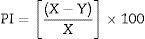Fourteen Trichoderma isolates were evaluated for their tolerance to two heavy metals, nickel and cadmium. Three isolates, MT-4, UBT-18, and IBT-I, showed high levels of nickel tolerance, whereas MT-4, UBT-18, and IBT-II showed better tolerance of cadmium than the other isolates. Under nickel stress, biomass production increased up to a Ni concentration of 60ppm in all strains but then decreased as the concentrations of nickel were further increased. Among the nickel-tolerant isolates, UBT-18 produced significantly higher biomass upon exposure to nickel (up to 150ppm); however, the minimum concentration of nickel required to inhibit 50% of growth (MIC50) was highest in IBT-I. Among the cadmium-tolerant isolates, IBT-II showed both maximum biomass production and a maximum MIC50 value in cadmium stress. As the biomass of the Trichoderma isolates increased, a higher percentage of nickel removal was observed up to a concentration of 40ppm, followed by an increase in residual nickel and a decrease in biomass production at higher nickel concentrations in the medium. The increase in cadmium concentrations resulted in a decrease in biomass production and positively correlated with an increase in residual cadmium in the culture broth. Nickel and cadmium stress also influenced the sensitivity of the Trichoderma isolates to soil fungistasis. Isolates IBT-I and UBT-18 were most tolerant to fungistasis under nickel and cadmium stress, respectively.
Trichoderma species are imperfect filamentous fungi with teleomorphs and belong to the order Hypocreales in the Ascomycete division. Trichoderma spp. are among the most frequently isolated soil fungi and are well known for their biocontrol ability against a wide range of plant pathogenic fungi,1 induction of localized and systemic defense responses in plants,2 and plant growth enhancement.3,4 They play an important role in ecology by taking part in decomposition of plant residues, as well as in biodegradation of man-made chemicals and bioaccumulation of high amounts of various metals from wastewater and soil.5,6 Metal-containing pollutants are increasingly released into soil from industrial wastewater, as well as from wastes derived from chemical fertilizers and pesticides used in agriculture.7 Some metal-containing pollutants are not biodegradable; they enter the food chain and lead to bioaccumulation.8 Minute amounts of metals, except those that are non-essential for biological functions, such as mercury, arsenic, lead and cadmium, influence vital metabolic processes and are required by all forms of life. However, metals may be toxic at concentrations higher than nutritional requirements. Evidence has suggested that Trichoderma spp. exhibit considerable tolerance for metals and accumulate high amounts of metals from polluted habitats.1,8 Therefore, metal-tolerant Trichoderma spp. may become dominant organisms in some polluted environments and may play an important role in eco-friendly metal removal technology.7 As a component of soil fungistasis, metal ions may influence the growth, sporulation and enzymatic activities of Trichoderma.9,10 This can cause changes in the quantities of extracellular enzymes and metabolites,11,12 as well as in overall biocontrol activities against plant pathogenic fungi and in plant growth-stimulating activities. Katayama and Matsumura13 demonstrated a degradation potential of a rhizosphere-competent Trichoderma sp. for several synthetic dyes, pentachlorophenol, endosulfan, and dichlorodiphenyltrichloroethane (DDT). Thus, Trichoderma spp. have acquired an exceptional role as part of a sustainable approach to bioremediation of herbicide/pesticide-laden soils.
However, the microhabitat behavior of Trichoderma spp. upon exposure to metal-containing compounds may differ, depending on the type of the metal and the Trichoderma isolate, and very little information in this regard is available. Hence, an attempt was made to screen Trichoderma isolates for nickel and cadmium tolerance and identify strains that can potentially be used for bioremediation of soils polluted with these metals.
Materials and methodsIsolation of Trichoderma spp. from soilTrichoderma spp. were isolated from soil on a modified Trichoderma-specific medium (TSM)14 using a dilution plate method.15 Soil samples were collected from different sugarcane growing areas of Manipur and from tea industry areas in northern districts of West Bengal, where indiscriminate use of chemicals and effluents has caused heavy-metal soil toxicity. The samples were air-dried and ground to powder using a mortar and a pestle. Soil suspensions (1mL of 10–3, 10–4, and 10–5 dilutions) were plated in Petri plates containing 20mL of modified TSM. The suspensions were distributed uniformly over the medium surface by horizontal shaking and incubated at 28±1°C for seven days. Green colonies of the antagonist usually appeared after four or five days of incubation. Each colony was observed under a microscope using lactophenol cotton blue stain and identified to the genus level based on the available taxonomic literature.16 The shape, size and aggregation of phialospores and phialides were used as main identification criteria, along with cultural characteristics on potato dextrose agar (PDA). The colonies identified as Trichoderma spp. were transferred onto PDA slants and kept at 4°C for further use. The isolates from Manipur were designated as MT, and the isolates from the tea plantation areas were designated as IBT, followed by a number. One isolate, namely, UBT-18, was obtained from the culture collection of the Department of Plant Pathology, Uttar Banga Krishi Viswavidyalaya.
