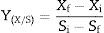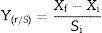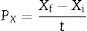Edible mushroom species are considered as an adequate source of food in a healthy diet due to high content of protein, fiber, vitamins, and a variety of minerals. The representatives of Pleurotus genus are characterized by distinct gastronomic, nutritional, and medicinal properties among the edible mushrooms commercialized worldwide. In the present study, the growth of mycelial biomass of Pleurotus albidus cultivated in submerged fermentation was evaluated. Saccharose, fructose, and maltose were the three main carbon sources for mycelial biomass formation with corresponding yields of 7.28gL−1, 7.07gL−1, and 6.99gL−1. Inorganic nitrogen sources did not stimulate growth and the optimal yield was significantly higher with yeast extract (7.98gL−1). The factorial design used to evaluate the influence of saccharose and yeast extract concentration, agitation speed, and initial pH indicated that all variables significantly influenced the production of biomass, especially the concentration of saccharose. The greater amount of saccharose resulted in the production of significantly more biomass. The highest mycelial biomass production (9.81gL−1) was reached in the medium formulated with 30.0gL−1 saccharose, 2.5gL−1 yeast extract, pH 7.0, and a speed of agitation at 180rpm. Furthermore, P. albidus manifested different aspects of morphology and physiology under the growth conditions employed. Media composition affected mycelial biomass production indicating that the diversification of carbon sources promoted its improvement and can be used as food or supplement.
Pleurotus albidus (Berk.) is a species that occurs in Mexico, Argentina, and Brazil. It is glabrous, white to cream colored and presents infundibuliform and circular pileus, deeply decurrent lamella, stipe fluted upward through lamellar bases, tapering downward, usually curved-ascendant, dull white and occasionally with hairs at the base.1–3 The Pleurotus species, commonly known as oyster mushrooms, have gastronomic, nutritional, and medicinal properties and are grown in the temperate and tropical rainforests. Nowadays, seventy species of this genus are reported, but only few of them are commercialized, including P. ostreatus, P. florida, P. sajor-caju, and P. eryngii.4
It has long been known that mushrooms develop biomass through degrading cellulose and lignin by the action of specific enzymes under optimal conditions.5,6 They can be cultivated at large scale using techniques of solid state and submerged cultures. Both methods use agroindustrial waste, although in submerged fermentation, the conditions are monitored with greater precision when compared to solid state fermentation.7,8
Submerged fermentation is a viable alternative for efficient Pleurotus biomass production. This process has a number of advantages, such as a better control of physicochemical parameters, as well as the potential for high biomass production in a compact space, shorter growth time, and independent of seasonality.9,10
In submerged fermentation of Pleurotus, no fruiting body production occurs, and the mycelial biomass develops in two forms: filamentous and pellet. Pellets are characterized by mycelia development into spherical aggregates, which are a branched and partially intertwined network of hyphae.11,12 The filamentous form consists of hyphae homogenously spread in the culture medium which is more viscous than that of the pellet form. The mycelial biomass produced by submerged fermentation can be used as an inoculum source for mushroom production in semi-solid fermentations,1 in food additives, and for extraction of antimicrobial compounds, flavorings, polysaccharides, antioxidants, etc.10
Aimed at the improvement of Pleurotus cultivation technology, the present study investigated the influence of physicochemical parameters of synthetic media on mycelial biomass production by P. albidus in submerged fermentation.
