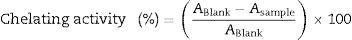Algae can tolerate a broad range of growing conditions but extreme conditions may lead to the generation of highly dangerous reactive oxygen species (ROS), which may cause the deterioration of cell metabolism and damage cellular components. The antioxidants produced by algae alleviate the harmful effects of ROS. While the enhancement of antioxidant production in blue green algae under stress has been reported, the antioxidant response to changes in pH levels requires further investigation. This study presents the effect of pH changes on the antioxidant activity and productivity of the blue green alga Spirulina (Arthrospira) platensis. The algal dry weight (DW) was greatly enhanced at pH 9.0. The highest content of chlorophyll a and carotenoids (10.6 and 2.4mg/g DW, respectively) was recorded at pH 8.5. The highest phenolic content (12.1mg gallic acid equivalent (GAE)/g DW) was recorded at pH 9.5. The maximum production of total phycobiliprotein (159mg/g DW) was obtained at pH 9.0. The antioxidant activities of radical scavenging activity, reducing power and chelating activity were highest at pH 9.0 with an increase of 567, 250 and 206% compared to the positive control, respectively. Variation in the activity of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) was also reported. While the high alkaline pH may favor the overproduction of antioxidants, normal cell metabolism and membrane function is unaffected, as shown by growth and chlorophyll content, which suggests that these conditions are suitable for further studies on the harvest of antioxidants from S. platensis.
Algae can live in a broad range of habitats, which exposes them to abiotic stress such as extreme levels of pH, heavy metals, and salinity. These extreme conditions may lead to the generation of highly dangerous reactive oxygen species (ROS). Unless the ROS can be restrained, it may lead to severe consequences including the deterioration of cell metabolism and damage to cellular components. Algae have developed several mechanisms to alleviate the harmful effects of ROS, including non-enzymatic antioxidants, such as chlorophyll,1 carotenoids,2 phycobiliprotein,3 phenolics,4 and enzymatic antioxidants.5,6 A better understanding of the mechanisms by which algae use these enzymes and non-enzymatic contents may ultimately lead to the production of these antioxidants for the pharmaceutical industry.
While previous studies investigated the effect of pH on the algal growth, pigment production, and protein content of Spirulina sp.,7,8 the direct effect of pH on the antioxidant system requires further elucidation.
The growth of algae may be affected by variations in pH levels in two ways: available carbon alteration, which may interfere with photosynthesis, or through the disruption of cell membrane processes. This may have a direct impact on the accumulation of antioxidants by algae.9 Moreover, factors such as nutrient availability, ionization and heavy metal toxicity, which have large impacts on algal metabolism, are related to both the pH and redox potential of the environment.10 Algae that can tolerate these conditions must have mechanisms that protect cell homeostasis and continue to produce antioxidants.
Spirulina (Arthrospira) platensis, a blue-green alga, is considered a valuable source of natural antioxidants, such as water-soluble phycocyanin pigments, carotenoids, and phenolic compounds, in addition to antioxidant enzymes, such as superoxide dismutase, catalase and peroxidase.11Spirulina species are widely cultivated, not only because they are a biologically active food source but also because of their therapeutic characteristics.12 Several reports claim the ability of Spirulina preparations to reduce blood cholesterol levels, stimulate the immunological system,13 prevent and inhibit cancers,14 reduce the nephrotoxicity of pharmaceuticals and toxic metals15 and provide protection against the harmful effects of radiation.12 These activities are thought to result from the antioxidants produced by Spirulina spp. Therefore, the production of antioxidants is in high demand and has focused on these blue-green algae as a source of biologically active compounds.
The enhancement of antioxidant production in Spirulina sp. under abiotic stress has been previously reported11,16,17; however, the antioxidant response to changes in pH levels requires further investigation. Therefore, the goal of this study was to monitor the effect of pH on the production and activity of various types of antioxidants produced by S. platensis.
Materials and methodsAlgal species, culturing, and experimental designSpirulina platensis (Gomont) Geitler (MIYE 101) was obtained from the Phycology Lab, Faculty of Science, Zagazig University, Egypt and was grown in Zarrouk medium.18 Growth of the experimental organism was conducted as follows. Forty-nine mL of culture media was sterilized in 125mL flasks. Different pH values, viz., 7.5, 8.0, 8.5 (Zarrouk control), 9.0, 9.5, 10.0, 10.5 and 11.0, were set for the experiment (based on preliminary data). The pH was adjusted at the beginning of the experiment with the help of an 8M NaOH or 1N HCl solution; it has been shown that pH varies only slightly during cultivation.19 Under aseptic conditions, the flasks were inoculated with 1mL (DW, ca. 0.7mg) of previously grown algae (from the mid-log phase of growth). The incubation was conducted at 31±0.5°C with continuous cooling white fluorescent lights (60μmol photonsm−2s−1, measured by using LI-190SB quantum sensor attached to a LI-185B quantum/radiometer/photometer, LI-COR, Inc., USA), and the cultures were hand shaken once daily. The experiments (including the analyses) were conducted in triplicate.
Growth, biomass and pigment analysesThe growth was measured by monitoring the change in absorbance at 560nm (OD560nm) with a spectrophotometer.20 The algal cells were harvested at the late log-phase (after 14 days) of incubation by centrifugation at 10,000rpm for 10min at 4°C and washed thoroughly with 10mM Na2-EDTA, followed by sterile distilled water (twice). The biomass yield (DW) was determined following the procedure of Dönmez et al.21 The quantitative determination of chlorophyll and carotenoid pigments was conducted according to the American Public Health Association.22 The concentration of C-phycocyanin (CPC) and total phycobiliprotein pigment of the cyanobacterial cells was extracted in 0.1M Na-phosphate buffer (pH 7.0) and spectrophotometrically calculated according to the formulae of Bennett and Bogorad.23
Preparation of aqueous extracts of algaeThe aqueous extracts were prepared by homogenizing 100mg of algal DW and mixed with an equal volume of glass beads (0.45–0.50mm diameter) in 2mL sterile distilled water at 4°C. The total time for homogenization was optimized using a light microscope (Leitz Wetzlar, Germany) to ensure complete cell breakage. The homogenates were then centrifuged at 10,000rpm for 10min at 4°C and stored at −20°C until ready for the bioassay.
Determination of the total phenolic compound contentThe total phenolic compound content of algal extracts was determined using a modification of the Folin–Ciocalteu method as described by Kuda et al.24 Briefly, 0.4mL of 10% Folin–Ciocalteu solution was added to 0.2mL of the algal extract. After 3min, 0.8mL of the 10% sodium carbonate was added. The mixture was allowed to stand for 1h at ambient temperature in the dark, and the absorbance was then measured at 750nm. The content of phenolic compounds was expressed as GAE/g DW (using a calibration curve, R2=0.993).
Antioxidant activitiesRadical scavenging activityThe radical scavenging activity was measured according to Sánchez-Moreno et al.25 Briefly, 23.5mg of DPPH (1,1-diphenyl-2-picrylhydrazyl, Sigma–Aldrich, Steinheim, Germany) was dissolved in 100mL of absolute methanol and stored at 4°C until ready for use. This stock solution was diluted 1:10 in methanol for the direct assay. One hundred microliters of each algal extract was added to 3.9mL of diluted DPPH solution in 15mL screw-cap tubes. Due to the coloration of the extracts, it was necessary to prepare a background blank, which consisted of 100μL of each algal extract added to 3.9mL of methanol (without DPPH). The tubes were left on a shaker (200rpm) in the dark for 4h at room temperature, and the absorbance was measured at 515nm using a spectronic 20 (Milton Roy Company, USA). Methanol was used to set the spectrophotometric zero. The radical scavenging activity of the sample is expressed as the percentage discoloration of the DPPH solution using the following equation:
where ABlank is the absorbance of the DPPH solution alone, ASample is the absorbance of the sample within the DPPH solution, ABackground is the absorbance of the sample within the methanol solution excluding the DPPH and at 515nm after 4h. The values obtained were normalized (set at 100) to BHT (2.5μg) as a positive control.Reducing powerThe reducing power was determined as described by Zhu et al.26 Briefly, 0.2mL from the algal extracts was mixed with 0.2mL of 0.2M phosphate buffer (pH 6.6) and 0.2mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was then incubated at 50°C for 20min, and then the tubes were permitted to adjust to room temperature and 0.2mL of 10% trichloroacetic acid was added to the mixture, followed by 0.2mL of 0.1% FeCl3 and mixed and incubated for 5min. Finally, the total volume was achieved by adding 2mL of distilled water and the absorbance was measured at 655nm. The increased absorbance at 700nm of the reaction mixture indicated increased reducing power. The reducing power was calculated as the ΔO.D./mg DW. The values obtained were normalized (set at 100) to BHT (2.5μg) as a positive control.
Chelating activityThe ability of the aqueous extract to chelate Fe2+ was determined using the method described by Puntel et al.27 Briefly, 150μL of freshly prepared 500μM FeSO4 were added to a reaction mixture containing 168μL of 0.1M Tris–HCl (pH 7.4) and 218μL of the aqueous extract of algae. The reaction mixture was incubated for 5min at room temperature before the addition of 13μL of 0.25% 1,10-phenanthroline. The absorbance was subsequently measured at 510nm.
The chelating activity was calculated as:
where ABlank is the absorbance of the ferrous solution alone, and Asample is the absorbance of the sample within the ferrous solution at 510nm. The values obtained were normalized (set at 100) to BHT (2.5μg) as a positive control.Antioxidant enzyme extraction and assayAfter the incubation period, S. platensis cultures were harvested by centrifugation at 10,000rpm for 10min at 4°C, and the pellets obtained were washed with 10mM Na2-EDTA and then twice with distilled water. The algal pellets were homogenized with an equal volume of glass beads at 4°C in 2mL of extraction buffer containing 50mM of phosphate buffer (pH 7.0), 1% polyvinylpyrrolidone (PVP), 0.5% Triton X-100 and 1mM Na2-EDTA. The homogenate was centrifuged at 10,000rpm for 10min at 4°C. The supernatant was used to measure the protein content according to Lowry et al.,28 and the activity of the antioxidant enzymes was determined as follows.
Superoxide dismutase activity (SOD, EC 1.15.1.1)The SOD activity was measured according to Beauchamp and Fridovich.29 One unit of SOD activity was defined as the corrected amount of enzyme (by the negative control) required to result in a 50% inhibition of the nitroblue tetrazolium (NBT) reduction measured at 560nm in comparison with the positive control under the assay conditions described. The activity was expressed in U/mg protein.
Catalase (CAT, EC 1.11.1.6) and peroxidase (POD, EC 1.11.1.7) activitiesThe activity of the catalase and peroxidase was assayed after the method of Kar and Mishra.30 One unit of catalase activity is defined as the amount of enzyme that decomposes 1mmol of H2O2 in 1min under the assay conditions described, whereas one unit of peroxidase activity was defined as the amount of enzyme that produces 1 absorbance change at 420nm per min in the above assay conditions described. The activity was expressed in U/mg protein.
Statistical analysisThe data employed herein were represented as the mean±standard deviation (SD) of at least three independent experiments. All of the statistical analyses were conducted using SPSS 10.0 software (SPSS, Richmond, VA, USA) as described by Dytham.31 The one-way analysis of variance (ANOVA) with Duncan's multiple range tests was used for comparison of the significance level between values at p<0.05. Data that have different letters within each assay were significantly different. Statistical values of p<0.05 were considered significant.
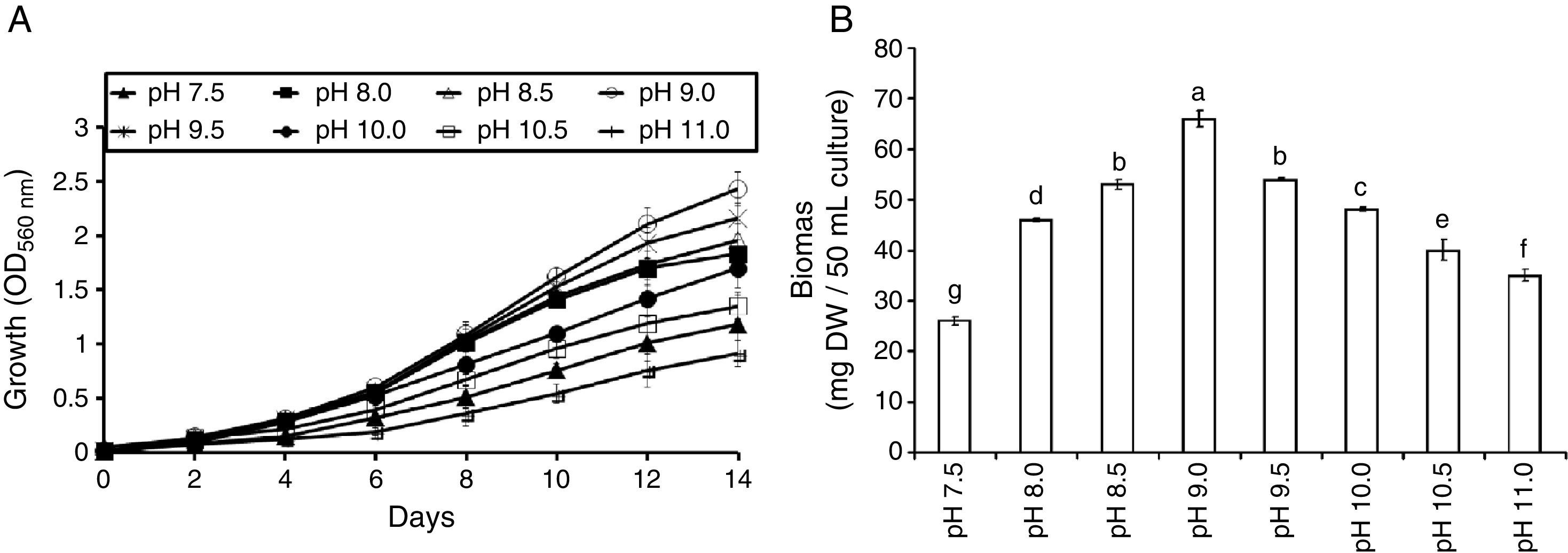
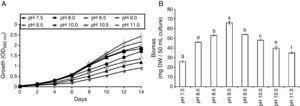
ResultsAlgal growth and biomass productivityThe growth curve of S. platensis showed lag, log and stationary phases. The lag-phase continued for the first two (or the four) days of culturing followed by the exponential phase (log-phase) (Fig. 1A) and lasted until the 14th day of growth when the stationary phase began. The pH level of the medium affects the growth of S. platensis, and the optimum pH for growth was recorded at pH 9.0. A pH below 8.5 or above 9.5 was accompanied by a significant growth reduction, and therefore, these pH levels were assumed to cause stress. Over the wide range of the tested pHs, the highest value of the biomass production was obtained at pH 9.0 (66mg DW/50mL culture, Fig. 1B), which is consistent with Fig. 1A, and a decline in dry biomass was observed at other pH levels.
The growth curves of S. platensis at different pH levels (A). Each point represents the mean value of three replicate determinations; bars indicate standard deviations. Biomass productivity of S. platensis at different pH levels after the 14th day of growth (B). The bars represent the mean±SD of at least three independent experiments. The different letters represent significant differences at p<0.05 (Duncan's).
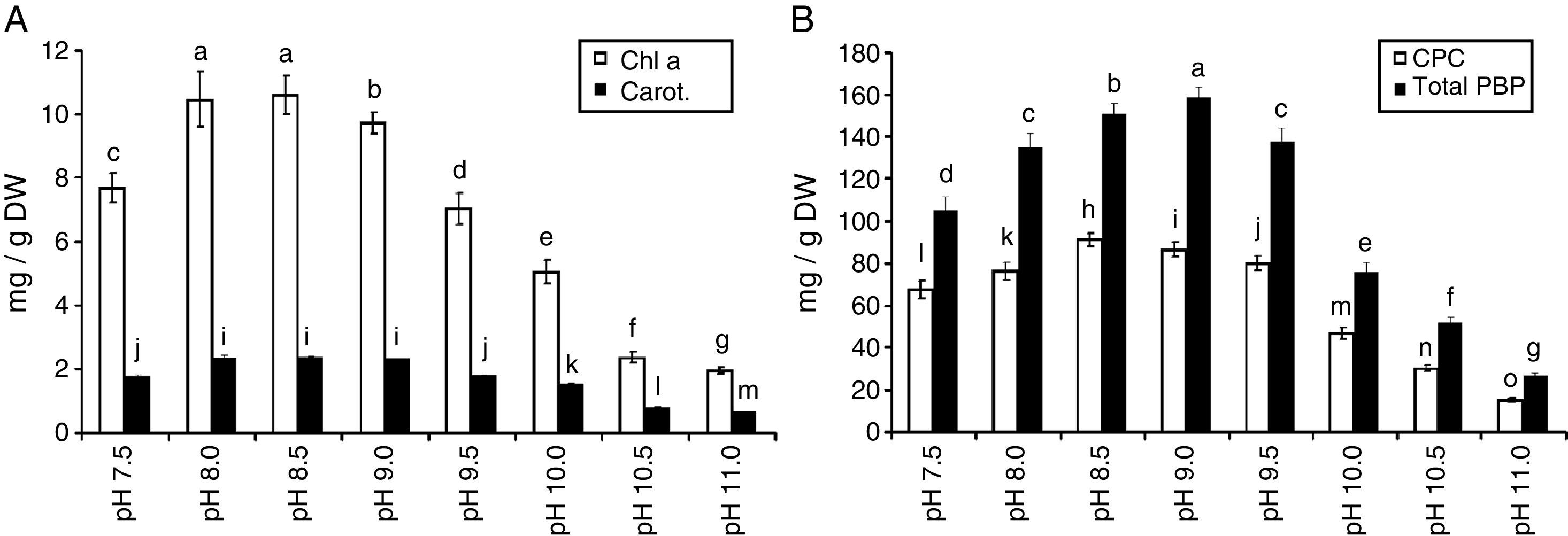
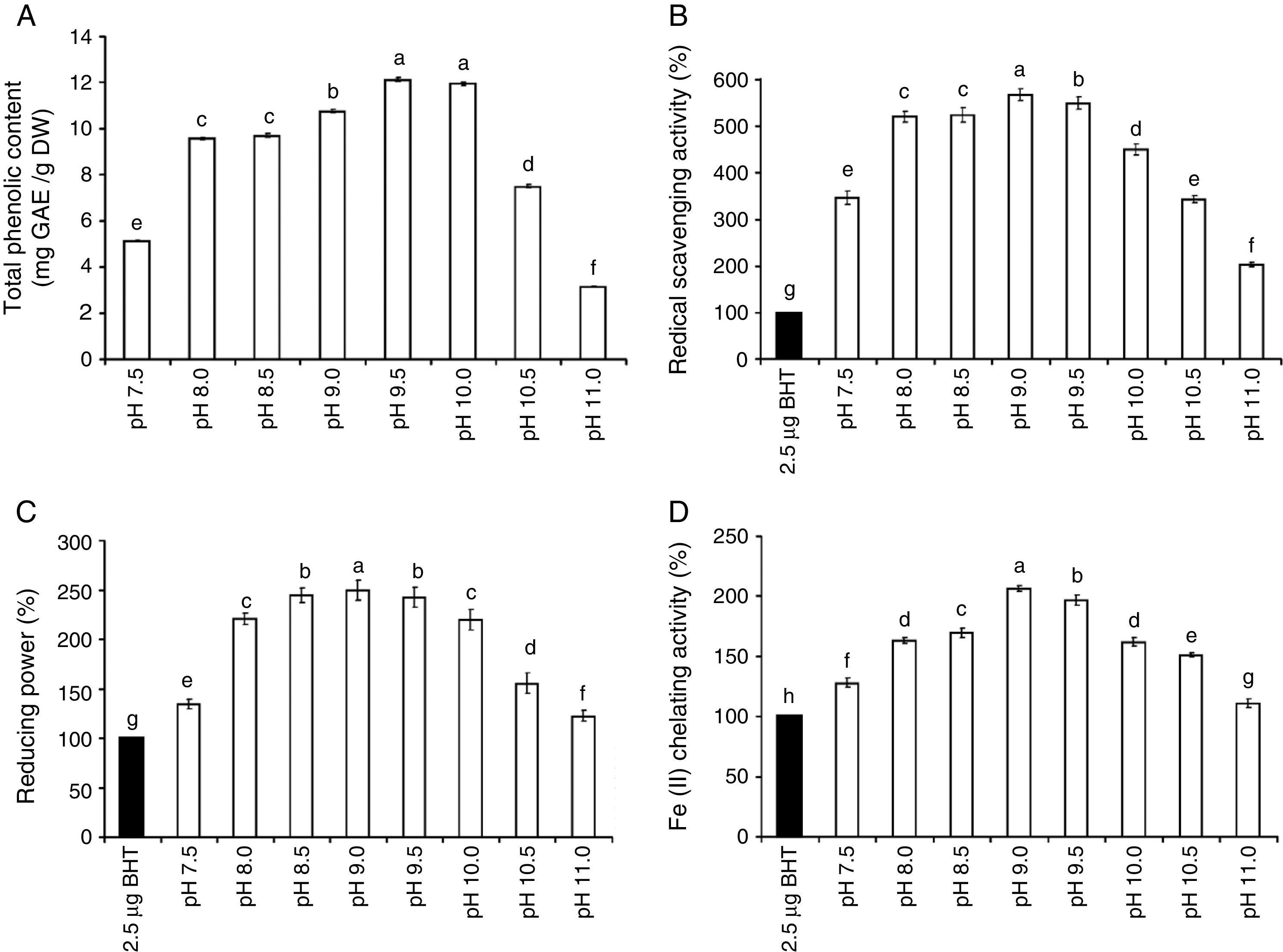
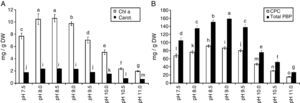
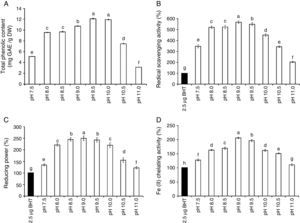
The amount of chlorophyll a (Chl a) produced by S. platensis was highest at pH 8.5 (10.6mg/g DW), but Chl a was significantly lower at all of the other pH levels (Fig. 2A). The highest amount of carotenoids was obtained at pH 8.5 (2.4mg/g DW). The highest C-phycocyanin content was recorded at pH 8.5 (91mg/g DW), and the highest amount of total phycobiliprotein content (159mg/g DW) was obtained at pH 9.0 (Fig. 2B). The total phenolic content of S. platensis was significantly higher at pH 9.5 (12.1mg gallic acid equivalent (GAE)/g DW) and pH 10.0 (11.9mg GAE/g DW) than all of the other pH levels (Fig. 3A).
The total phenolic content (TPC) (A), the radical scavenging activity (B), the reducing power (C), and the Fe(II) chelating activity (D) of S. platensis at different pH levels. The values are expressed as the mean±SD of three independent experiments and were normalized to 2.5μg BHT (
) as a positive control. The different letters represent significant differences at p<0.05 (Duncan's).S. platensis had stronger antioxidant activity than the positive control (2.5μg BHT) at a wide range of pH levels from pH 7.5 to pH 11.0 (Fig. 3B–D). The radical scavenging activity, reducing power and chelating activities all showed the highest value at pH 9.0 with a percent increase of 567, 250 and 206% compared to the positive control, respectively. A reduction in antioxidant activity was recorded when the pH value was shifted either towards high alkalinity or neutrality.
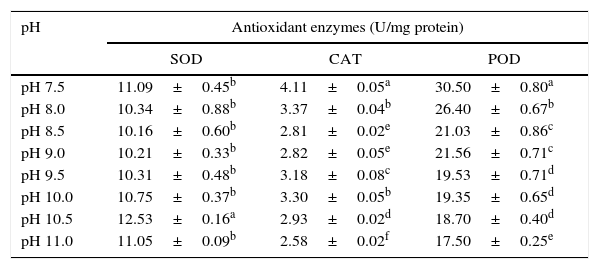
Antioxidant enzymesThe activities of antioxidant enzymes (SOD, CAT and POD) varied at different pH levels (Table 1). The SOD activity was highest at pH 10.5, and the level of activity was lowest at pH 8.5. However, the CAT activity was highest at pH 7.5 and lowest at pH 11.0. The activity of POD was highest at pH 7.5 and lowest at pH 11.0.
The effect of pH on antioxidant enzyme activity of S. platensis.
| pH | Antioxidant enzymes (U/mg protein) | ||
|---|---|---|---|
| SOD | CAT | POD | |
| pH 7.5 | 11.09±0.45b | 4.11±0.05a | 30.50±0.80a |
| pH 8.0 | 10.34±0.88b | 3.37±0.04b | 26.40±0.67b |
| pH 8.5 | 10.16±0.60b | 2.81±0.02e | 21.03±0.86c |
| pH 9.0 | 10.21±0.33b | 2.82±0.05e | 21.56±0.71c |
| pH 9.5 | 10.31±0.48b | 3.18±0.08c | 19.53±0.71d |
| pH 10.0 | 10.75±0.37b | 3.30±0.05b | 19.35±0.65d |
| pH 10.5 | 12.53±0.16a | 2.93±0.02d | 18.70±0.40d |
| pH 11.0 | 11.05±0.09b | 2.58±0.02f | 17.50±0.25e |
The values are expressed as the mean±SD of at least three independent experiments. The different superscript letters within a column indicate significant differences at p<0.05 (Duncan's).
The cyanobacterium Spirulina sp. has been investigated due to the potential for industrial application of antioxidants. This is the first work to study the effect of growth medium pH on antioxidant activity and productivity of S. platensis.
The variability in the chemical composition of algae is dependent on many factors, including the growth medium nutrients and laboratory conditions, which reflect their natural habitat conditions. For instance, the growth and biomass yield of S. platensis were clearly affected by the pH of the growth medium. Although S. platensis can withstand a wide pH range, the growth decreased by shifting the pH above 10 and the cells turned pale in color. The decrease in growth may be related to the inhibition of photosynthetic activity at very high pH where no carbon dioxide is accessible for algal metabolism.6
The optimum pH for algal growth differs according to many factors including algal species, media type, and the laboratory cultivation (e.g., temperature).7 However, shifting the pH away from the optimum value may result in inhibition of chlorophyll synthesis and carotenoids,32 which ultimately affect algal growth.