Selection of nickel- and cadmium-tolerant isolates of Trichoderma spp.In vitro tolerance of Trichoderma spp. to different concentrations of nickel and cadmium was determined by the poisoned food technique.15 PDA medium (100mL) was prepared in 250-mL conical flasks, then appropriate quantities of nickel and cadmium stock solutions were added to molten PDA to get the required concentrations (40, 60, 100, 150, and 200mg/L), and the resulting media were poured into Petri plates after gentle shaking. The non-amended medium served as a control. The plates were inoculated by placing 6-mm mycelial discs of 4-day-old cultures of the Trichoderma isolates on the agar surface and incubated at 28±1°C for 2–3 days. Isolates showing maximum radial growth on the media, irrespective of the metal concentration, were selected for further studies.
Biomass production by Trichoderma isolates and determination of minimum inhibitory concentration of the metalsFor biomass preparation, selected Trichoderma isolates were inoculated on PDA plates and incubated at room temperature (27±1°C). After five days, a small portion (0.5mm) from the fungal mass was cut, transferred into a 250-mL conical flask containing 50mL of potato dextrose (PD) broth supplemented with different concentrations of a metal (0, 40, 60, 100, 150, and 200ppm), and incubated in triplicates at 27±1°C for seven days. The biomass was harvested by filtering through Whatman no. 1 filter paper and then washed thoroughly with deionized water to remove the growth medium. The harvested mycelia were oven-dried at 60°C for 48h and the dry weight was measured using a Sartorius LA8200S digital weight balance with an accuracy of 0.1mg. The inhibition of biomass production was calculated based on the dry weight using the following formula:
where PI is the percentage of inhibition; X is biomass in the control (0ppm) broth; and Y is biomass in the metal-containing broth.The minimum inhibitory concentration of each metal, causing 50% of growth inhibition (MIC50) of the selected Trichoderma isolates, was calculated from the growth inhibition results.
Estimation of residual metals in culture brothAfter harvesting the biomass of each isolate grown in the PD broth amended with different concentrations of nickel or cadmium (0, 10, 25, 50 and 100ppm), the culture broth was assayed for residual metal, following the method described by Tandon.17 Five milliliters of the culture broth was placed in a 100-mL clean beaker, followed by the addition of 10mL of a triacid mixture (HNO3:H2SO4:HClO4, 9:4:1, v/v/v), and the content was mixed by swirling and kept overnight. The mixture was digested in a digestion chamber at 60°C followed by heating at 90°C until the production of red NO2 fumes ceased. The content was further evaporated until the volume was reduced to about 2–3mL. The completion of digestion was confirmed when the liquid became colorless. After cooling the beaker, its content was transferred quantitatively to a 50-mL capacity volumetric flask, diluted to 50mL with distilled water, and kept overnight. On the next day, it was filtered through Whatman no. 44 filter paper. The filtrates were analyzed for cadmium or nickel using a Perkin Elmer Analyst 200AA flame spectrophotometer. Each sample was analyzed two or three times at a wavelength of 445nm for nickel or 229nm for cadmium. Residual metal concentrations were expressed in μg/mL of culture broth.
Tolerance of Trichoderma isolates to soil fungistasis under heavy metal stressFungistatic effects of soil were studied using the soil cellophane agar disk method18 with a slight modification. In this experiment, soil samples were amended with different concentrations of nickel or cadmium to adjust the metal contamination levels to 50, 100 or 150ppm and kept for one month for stabilization. Well-saturated, metal-contaminated soil (100g) was filled in a plastic cup, and the upper surface was smoothed using thumb pressure. Cellophane paper was cut to the diameter of the cup and boiled to eliminate plasticizer effects. A single piece of cellophane was placed on the smooth soil surface, and a disc (1cm in diameter) of 2% water agar was placed on the cellophane paper. The entire system was refrigerated for 24h to activate the agar disc. On the next day, a conidial suspension (103 cfu/mL) of the selected metal-tolerant Trichoderma isolate was applied to the agar disc and incubated at 28±1°C for 20h. After the incubation, the disc was transferred to a glass slide and stained with 0.1% lactophenol cotton blue to examine conidial germination under a light microscope at 20× magnification. The percentage of germinated conidia was recorded, and the percent of germination inhibition was calculated.