Material and methodsFungusP. albidus DPUA 1692 was obtained from Federal University of Amazonas DPUA Culture Collection, Brazil. All stock cultures were maintained on a potato dextrose agar (PDA) supplemented with 0.5% (w/v) yeast extract (YE) (HiMedia, Mumbai, India) slant, subcultured and incubated at 25°C for eight days in the dark and then stored at 4°C.13
Effect of inoculum volume on mycelial biomass productionAn aqueous homogenate of mycelium, previously subcultured on PDA+YE, was prepared using 50mL of sterilized distilled water (per dish) in a blender. Different volumes (0.5, 1.0, 2.0, 4.0, and 6.0mL) of the mycelial homogenate were added into Erlenmeyer flasks (125mL) containing 50mL of standard medium (SM): KH2PO4 (1.0gL−1), MgSO4·7H2O (0.5gL−1), peptone (10.0gL−1), and glucose (20.0gL−1) at pH 6.0. The flasks were incubated for five days at 25°C, under aerobic conditions at 150rpm, and tests were carried out in triplicate.14
Effect of carbon and nitrogen sources on mycelial biomass productionFollowing nutrients were analyzed: glucose, fructose, maltose, saccharose, and lactose, as carbon sources; and peptone, yeast extract, tryptone, sodium nitrate and ammonium sulfate, as nitrogen sources. The glucose and peptone in the SM were replaced separately according to the carbon and nitrogen sources mentioned. The aqueous homogenate of mycelium (6.0mL) was added to the flasks, and submerged fermentation was performed as mentioned above.
Determination of dry cell massAt the end of fermentation, biomass was collected by vacuum filtration, washed three times with distilled water, and dried at 70°C for dry-weight quantification.15
Determination of total carbohydratesThe total carbohydrate concentration was determined colorimetrically by the phenol-sulfuric acid method according to the protocol described by Dubois et al.16
Conversion factor of substrate to biomass, yield and productivityThe conversion factor of substrate to biomass and the yield and productivity were calculated according to Confortin et al.17
The conversion factor of substrate to biomass was expressed in grams of mycelial biomass produced per gram of substrate consumed (gg−1):
where Xf and Xi are final and initial biomass concentration, respectively, and Si and Sf are initial and final sugar concentration, respectively.The yield was expressed in grams of mycelial biomass produced per gram of substrate initially present in the culture medium (gg−1):
The productivity in biomass was expressed in grams of mycelial biomass produced per time (gL−1h−1)17:
where t is the incubation time.Factorial design and statistical analysisA 24 factorial design was used to evaluate saccharose concentration, yeast extract concentration, agitation speed, and initial pH (independent variables) on mycelial biomass production (dependent variable).18 In previous experiments, saccharose and yeast extract were selected as the best carbon and nitrogen sources, respectively. SM was used with saccharose and yeast extract at different experimental concentrations. Submerged fermentation was conducted, and the biomass quantified as described previously. All statistical analyses were performed via Statistic 8.0 software.
All results of the submerged fermentation experiments were subjected to variance analysis (one-way ANOVA), and the values were compared by Tukey's test at p≤0.05.
Morphology of the mycelial biomassThe morphology of the mycelial biomass produced under the factorial design was evaluated using the software Analysis FIVE Olympus and a stereomicroscope (Olympus SZ61, Ltd., Tokyo, Japan). The samples were fixed with a solution composed of 13mL of 40% (v/v) formaldehyde, 5mL of acetic acid, and 200mL of 50% (v/v) ethanol.19
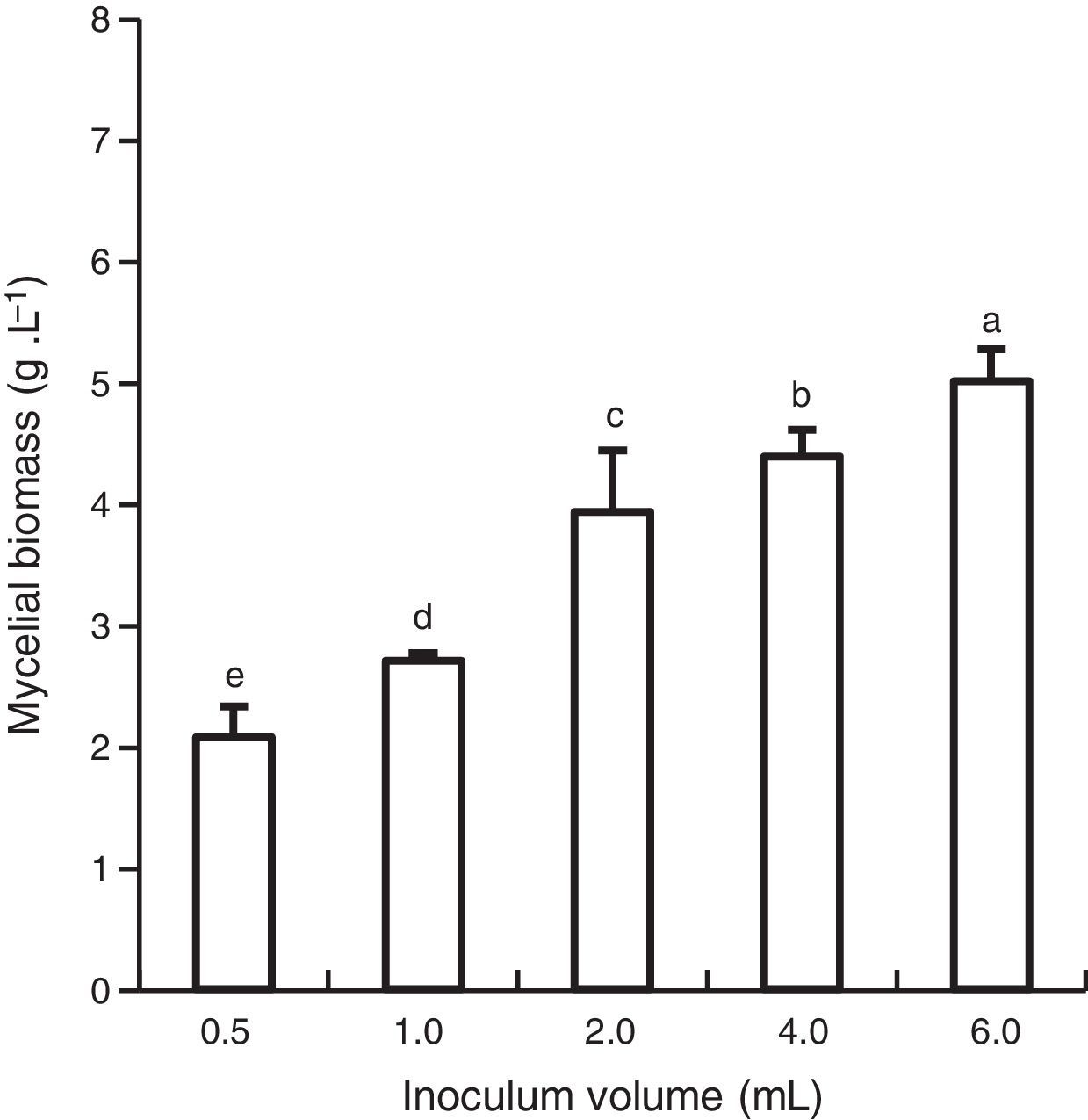
Results and discussionEffect of inoculum sizeMycelial biomass production varied significantly depending on the inoculum size (Fig. 1). The volume of 6.0mL induced higher biomass production (5.00gL−1). Therefore, this volume was used for further experiments.
In investigations on P. tuber-regium14 and Grifola frondosa,20 the inoculum size with a cell suspension volume ranging from 0.5 to 12.0mL, or 2 to 6%, had a higher positive influence on mycelial growth in comparison to the medium volume in submerged fermentation.
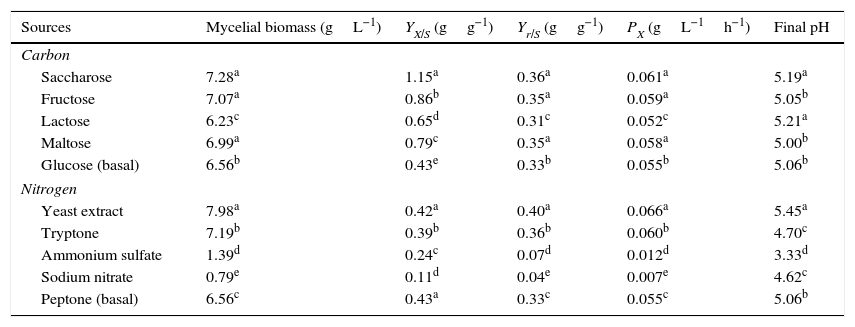
Effect of carbon and nitrogen sourcesAll carbon sources tested promoted P. albidus growth (Table 1). However, saccharose (7.28gL−1), fructose (7.07gL−1), and maltose (6.99gL−1) were the carbon sources that promoted the most considerable growth. Although the mushroom manifested the highest mycelial biomass production in the treatment with saccharose, the consumption of this sugar during the fermentation process was twice lower than that of fructose. This result might have been caused by the nature of the carbon source, because saccharose is a disaccharide which is necessary for invertase excretion, and after decomposition into glucose and fructose, it can be absorbed by the fungus.27
Mycelial biomass, conversion factor of substrate to biomass (YX/S), yield (Yr/S) and productivity (PX) of P. albidus under different carbon and nitrogen sources.
| Sources | Mycelial biomass (gL−1) | YX/S (gg−1) | Yr/S (gg−1) | PX (gL−1h−1) | Final pH |
|---|---|---|---|---|---|
| Carbon | |||||
| Saccharose | 7.28a | 1.15a | 0.36a | 0.061a | 5.19a |
| Fructose | 7.07a | 0.86b | 0.35a | 0.059a | 5.05b |
| Lactose | 6.23c | 0.65d | 0.31c | 0.052c | 5.21a |
| Maltose | 6.99a | 0.79c | 0.35a | 0.058a | 5.00b |
| Glucose (basal) | 6.56b | 0.43e | 0.33b | 0.055b | 5.06b |
| Nitrogen | |||||
| Yeast extract | 7.98a | 0.42a | 0.40a | 0.066a | 5.45a |
| Tryptone | 7.19b | 0.39b | 0.36b | 0.060b | 4.70c |
| Ammonium sulfate | 1.39d | 0.24c | 0.07d | 0.012d | 3.33d |
| Sodium nitrate | 0.79e | 0.11d | 0.04e | 0.007e | 4.62c |
| Peptone (basal) | 6.56c | 0.43a | 0.33c | 0.055c | 5.06b |
Each value represents an average of three replicates. Means followed by the same letter(s) are not significantly different by Tukey's test (p≤0.05).
Monosaccharides, such as fructose and glucose, are carbon sources that stimulate Pleurotus growth in submerged fermentation.21,22 However, the preference for saccharose as a carbon source has also been reported for Antrodia cinnamomea,23Cordyceps militaris,24C. sinensis,15 and C. jiangxiensis.25 Considering costs and ease of production, saccharose can be considered a more suitable substrate compared to other synthetic sources available on the market.
In our study, saccharose was more efficiently converted into biomass (1.15gg−1) with a higher productivity (0.061gL−1h−1), whereas biomass and productivity were 0.86gg−1 and 0.059gL−1h−1, respectively, for fructose. These values were higher than those reported for Pleurotus sajor-caju (0.82gg−1 and 0.085gL−1h−1) in a liquid medium containing an initial concentration of 10gL−1 glucose and 0.59gg−1 and 0.041gL−1h−1 for 10gL−1 saccharose.17
The addition of sodium nitrate and ammonium sulfate in the culture media did not favor mycelial biomass production. The maximum values of biomass formation were 0.79 and 1.39gL−1, respectively (Table 1). These findings are in accordance with those obtained for C. militaris24 and G. frondosa.20 Overall, it seems that inorganic nitrogen does not promote growth of several fungi species, in contrast to organic sources.24,26
Deacon27 describes that as a result of ammonia metabolism, H+ ions are released, and the pH of the medium is reduced, inhibiting the growth of some mushroom species. This was also observed when, at the end of fermentation process, the pH of culture media in which ammonium sulfate and sodium nitrate were utilized was below 4.6 (Table 1).
Our results indicated that YE (7.98gL−1) was the nitrogen source that stimulated most pronouncedly mycelial biomass production, which was due to presence of several other nutrients, including carbon sources, growth factors, and vitamins present in YE. The biomass of P. albidus was higher in the culture medium containing YE, and the values obtained for yield and productivity were 0.40gg−1 and 0.066gL−1h−1, respectively, which were significantly higher when compared with the other nitrogen sources evaluated. However, no statistical differences (p≤0.05) were observed between the values for yeast extract and control concerning the values of the conversion factor of substrate to biomass.
The presence of tryptone (7.19gL−1) and peptone (6.56gL1) in culture media also favored biomass production with values that were statistically different (p≤0.05) from those of the inorganic sources of nitrogen. Various organic nitrogen sources have been reported as suitable for mushrooms growth, including meat peptone, polypeptone, yeast extract, and soybean concentrates.20,23,28
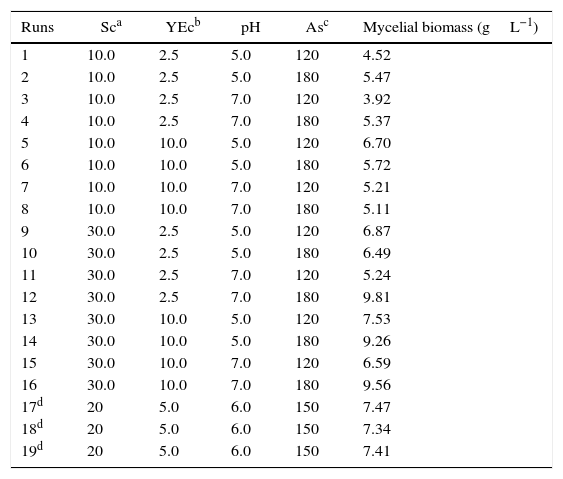
Factorial designThe variables and the results obtained from the runs of the 24 full factorial design are listed in Table 2. The mycelial biomass production ranged from 3.92gL−1 to 9.81gL−1 represented by runs 3 and 12, respectively.
Results of the full factorial design 24 for mycelial biomass production by P. albidus.
| Runs | Sca | YEcb | pH | Asc | Mycelial biomass (gL−1) |
|---|---|---|---|---|---|
| 1 | 10.0 | 2.5 | 5.0 | 120 | 4.52 |
| 2 | 10.0 | 2.5 | 5.0 | 180 | 5.47 |
| 3 | 10.0 | 2.5 | 7.0 | 120 | 3.92 |
| 4 | 10.0 | 2.5 | 7.0 | 180 | 5.37 |
| 5 | 10.0 | 10.0 | 5.0 | 120 | 6.70 |
| 6 | 10.0 | 10.0 | 5.0 | 180 | 5.72 |
| 7 | 10.0 | 10.0 | 7.0 | 120 | 5.21 |
| 8 | 10.0 | 10.0 | 7.0 | 180 | 5.11 |
| 9 | 30.0 | 2.5 | 5.0 | 120 | 6.87 |
| 10 | 30.0 | 2.5 | 5.0 | 180 | 6.49 |
| 11 | 30.0 | 2.5 | 7.0 | 120 | 5.24 |
| 12 | 30.0 | 2.5 | 7.0 | 180 | 9.81 |
| 13 | 30.0 | 10.0 | 5.0 | 120 | 7.53 |
| 14 | 30.0 | 10.0 | 5.0 | 180 | 9.26 |
| 15 | 30.0 | 10.0 | 7.0 | 120 | 6.59 |
| 16 | 30.0 | 10.0 | 7.0 | 180 | 9.56 |
| 17d | 20 | 5.0 | 6.0 | 150 | 7.47 |
| 18d | 20 | 5.0 | 6.0 | 150 | 7.34 |
| 19d | 20 | 5.0 | 6.0 | 150 | 7.41 |
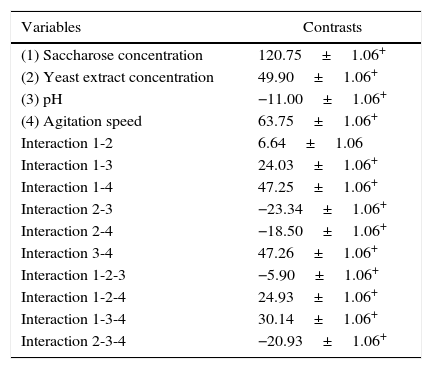
The contrast values were calculated for each of the variables and their interactions from the experiments of factorial design (Table 3). All variables had a substantial influence on mycelial biomass production, and, in terms of their impact, they can be classified in the following decreasing order of significance: saccharose concentration, agitation speed, yeast extract concentration, and pH. For the first three variables the effect was positive, which indicated that the increase of this lower level to the higher level favored biomass production of P. albidus in the fermentation process. The pH was also significant for this response; however, the negative effect showed that the reduction of the initial pH promoted more favorable conditions for production of mycelial biomass. The interactions of pH and agitation speed, and saccharose concentration and agitation speed on the higher level, also favored P. albidus growth.
