the inoculation in contaminated soil indicated that this group can be related with the biodegradation of hydrocarbons. This observation was consistent with the experiments of Sanni et al.,23 who revealed that prior exposure of microbial communities induces a physiological selection and adaptation of microbial populations capable of catabolizing it. Fungal was the group with major increase after the inoculation (Inoculants A, B and C) (Fig. 3), therefore, we suggest that the greater hydrocarbonoclastic abilities in soil may be provided by fungal populations.
The extended lag phase observed in the production of CO2 on the uninoculated treatment may indicate a toxic effect of gasoline on the native soil organisms24,25 or even at a low initial density. In the Brazilian commercial gasoline, both monoaromatic hydrocarbons benzene, toluene, xylene, and ethanol (BTEX) contribute to the toxicity of the fuel. Due to the lipophilic property of BTX, they accumulate in the cell plasma membrane, causing unspecific permeabilization thereof.26–30 Thus, these compounds interfere with the membrane integrity as well as their function as a barrier matrix for enzymes and as an energy transducer.27,28
The enumeration of heterotrophic bacteria through plate culture after storage with and without refrigeration (Fig. 5) confirmed that cell storage at low temperatures can be considered in routine practice for maintaining cell viability over a long period of time. However, in soils receiving inoculant A after storage, a heterotrophic bacterial density of approximately 30% greater was noted in the treatment with inoculants stored at room temperature in comparison with inoculant stored under refrigeration (Fig. 9). Given these population data, the maximum CO2 emission was noted in the treatment with the highest density of cultivable heterotrophic bacteria. However, this relationship was not confirmed, and the greater evolution of CO2 was observed for treatments when the inoculants were stored under refrigeration. These data reveal that bacterial populations of uncultivable, cultivable cell populations, viable but not cultivable (VBNC), and others (e.g., fungi and Archaea) were better in the degradation of hydrocarbons from gasoline studied in the microcosm system. The results emphasized the advantage of using microbial inoculants as MSWC because it may contain uncultivable microbial populations important for the biodegradation of contaminant complexes, as exemplified by the mixture of hydrocarbons detected in gasoline. Specifically for hydrocarbons, no work related to VBNC state cell activity has been reported for the degradation of these compounds. In this state cell, bacterial cells are alive and capable of performing metabolic activity, albeit unable to grow in culture media employed routinely for growth.31,32 However, some authors have reported that bacterial cells in the VBNC state can degrade polychlorinated biphenyls33–35 as well as some hydrocarbons, which are highly recalcitrant compounds.36 Thus, it can be inferred that cells in this state can also participate in the catabolism of hydrocarbons in contaminated environment. Zhang et al.37 showed that, in oil-contaminated soils, the physiologically active microbial community was larger than the cultivable bacterial community. The ability to grow in a culture media is not necessarily directly related to the abundance of a particular microorganism in the soil. Stefani et al.38 analyzed oil-contaminated soil by dependent and independent cultivation methods and found that some taxa, although being present in great abundance in the soil, cannot be cultivated even when different culture media is used.
The changes in the fatty acid profile of the inoculated soil indicate that the microorganisms present in the inoculants could thrive in the gasoline-contaminated soil (Figs. 3 and 10). The effects of inoculation in the soil were the appearance of fatty acid marker Gram-negative bacteria (16:1ω5c) in some treatments, reduced actinobacteria markers, and increased fungi markers. The increased relative proportion of fungi markers seems to be related to the prevalence of this group in MSWC (Fig. 6) as well as its hydrocarbonoclastic and adaptative abilities in different storage temperatures. Although actinobacteria are extremely versatile and efficient in degrading a wide range of hydrocarbons,37,39 inoculation may have reduced some populations of this group, possibly as a result of competition from other microbial populations originating from inoculants.
The storage temperature of the inoculants influences their efficiency in stimulating hydrocarbon degradation in soil. Comparing the same treatments with the application of inoculants to gasoline-contaminated soil before and after storage revealed a reduction in the degradation rate and total degradation of the fuel in the treatments applied with the stored inoculants. This observation may be related to the reduction of cultivable bacterial population in the inoculants after storage (Fig. 6).
Changes in the relative proportion of the fatty acid markers after inoculation of soil with inoculants stored for 103 days included the appearance of Gram-negative bacteria markers, reduction in the counts of Gram-positive markers, and increased fungal markers (Fig. 10). This effect was similar to that observed when newly produced inoculants were applied to soil, (Fig. 3) except when no significant reduction in the count of Gram-positive bacteria was noted (in various treatments, the effect was the opposite, that is, an increased number of Gram-positive markers were recorded). The absence of Gram-negative bacterial markers in gasoline-contaminated soil and uninoculated soil were noted, although a wide range of representatives of this group possess the ability to catabolize hydrocarbons,40,41 suggesting that this bacterial group was either absent or present in negligible quantity in the soil or MSWC. This observation can be attributed to the soil and MSWC storage conditions used that were dry at room temperature for a few weeks prior to this study. The osmotic stress subjected to the soil and MSWC favored the reduction/disappearance of Gram-negative bacteria, while Gram-positive, actinobacteria, and fungi were found to be more resistant to this stress.42,43 In addition, gasoline-contaminated soil and the toxic effects of light hydrocarbons in this fuel over the microbial cells may have led to the extinction of the few members of some population of Gram-negative bacteria in the soil.
ConclusionsMSWC is a suitable substrate for the development of microbial inoculants for the bioremediation of gasoline-contaminated soils. The efficiency of microbial inoculants for the degradation of hydrocarbons from gasoline is influenced by the inoculant dose applied to the soil. At doses >10g/kg, inoculants enriched with frequent additions of gasoline were more efficient than only MSWC enriched with mineral nutrients (N, P) in degradation. The storage temperature influenced the efficiency of inoculants on hydrocarbon biodegradation in the gasoline constituents of soil, of which refrigerated storage was found to be more efficient than storage under room temperature.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq).