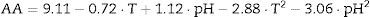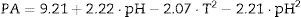The simultaneous production of amylase (AA) and protease (PA) activity by Bacillus subtilis UO-01 in brewery wastes was studied by combining the response surface methodology with the kinetic study of the process. The optimum conditions (T=36.0°C and pH=6.8) for high biomass production (0.92g/L) were similar to the conditions (T=36.8°C and pH=6.6) for high AA synthesis (9.26EU/mL). However, the maximum PA level (9.77EU/mL) was obtained at pH 7.1 and 37.8°C. Under these conditions, a considerably high reduction (between 69.9 and 77.8%) of the initial chemical oxygen demand of the waste was achieved. In verification experiments under the optimized conditions for production of each enzyme, the AA and PA obtained after 15h of incubation were, respectively, 9.35 and 9.87EU/mL. By using the Luedeking and Piret model, both enzymes were classified as growth-associated metabolites. Protease production delay seemed to be related to the consumption of non-protein and protein nitrogen. These results indicate that the brewery waste could be successfully used for a high scale production of amylases and proteases at a low cost.
The amylases and proteases produced by the Bacillus species are of world-wide interest for their important industrial applications.1 Therefore, production of these enzymes should be carried out at a low production cost by using economically available culture media (such as food wastes or agro-industrial residues) and optimized fermentation conditions.2,3
Different studies have shown that production of amylases and proteases is affected by a variety of physicochemical factors, including the type and composition of the substrate, incubation time and temperature, pH, agitation and the concentration and type of the carbon and nitrogen sources.1,3 However, in some cases, the effects of these variables on enzyme production have been studied by using the “one variable at a time” method1,3,4 rather than using response surface methodology (RSM). The first method is time consuming and could lead to an incomplete interpretation of the behavior of the system, resulting in a lack of predictive ability, mainly when there are interactions between the independent factors.5 In contrast, the use of RSM allows the reduction in the number of experiments and obtains empirical mathematical models describing the effect (both linear and quadratic) of each independent factor and their interactions on the response variables.5
Considering the substantial availability of brewery wastes (BWs) with a high chemical oxygen demand (COD) at very low prices from a brewery plant in Santiago de Cuba (Cuba), the use of this effluent as a fermentation substrate could offer an attractive alternative for the low cost production of amylases and proteases by Bacillus subtilis UO-01. However, to the best of our knowledge, there is no information available on the use of brewery waste for production of amylases and proteases by Bacillus strains.
In the present study, we investigated the suitability of this waste to support both the growth and enzyme production by B. subtilis UO-01 at different initial values of pH and incubation temperatures. For this purpose, the time courses of the synthesis of amylases, proteases, biomass and total sugars consumption were followed for 30h. Subsequently, the concentrations of enzymes and biomass obtained were modeled by using the corresponding logistic model to smooth the experimental data obtained and reduce the experimental error. The data predicted by the logistic model in each case at the appropriate fermentation time were then used for the RSM analysis to obtain the optimum pH and temperature values for high production of amylases, proteases and biomass. In addition, the enzyme production system was studied under the optimum conditions to verify the effectiveness and the accuracy of the empirical enzyme model obtained. After typifying both the amylase and protease production with the Luedeking and Piret model,6 the relationship between protease production and consumption of total nitrogen and proteins was studied at different culture pH values and at the optimum temperature for growth of B. subtilis UO-01.
Materials and methodsBacterial culturesB. subtilis UO-01, the amylase- and protease-producing strain, was acquired from the Biotechnology Center of the University of Oriente (Santiago de Cuba, Cuba). Stock cultures were maintained at 4°C on nutrient agar slants. The working cultures were prepared monthly from frozen stock cultures and maintained at 4°C on nutrient agar (Cultimed Panreac Química S.A.).
Culture medium preparation, inoculum and fermentation conditionsBrewery wastes (BWs), which were used to prepare the culture media, were obtained from a local brewery in Santiago de Cuba, Cuba. The wastes were centrifuged at 12,000×g/15min to remove the solids in suspension. The supernatant obtained from the BWs contained (g/L): COD, 3.40; total sugars, 1.98; reducing sugars, 1.46; total nitrogen, 0.095; total phosphorus, 0.034.
Inoculum was prepared by transferring (with a sterile inoculation loop) some colonies of a 24-h old slant culture into 250mL Erlenmeyer flasks containing 50mL of sterile medium composed of (g/L): glucose, 20; bacteriological peptone, 2.5; KH2PO4·3H2O, 1.5; Na2SO4, 1.5; MgSO4·7H2O, 0.15, FeSO4·7H2O, 0.03; MnCl2·4H2O, 0.1; CaCl2·2H2O, 0.45. After inoculation, the medium was adjusted to pH 6.8 and sterilized at 121°C/15min. The inoculated culture was then incubated at 36°C/12h (200rpm).
The production BWs medium was supplemented with the same ingredients as the medium used to prepare the inoculum, but in this case, soluble potato starch (at a concentration of 10g/L) was used instead of glucose. The media were buffered at different initial pH values with the appropriate buffer (0.1M potassium hydrogen phthalate–HCl buffer for pH values of 4.0, 4.5 and 6.2 or 0.1M sodium phosphate buffer for pH values of 8.0 and 8.5) according to the experimental design defined in Table 1 and then sterilized (121°C/15min).
Batch cultures were performed in triplicate in 250mL Erlenmeyer flasks containing 50mL of the corresponding buffered medium. Each flask was inoculated with a 2% (v/v) inoculum level (with an absorbance of 0.5 at 600nm) of a 12-h inoculum culture and incubated in an orbital shaker (200rpm) for 30h at the corresponding temperature according to the experimental matrix defined in Table 1.
To study the relationship between nitrogen and protein consumption and protease production by strain UO-01 at different initial pH values, the BWs media were buffered with the same buffers used in the former experiment to obtain initial pH values of 4, 5, 6, 7, and 8. The media were incubated (200rpm) at the optimum temperature for growth of the enzyme-producing bacterium for 21h.
Analytical methodsIn each fermentation, three flasks were collected each 3h, and triplicate samples (runs) were taken from each flask to perform analytical determinations. The COD values were measured at the end of the culture period (30h).
Growth was monitored by absorbance at 600nm and converted to cell dry weight (CDW) from a standard curve. Cells were harvested by centrifugation (12,000×g for 15min at 4°C) of culture samples and washed twice with saline (0.8% NaCl). The culture supernatants were used to measure total sugars (TS), total nitrogen (TN), proteins, enzyme production and COD. The methods for determining total sugars (phenol–sulfuric acid method), nitrogen (micro-Kjeldahl, substituting distillation for a spectrophotometric method) and proteins (method of Lowry) were described in a previous work.7
Amylase activity (AA) of the cell-free medium was determined at 40°C by appropriately mixing the diluted crude enzyme extract (80μL) with 400μL of 0.15M citrate–phosphate buffer (pH 5.0) and 800μL of 4% soluble starch previously maintained at 40°C/15min. The reaction was stopped by immediately adding 480μL of 3,5-dinitrosalicylic acid (DNS). The reducing sugars (glucose equivalents) released from the enzymatic reaction were determine after 10min by using the DNS method. One unit of amylase activity (enzymatic units (EU)/mL) was defined as the amount of enzyme releasing 1mg/mL of reducing sugars/min under the assay conditions.8
Protease activity (PA) of the cell-free medium was determined as described by Tekin et al.9 The reaction mixture with 1mL of 1% (w/v) casein in 0.02M NaOH, 2mL of 0.4M phosphate buffer pH 6, and 1mL of cell-free medium (suitably diluted) was incubated at 30°C/10min. The reaction was stopped with 3mL of 10% trichloroacetic acid, mixed and after 5min, centrifuged at 12,000×g for 5min, then 0.5mL of the supernatants were incubated with 2.5mL of 0.1M NaOH in 2% (w/v) Na2CO3 for 10min. Thereafter, 0.25mL of Folin phenolic reagent (commercial solution diluted 1:1 in distilled water) was added, mixed and held for 30min at room temperature. The absorbance measured at 750nm was converted to mg of tyrosine/L using a calibration curve (mg tyrosine/L vs. absorbance). The tyrosine solutions dissolved in 0.01M HCl were treated in the same manner as the cell-free medium. One unit of protease (EU/mL) was defined as the amount of enzyme that produced an absorbance at 750nm equivalent to 1μmol of tyrosine/min under the assay conditions.