Contrast values calculated according to factorial design of P. albidus mycelial biomass production.
| Variables | Contrasts |
|---|---|
| (1) Saccharose concentration | 120.75±1.06+ |
| (2) Yeast extract concentration | 49.90±1.06+ |
| (3) pH | −11.00±1.06+ |
| (4) Agitation speed | 63.75±1.06+ |
| Interaction 1-2 | 6.64±1.06 |
| Interaction 1-3 | 24.03±1.06+ |
| Interaction 1-4 | 47.25±1.06+ |
| Interaction 2-3 | −23.34±1.06+ |
| Interaction 2-4 | −18.50±1.06+ |
| Interaction 3-4 | 47.26±1.06+ |
| Interaction 1-2-3 | −5.90±1.06+ |
| Interaction 1-2-4 | 24.93±1.06+ |
| Interaction 1-3-4 | 30.14±1.06+ |
| Interaction 2-3-4 | −20.93±1.06+ |
The highest biomass production was 9.81gL−1, as determined in run number 12 with the culture medium formulated with saccharose (30.0gL−1), yeast extract (2.5gL−1), pH 7.0, and an agitation speed of 180rpm. An R-square value of 0.9341 was obtained for the biomass, indicating that the model explained approximately 93% of the variability data.
Park et al.11 evaluated the influence of different physicochemical parameters on the growth and production of polysaccharides by submerged fermentation in another mushroom species (C. militaris). Their results indicated that among the carbon sources tested, saccharose also exerted a favorable effect on mycelial growth. The growth was proportional to the increase of the initial concentration of this carbon source, ranging from 10gL−1 to 60gL−1 and expressing the maximum growth in the medium containing 60gL−1 of saccharose.
According to Tang et al.,5 the agitated culture medium promoted proper homogenization of components, increased the oxygenation, and improved the mass and heat transfer. These conditions had a positive influence on mushroom mycelial growth by submerged fermentation. Nevertheless, extremely high agitation speeds may also cause shear forces that can damage the hyphae, causing changes in the mycelium, variations in growth rate, and interfere with product formation. The agitation speed of 180rpm in P. albidus cultures was that the most suitable for the mycelial growth in comparison to the others speeds tested. These results differ from those obtained by Kim et al.29 who evaluated the effect of agitation speed and aeration on the mycelial growth of Ganoderma resinarum. The authors reported that when agitation speed increased from 50 to 250rpm, biomass production was reduced by approximately 30% (from 12.7gL−1 to 8.9gL−1).