Statistical analysisThe experiments were conducted using a factorial, completely randomized design with three replications, considering the isolates as factor A and the metal concentrations as factor B. An analysis of variance (ANOVA) was performed for all parameters using the INDOSTAT package. Comparison of means was done by Duncan's multiple range test at the p<0.05 level of significance.19 The identical letters in the results denote non-significant differences among the treatments within each isolate.
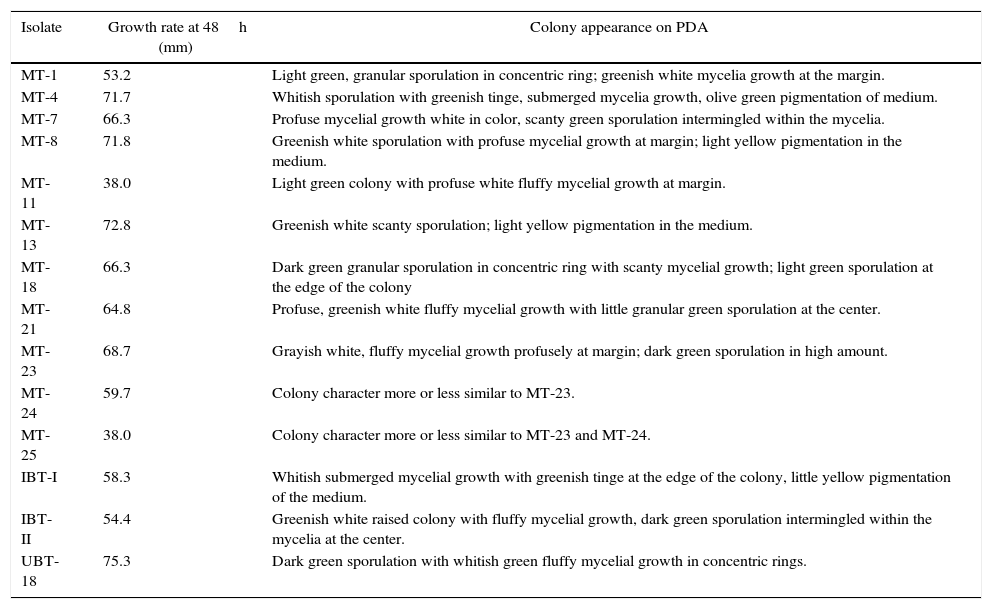
Results and discussionTrichoderma spp. are ubiquitous microorganisms distributed in almost all types of crop rhizosphere20,21 and have even been found in metal-polluted ecosystems.22,23 In this study, 14 Trichoderma isolates, namely, MT-1, MT-4, MT-7, MT-8, MT-11, MT-13, MT-18, MT-21, MT-23, MT-24, MT-25, IBT-I, IBT-II, and UBT-18, were identified and selected for further study, based on their cultural variability and growth rates. Cultural variability existed among the isolates with respect to their mycelial growth pattern, color of sporulation, and pigmentation of the medium (Table 1), indicating that the isolates might be able to produce secondary metabolites. The maximum growth rate was demonstrated by UBT-18, followed by MT-13, MT-8, MT-4, and MT-23.
Cultural variability of the Trichoderma isolates.
| Isolate | Growth rate at 48h (mm) | Colony appearance on PDA |
|---|---|---|
| MT-1 | 53.2 | Light green, granular sporulation in concentric ring; greenish white mycelia growth at the margin. |
| MT-4 | 71.7 | Whitish sporulation with greenish tinge, submerged mycelia growth, olive green pigmentation of medium. |
| MT-7 | 66.3 | Profuse mycelial growth white in color, scanty green sporulation intermingled within the mycelia. |
| MT-8 | 71.8 | Greenish white sporulation with profuse mycelial growth at margin; light yellow pigmentation in the medium. |
| MT-11 | 38.0 | Light green colony with profuse white fluffy mycelial growth at margin. |
| MT-13 | 72.8 | Greenish white scanty sporulation; light yellow pigmentation in the medium. |
| MT-18 | 66.3 | Dark green granular sporulation in concentric ring with scanty mycelial growth; light green sporulation at the edge of the colony |
| MT-21 | 64.8 | Profuse, greenish white fluffy mycelial growth with little granular green sporulation at the center. |
| MT-23 | 68.7 | Grayish white, fluffy mycelial growth profusely at margin; dark green sporulation in high amount. |
| MT-24 | 59.7 | Colony character more or less similar to MT-23. |
| MT-25 | 38.0 | Colony character more or less similar to MT-23 and MT-24. |
| IBT-I | 58.3 | Whitish submerged mycelial growth with greenish tinge at the edge of the colony, little yellow pigmentation of the medium. |
| IBT-II | 54.4 | Greenish white raised colony with fluffy mycelial growth, dark green sporulation intermingled within the mycelia at the center. |
| UBT-18 | 75.3 | Dark green sporulation with whitish green fluffy mycelial growth in concentric rings. |
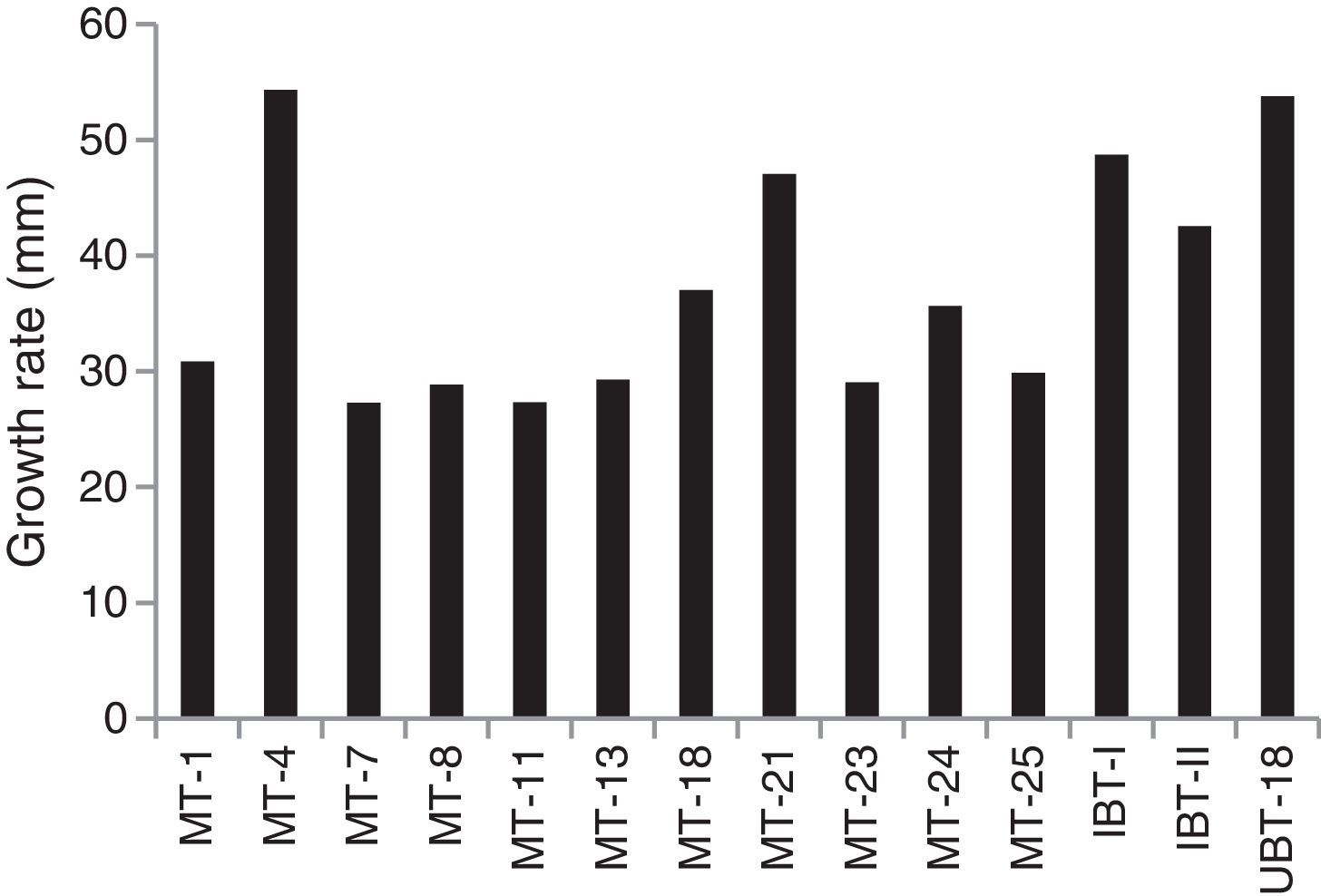
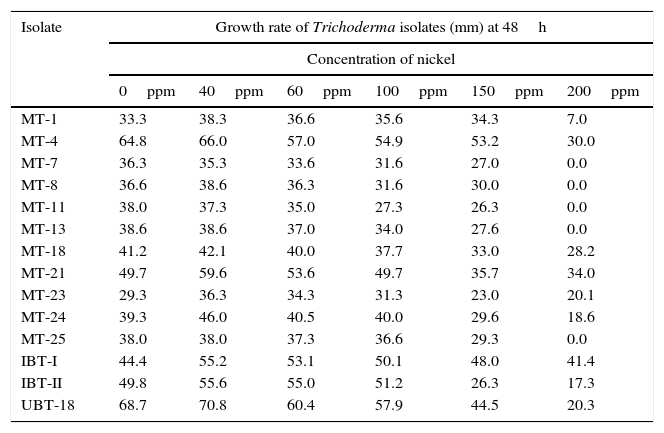