COD analyses were carried out in triplicate in the culture supernatants at the end of each fermentation (30h) using the Standard Methods for the Examination of Water and Wastewater.10
Statistical analysesAfter obtaining the experimental concentrations of biomass (CDW), AA and PA for each sampling time, the data were smoothed by using, in case of growth and PA production, the corresponding form of the logistic model6 and a modified form of the logistic model, in the case of AA:
where CDWmax and CDW0 are, respectively, the maximum and the initial cell dry weight concentration (g/L), b is a constant of proportionality (h−1), t is the time (h) and A0, A1, A2, A3 and A4 are constants in models (2) and (3). The latter models were also adjusted to describe AA production. However, the best fits were obtained with the use of model (2) that includes a term (e(−A1⋅t)) that considers the AA decay observed from the 18h of incubation until the end of the culture. After smoothing the cell growth and enzyme (AA and PA) production curves, the best sampling time to take the data for the corresponding experimental design was selected for studying the combined effect of pH and temperature on the growth and enzyme synthesis by B. subtilis UO-01 in the BWs medium.The central composite (orthogonal) design was based on two levels and two variables and consisted of 13 experiments with four (22) factorial points, four axial points to form a central composite design with α=1.267 and five center points for replication (Table 1). The corresponding smoothed data obtained at 15h of fermentation were used in each case. The results were analyzed by the Experimental Design Module of the Statistica software package (Statistica 12.0 for Windows computer program manual; StatSoft Inc., 2013, Tulsa, OK, USA). The coefficients of the models with p values lower than 0.05 were considered statistically significant. The Fisher's F-test for analysis of variance (ANOVA) was performed on experimental data to evaluate the statistical significance of the model.
Modeling the enzymes production with the Luedeking and Piret modelBoth AA and PA production were modeled by using the Luedeking and Piret model.6 The model parameters in each case were obtained by using the non-linear curve-fitting software of SigmaPlot for Windows version 12.0 (Systat Software, Inc., 2011). The coefficients of the models with p values lower than 0.05 were considered statistically significant.
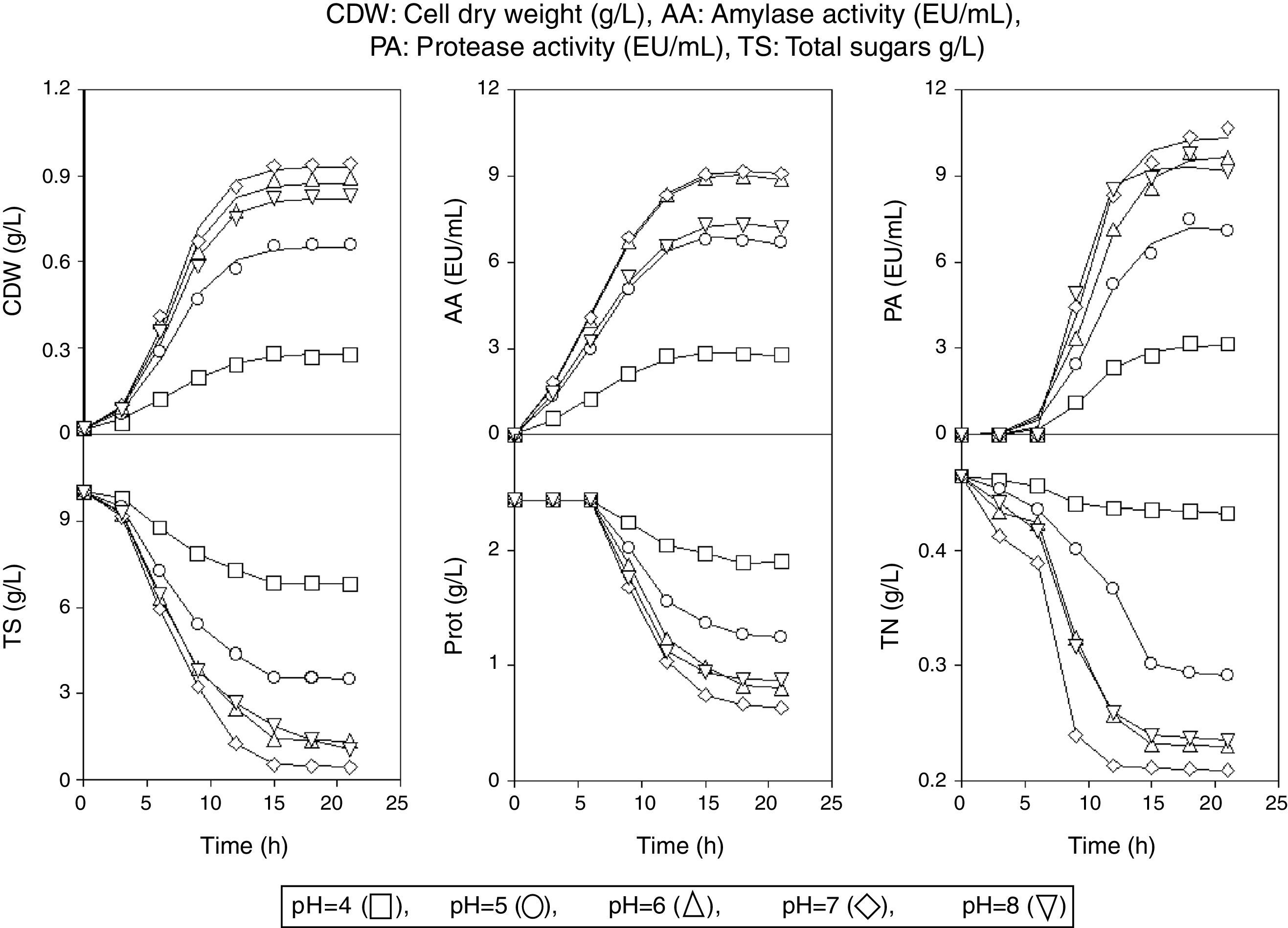
Results and discussionKinetics of growth, enzyme production and total sugars consumption by B. subtilis UO-01 in BWs mediaThe kinetics of growth (CDW), production of amylase (AA) and protease (PA) activity and total sugars (TS) consumption by B. subtilis UO-01 in BWs media was followed in batch cultures at different initial pH values and incubation temperatures (Fig. 1) according to the experimental design defined in Table 1.
Kinetic of production of cell dry weight, amylase and protease activity, and consumption of total sugars by B. subtilis UO-01 at the initial pH and T values, as defined in Table 1. Each data point represents the mean of three independent cultures (the standard errors were less than 5% of the means).
According to the predictions of model (1), biomass production increased until 15h of incubation and remained constant afterwards in all the cultures although, in some fermentations, the total sugars were not totally consumed (Fig. 1). However, the evolution of enzyme (AA and PA) synthesis and biomass production showed some differences. Thus, AA levels increased until reaching the maximum concentration at 15h of culture, then the AA concentration was maintained almost constant until 18h and decreased slightly afterwards (Fig. 1), although not always significantly, because the values obtained for the coefficient A1 in model (2) were not always significant.
Because the presence of starch induces the production of α-amylases,2 during the first 15h of fermentation, AA was synthesized by B. subtilis UO-01 to hydrolyze the starch present in the medium and produce more easily assimilable sugars (glucose and maltose) for the growing strain.1
The observed decline in AA synthesis after 18h of incubation could be related to a possible proteolytic degradation of the amylases by the action of the extracellular proteases produced by the B. subtilis strain.1,2 However, other researchers attributed this AA decrease to a possible denaturation and/or decomposition of the enzyme due to interactions with other compounds in the fermented medium.3
Protease production that reached the maximum PA levels at 15h of incubation (Fig. 1) was only detected after 9h of fermentation in all cultures, when the cells entered the post-exponential phase, as it was observed before for other Bacillus species.11 This behavior has been attributed to the need for obtaining nutrients for post-exponential survival11 or to an increased need for turnover of cell proteins at the slower growth rate.1
Because all the media were buffered at the corresponding initial pH values, the cessation of growth from 15h of fermentation could be related to the following reasons:
- 1.
In the cultures at initial pH≥6.2, the carbon source reached levels considerably lower (<3g/L), except for the cultures at initial pH 8.0 and T=45.5°C and at initial pH 6.2 and T=47.6°C (Fig. 1). These low TS levels could lead to a low availability of the carbon source12 that limits the growth of B. subtilis UO-01.
- 2.
The exhaustion in all the cultures of some nutrient (the sources of nitrogen and/or phosphorus) or micronutrient (vitamins, minerals amino acids or cations) essential for the growth of the enzyme-producing strain, as it was observed before for other bacteria.12,13
The removal percentages of initial COD load in the fermented media are shown in Table 2. The higher COD reductions (69.9–77.8%) were obtained in the cultures characterized by the highest biomass production in the center points of the experimental design, suggesting that B. subtilis UO-01 was able to utilize the organic matter present in the brewery wastewater as a source of nutrients. Thus, bacterial growth and enzyme production by B. subtilis UO-01 could be used as an alternative for removing the initial COD load present in brewery wastes.
Concentrations of biomass (CDW in g/L), amylase (AA in EU/mL) and protease (PA in EU/mL) activity obtained at 15h of incubation and chemical oxygen demand (COD) reduction percentages (%) at the end of the cultures (30h). No.: number of the experiment.
| No. | Coded values | Actual values | Response variable values | |||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | T (°C) | pH | CDW | AA | PA | COD reduction | |
| 1 | 1 | 1 | 45.5 | 8.0 | 0.49 | 3.97 | 7.16 | 40.4 |
| 2 | 1 | –1 | 45.5 | 4.5 | 0.10 | 1.73 | 2.72 | 8.1 |
| 3 | –1 | 1 | 30.0 | 8.0 | 0.68 | 5.30 | 7.50 | 56.3 |
| 4 | –1 | –1 | 30.0 | 4.5 | 0.37 | 3.20 | 3.06 | 30.1 |
| 5 | 1.267 | 0 | 47.6 | 6.2 | 0.35 | 3.08 | 5.42 | 28.8 |
| 6 | –1.267 | 0 | 28.0 | 6.2 | 0.63 | 4.94 | 5.91 | 51.3 |
| 7 | 0 | 1.267 | 37.8 | 8.5 | 0.68 | 5.20 | 8.26 | 56.7 |
| 8 | 0 | –1.267 | 37.8 | 4.0 | 0.25 | 2.25 | 2.64 | 19.9 |
| 9 | 0 | 0 | 37.8 | 6.2 | 0.91 | 9.40 | 8.84 | 74.4 |
| 10 | 0 | 0 | 37.8 | 6.2 | 0.89 | 9.27 | 8.81 | 73.1 |
| 11 | 0 | 0 | 37.8 | 6.2 | 0.93 | 9.40 | 9.48 | 77.8 |
| 12 | 0 | 0 | 37.8 | 6.2 | 0.81 | 8.75 | 9.63 | 69.9 |
| 13 | 0 | 0 | 37.8 | 6.2 | 0.87 | 9.10 | 9.48 | 72.1 |
To typify the production of the two enzymes (E), the classical Luedeking and Piret (LP) model6 was used:
where X is the biomass concentration (g of CDW/L), t is the time (h), qE is the specific enzyme production rate (EU/mg/h), μ is the specific growth rate (h−1), α is a growth-associated constant for enzyme production (EU/mg), and β is the non-growth-associated constant (EU/mg/h).The CDW, AA and PA values (symbols in Fig. 2) predicted by models (1), (2) and (3) (solid lines in Fig. 1) were used to calculate the values of the parameters of the LP model (5).
Modeling the production of amylase and protease activities by B. subtilis UO-01 in BWs media at different temperatures and pH values (experiments 1–13, Table 1). The symbols correspond to the amylase and protease levels obtained, respectively, with models (2) and (3). The solid lines drawn through the symbols were obtained according to the LP model (5). The parameters αAA and αPA are the growth associated constants (EU/mg) for AA and PA productions in the LP model, and R2AA and R2PA are the corresponding correlation coefficients.
The trajectories for the production dynamic of the two enzymes (solid lines in Fig. 2) were calculated by numerical integration of the qE values obtained with the LP model (5) for AA and PA synthesis in the corresponding batch cultures of B. subtilis UO-01.
For both enzymes, significant values (p<0.05) for α and nonsignificant values (p>0.05) for β were always obtained (Fig. 2). Thus, AA and PA can be characterized as growth-associated metabolites (α≠0, β=0). However, AA production (R2AA values between 0.985 and 0.999) was more satisfactorily described by the LP model than PA synthesis (R2PA values between 0.876 and 0.969), mainly because production of the latter enzyme started after 9h of incubation, while the growth and AA production started from the beginning of the culture (Fig. 2).
Effect of initial pH and incubation temperature on the growth and production of amylases and proteasesThe results on the effect of initial pH and temperature on production of biomass (CDW), AA and PA by B. subtilis UO-01 in BWs medium after 15h of incubation are shown in Table 2.
The empirical models obtained with their significant coefficients are:
Because the p values were higher than α (α=0.050) and the F values were lower than 6.390 for the three models, there is a statistically significant relationship between the response and the independent variables at the 95% confidence level. The higher values of the adjusted determination coefficient (adj R2=0.989, 0.983 and 0.989) obtained for these models also indicated their high significance and excellent adequacy for the experimental data.
The response surfaces generated with the empirical models (6), (7) and (8) are shown in Fig. 3A–C, respectively.
The highest biomass concentration (0.92g/L) predicted by model (6) was obtained at T=36.0°C and pH=6.8. These optimum T and pH values were almost similar to those (T=36.8°C and pH=6.6) obtained for high amylase production (9.26EU/mL). The same behavior was observed before for a B. subtilis strain isolated from soil samples3 and three mesophilic Bacillus (Bacillus sp., B. subtilis and B. amyloliquefaciens) isolates.14
However, comparisons of the optimum amylase level produced by B. subtilis UO-01 with the optimum enzyme productions for other Bacillus strains is very difficult due to the different methodologies used to determine the enzyme activity, the different forms to express the enzyme concentration and the different forms to define the enzyme activity unit. For example, in some cases, the enzyme activity was determined by quantifying the amounts of reducing sugars released from the hydrolysis of starch by incubating the reaction mixtures at temperatures of 40°C,1 50°C14 or 60°C15 for 10min. In these cases, the enzyme activity unit was defined as the amount of enzyme releasing 1μmol of glucose14,15 or maltose1 equivalents from the substrate per min under the assay conditions, and the reaction mixtures had different compositions.
With regard to protease production, the maximum level (9.77EU/mL) predicted by model (8) was obtained at pH=7.1 and T=37.8°C. Similar results were obtained for B. subtilis strain Rand, which produced the maximum relative protease activity (%) at pH=7 and T=37°C.16
As indicated above, comparison of the optimum protease level produced by B. subtilis strain UO-01 with the protease levels found by other researchers is very difficult as a consequence of the different forms of expressing the enzyme concentrations or defining the unit of protease activity. For example, protease levels have been expressed as relative production in U/log10CFU17 or as relative activity (%).16 In addition, the unit of protease activity was defined as the amount of enzyme required to: (i) produce an increase of 0.1 in optical density at 700nm,1 (ii) release 1μg of tyrosine per mL per minute,18 or (iii) to obtain an increase of 0.001 absorbance unit at 450nm per minute.16
The increase in temperature over its optimum value inhibited product (CDW, AA and PA) formation by B. subtilis strain UO-01, probably by suppression of cell viability19 and enzymatic inactivation.20 In contrast, low temperature values may slow down the metabolism of the microorganism20 and consequently, enzyme synthesis, because the latter products were synthesized as growth-associated (primary) metabolites, as it was demonstrated with the LP model (Fig. 2).