The use of YE as a nitrogen source in the culture medium to evaluate biomass production by G. frondosa was reported.20 The authors evidenced that the biomass increased as the concentration of the nitrogen source was elevated in the culture medium; and the yeast extract concentration of 6gL−1 was optimal for biomass production. A number of studies have indicated that mushrooms, when grown under the conditions of submerged fermentation, have preference for organic nitrogen sources, such as yeast extract and peptone rather than for the inorganic ones.30,31 Gbolagade et al.32 reported that this preference may be explained by the complex combination of amino acids and carbohydrates present in these organic compounds, resulting in the enhanced fungal growth.
Fermentation medium pH is an extremely important variable in the growth of fungi, as it affects the function of the cell membrane, the structure, and cellular morphology, as well as the processes of uptake of various nutrients and product biosynthesis.27,33 In this research, the results indicated that, in general, the use of lower initial pH in culture medium favored P. albidus growth. At the end of the fermentation process, the pH remained constant in the culture media where the initial pH was 5.0. However, when the fungus started its growth at initial pH 7.0, there was a reduction to pH 5.0. According to Papagianni,33 culture medium composition may affect the initial culture medium pH causing fluctuations in the pH range during fungal growth.
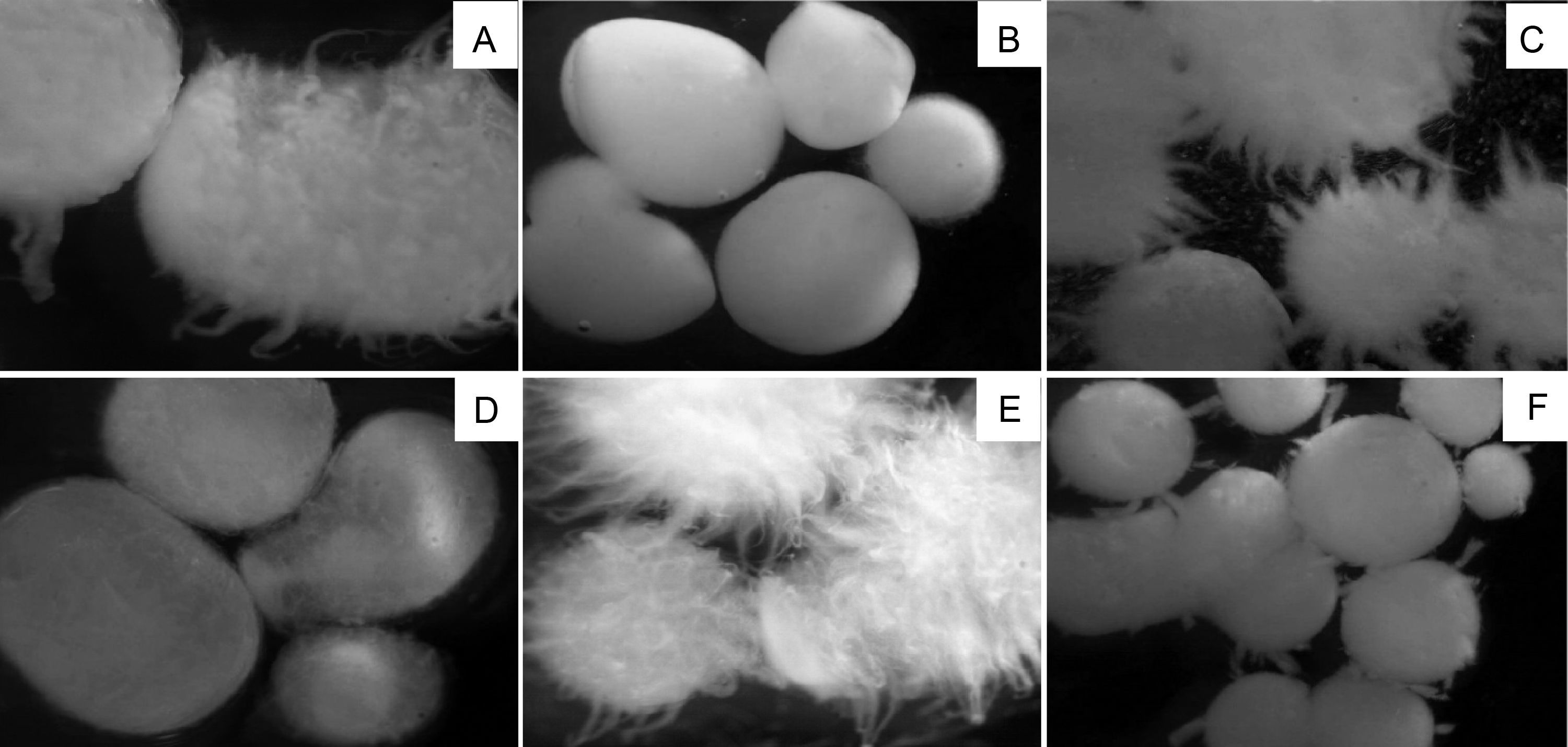
Morphological observations of mycelium in submerged fermentationZnidarsic and Pavko34 and Kim et al.29 reported that the characteristics of the mycelium produced by submerged fermentation are influenced by culture conditions, such as the composition and initial pH of the fermentation medium, the age and size of inoculum, aeration rate, and agitation speed.