In this study, the highest biomass yield was recorded at pH 9.0, which supported the requirement of alkaline conditions for the growth of Spirulina sp., where the optimum growth was previously recorded in cultures with pH 9–10,7,8,33,34 and above this pH, a sharp decline in the output rate was recorded.35 The decreased algal production and pigment content at the high pH can be explained if bicarbonate is the only source of carbon, which causes the culture pH to rise8 and free CO2 concentrations to eventually become limiting.36 Because of the stress from carbon dioxide deficiency at the high pH, the free radicals or ROS levels in algal cells may increase, which causes the algal cells to undergo oxidative stress.37 While high levels of cellular antioxidants such as Chl a, carotenoids, and phycobiliproteins reflected optimal algal growth, the increase in phenolics was possibly a result of ROS production to alleviate this stress. High phenolic contents recorded at pH levels 9.5 and 10.0 were thought to act as antioxidants by donating electrons either to free radical atoms (free radical scavengers) or to antioxidant enzyme substrate for the detoxification of H2O2 produced under stress conditions.38 The further reduction of phenolics at pH levels of 10.5 and 11.0 may have been the result of the inability of all cell systems to function under these extreme conditions. If the function of all cell systems was affected by these high pH levels, including phenolic production, algal growth and any protective systems would cease to function. This is supported by low algal growth and biomass production (Fig. 1) and the lowest pigment production (Fig. 2) at pH levels of 10.5 and 11.0.
The antioxidant activities (the radical scavenging activity, reducing power and chelating activity) were enhanced under stress conditions and at the pH level that supports optimal growth. The relative reduction of the antioxidant activity at the high pH levels where SOD enhancement was recorded may be because these antioxidant activities (e.g., radical scavenging activity) reflect the reduction of non-enzymatic antioxidants, e.g., phycocyanin and phenolics39 at high pH, and underestimate the antioxidant enzymes activities.
SOD, CAT and POD are important antioxidant enzymes in the algal cell that protect against the peroxidation system and maintain the redox state of the cell. The enhanced activity of SOD (at pH 10.5 and 11.0) and CAT (at pH 10.0) may suggest a cooperative role for these enzymes to detoxify ROS at higher pH levels.5 The SOD, which is the first cell defense line,40 dismutates superoxide anions (O2¿) into hydrogen peroxide and oxygen molecules. Inside the cells, the level of hydrogen peroxide was maintained by CAT and/or POD by catalyzing its decomposition into molecular oxygen and water. The reduction of POD activity at higher pH levels may be explained by the sensitivity of this enzyme to increased pH stress. Mizobutsi et al.41 showed the pH-dependence of peroxidase activity, for which the maximum enzyme activity observed at pH 6.5 was reduced when the pH was different from the optimal pH required for growth. The decreased POD production at a high pH is supported by Jin et al.42 who reported that at extreme pH levels (pH 10.5 and 11.0) algal cells began to collapse and likely resulted in reduction or failure of many cellular processes (including antioxidant machinery). The more or less constant activity of CAT during active growth between pH 8.5 and 10.0 may be explained if the cells rely mainly on CAT for H2O2 detoxification rather than POD. However, the stimulatory effect of all three of the antioxidant enzymes at pH 7.5 may counteract the production of ROS as a result of photosynthesis.43
In conclusion, while the activities were highest at optimal growth conditions, the overproduction of the enzymes was shown at the high alkaline pH, which favored the overproduction of SOD, and the neutral pH levels favored the overproduction of CAT and POD in S. platensis. These antioxidants resist the stress imposed at the extreme pH levels and maintain normal construction and cell homeostasis as proven by growth, chlorophyll and different antioxidant contents. In this case, the relative reduction of the growth of S. platensis at higher pH values could be compensated by the higher antioxidants content. This study demonstrates the importance of S. platensis as producers of natural and powerful antioxidants when pH levels are changed from optimal to extreme levels. With this knowledge, the overproduction of these algal antioxidants may be further explored for their use as medicinal products and additives in pharmaceutical, food, cosmetic or other industrial applications.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Prof. Dr. Mohsen Abdel-Tawwab for assistance in statistical analyses; Maged Ismaiel for writing assistance; the Faculty of Science, Zagazig University, Egypt, and EPICO (Egyptian International Pharmaceutical Industries Company) for technical and financial support; and the Department of Missions (Ministry of Higher Education and Scientific Research, Egypt) for providing financial support through a channel system scholarship to Mostafa M.S. Ismaiel.