The decrease in growth and enzyme (AA and PA) production observed at pH levels lower and higher than the optimum value could be related to a reduction in the metabolic activity of B. subtilis UO-01,3 probably caused by a limitation in micronutrients or nutrient transport.13,21 Inactivation or instability of the enzymes could be other reasons to explain the decrease observed in enzyme production.22
Verification of the predicted results in the optimal conditions for enzyme productionTriplicate incubation experiments were carried out under the optimized conditions for AA and PA productions to verify the results predicted by models (7) and (8). The experimental data for growth and production of AA and PA in both cases were smoothed by using the models (1), (2) and (3) (Fig. 4). Under the optimal conditions for AA production (T=36.8°C and pH=6.6), the predicted values of biomass, AA and PA after 15h of incubation were, respectively, 0.94g/L, 9.35EU/mL and 9.34EU/mL (left part of Fig. 4). In addition, under the optimum AA conditions, the concentrations of biomass and PA predicted by the empirical models (6) and (8) were 0.91g/L and 9.51EU/mL, respectively.
Kinetics of the production of cell dry weight, amylase and protease activity and consumption of total sugars by B. subtilis UO-01 under the optimum conditions for amylase and protease productions, respectively. Each data point represents the mean of three independent assays (the standard errors were less than 5% of the means).
In the same way, under the optimum conditions for PA production (T=37.8°C and pH=7.1), the mean concentrations of biomass, AA and PA after 15h of incubation predicted by the models (1), (2) and (3) were, respectively, 0.91g/L, 8.97EU/mL and 9.87EU/mL (right part of Fig. 4). Under the optimum PA conditions, the concentrations of biomass and AA predicted by the empirical models (6) and (7) were 0.90g/L and 8.90EU/mL, respectively. Therefore, the models (6), (7) and (8) can be considered to be accurate and reliable for predicting the growth and production of amylases and proteases by B. subtilis UO-01 as a function of initial pH and incubation temperature in BWs media.
As observed in the previous cultures (Fig. 1), protease production started after 9h of incubation in the cultures at the optimal conditions for AA and TA production (Fig. 4). In an attempt to explain the observed delay in protease production, the kinetics of growth and enzyme production were followed in parallel with the evolution of the corresponding inductor nutrient (total sugars and proteins) for each enzyme.
Because both the nutrient consumption and product formation by different bacteria (Lactococcus lactis, L. cremoris, Lactobacillus rhamnosus and Lact. casei) depended on the culture pH,13,21 a series of cultures with strain UO-01 in BWs media adjusted to initial pH values of 4, 5, 6, 7 and 8 was studied. The cultures were incubated at 36°C (the optimum temperature for biomass production) for 21h. Considering that the presence of protein and non-protein nitrogen in the culture media can affect protein consumption,7 the evolution of total nitrogen was also followed in the fermentations.
As observed in Fig. 5, production of biomass and AA increased in parallel with the decreases in TS and TN. In addition, a direct relationship between the time courses of proteins and PA production was observed. In the latter case, proteins decreased at 9h of fermentation when protease production was detected in the culture medium. However, total nitrogen was slightly consumed during the first 6h of incubation, but after this time, the concentration of total nitrogen decreased rapidly, coinciding with the start of the intense protein consumption.
If the time courses of proteins and TN are jointly analyzed, a direct relationship between both variables only exists after 9h of incubation. According to the manufacturers (Pronadisa, Laboratorios Conda, SA), the bacteriological peptone used as the nitrogen source in the BWs media has a total nitrogen and an amino nitrogen content of 15.5% and 3% (w/w), respectively. Thus, B. subtilis UO-01 seemed to consume first the non-protein nitrogen fraction (presumably easier to assimilate) and then the nitrogen present in the proteins of the culture media (Fig. 5), as it was observed before for other bacteria.7,23,24
These observations suggest that the delay in protease production could be closely related to the consumption of non-protein and protein nitrogen by B. subtilis UO-01 (Fig. 5). However, the consumption of both total nitrogen and proteins depended on the culture pH (Fig. 5) as observed before for nutrient (sugars, amino acids and phosphorus) transport in different strains of lactic acid bacteria.13,21
ConclusionsThe results obtained in this work showed that brewery wastes could be used for enzyme (amylases and proteases) production by B. subtilis UO-01, with the additional advantage of an effective and cost-effective reduction of the initial COD. Then, the methodological procedure described in this work and the results obtained could be used as a reference for the production of other important microbial metabolites in brewery wastes and in other effluents from the food industry.
Conflicts of interestThe authors declare no conflicts of interest.