The formation of pellets with different sizes and morphology were observed in all fermentation conditions. These pellet characteristics were more evident after variation in the agitation speed, following the alterations in the initial pH of the culture medium. When the process was performed at 120rpm, the pellets were large and few in numbers, whereas at 180rpm, they became smaller in size and larger in quantity. The variation in the size of pellets under different agitation conditions was also reported by other authors.29 They observed the presence of large pellets when Ganoderma resinaceum was subjected to an agitation speed of 50rpm, whereas under more vigorous agitation (300rpm), pellets became small at the end of the fermentation process.
At 120rpm and pH 7.0, growth of elongated and protruding hyphae on the surface of the pellets was observed (Fig. 2a). Under fermentation conditions of pH 5.0, the extensions of these hyphae exhibited more reduced length or were absent (Fig. 2b and d). In a study performed by Hwang et al.,35 the authors verified that Phellinus baumii grown in a liquid medium produced pellets with a starfish-like appearance at a more acidic pH. However, when the pH of the culture medium ranged from neutral to alkaline, pellets became more compact, and no free mycelium was available.
In cultures performed at a higher agitation speed (180rpm), except for run no. 6, pellets were present, and, in general, elongated hyphae were observed on the surface. At pH 7.0 (Fig. 2c and e), hyphae around the pellets were more numerous than at pH 5.0 (Fig. 2f).
Similar results were also obtained by Park et al.,11 who reported that the pellets of C. militaris formed during the fermentation period increased in size independently of the agitation speed. Nonetheless, when the fermentation was conducted under low agitation speed (50rpm), the pellets became large and fluffy, whereas at a high agitation speed (300rpm), the outer hairy regions of the pellets were removed and, consequently, became smaller and changed to a circular shape.
During the fermentation process, P. albidus biomass was observed to adhere strongly to the inside walls of the Erlenmeyer flasks in all growth conditions evaluated. This phenomenon was also observed in previous investigations on submerged fermentation of Ganoderma lucidum, in which the authors reported biomass adherence due to the polysaccharides production.36
When establishing a relationship between the production and biomass morphology of P. albidus, higher mycelial biomass production was found when the pellets were small in size with short hyphae.
ConclusionThe results obtained in this study elucidated some important aspects of the morphology and physiology of P. albidus. These findings can be considered pioneering for the production of mycelial biomass of this mushroom by submerged fermentation. The components of the culture media, including the concentration and type of carbon and nitrogen sources, initial pH, inoculum volume, and agitation speed are main factors that significantly affect biomass production. The experimental design was an effective tool for evaluating the culture factors that most influenced the growth and morphology of the mushroom biomass. This information may be useful for the production of P. albidus mycelial biomass for human consumption.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge the technical and financial support granted by the Culture Collection DPUA and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes)/Brazil.