The effects of different concentrations of Ni on mycelial growth of the 14 Trichoderma isolates were tested (Table 2), and the maximum radial growth was shown by UBT-18 (70.8mm) at a Ni concentration of 40ppm, followed by isolate MT-4 (66.0mm), with a significant difference between the two isolates. Six other isolates, namely, IBT-I, IBT-II, MT-1, MT-23, and MT-24, exhibited significantly higher mycelial growth at the same Ni concentration compared with their corresponding non-amended cultures. With a further increase of Ni concentration in the medium to 60ppm, only four isolates, IBT-I, IBT-II, MT-21, and MT-23, showed significantly higher mycelial growth versus their respective controls. At higher concentrations of Ni, from 100 to 200ppm, there was a significant reduction in mycelial growth of all isolates, although three of them, viz., MT-4, IBT-I, and UBT-18, consistently showed higher mycelial growth at concentrations of up to 150ppm. Although MT-21 showed a higher level of tolerance to Ni toxicity at 200ppm, its growth was comparatively low at concentrations from 60 to 150ppm compared with the other Trichoderma isolates.
Growth rate of the Trichoderma isolates on nickel amended PDA medium.
| Isolate | Growth rate of Trichoderma isolates (mm) at 48h | |||||
|---|---|---|---|---|---|---|
| Concentration of nickel | ||||||
| 0ppm | 40ppm | 60ppm | 100ppm | 150ppm | 200ppm | |
| MT-1 | 33.3 | 38.3 | 36.6 | 35.6 | 34.3 | 7.0 |
| MT-4 | 64.8 | 66.0 | 57.0 | 54.9 | 53.2 | 30.0 |
| MT-7 | 36.3 | 35.3 | 33.6 | 31.6 | 27.0 | 0.0 |
| MT-8 | 36.6 | 38.6 | 36.3 | 31.6 | 30.0 | 0.0 |
| MT-11 | 38.0 | 37.3 | 35.0 | 27.3 | 26.3 | 0.0 |
| MT-13 | 38.6 | 38.6 | 37.0 | 34.0 | 27.6 | 0.0 |
| MT-18 | 41.2 | 42.1 | 40.0 | 37.7 | 33.0 | 28.2 |
| MT-21 | 49.7 | 59.6 | 53.6 | 49.7 | 35.7 | 34.0 |
| MT-23 | 29.3 | 36.3 | 34.3 | 31.3 | 23.0 | 20.1 |
| MT-24 | 39.3 | 46.0 | 40.5 | 40.0 | 29.6 | 18.6 |
| MT-25 | 38.0 | 38.0 | 37.3 | 36.6 | 29.3 | 0.0 |
| IBT-I | 44.4 | 55.2 | 53.1 | 50.1 | 48.0 | 41.4 |
| IBT-II | 49.8 | 55.6 | 55.0 | 51.2 | 26.3 | 17.3 |
| UBT-18 | 68.7 | 70.8 | 60.4 | 57.9 | 44.5 | 20.3 |
SEM±1.23; LSD (p=0.05) 3.50.
PDA, potato dextrose agar.
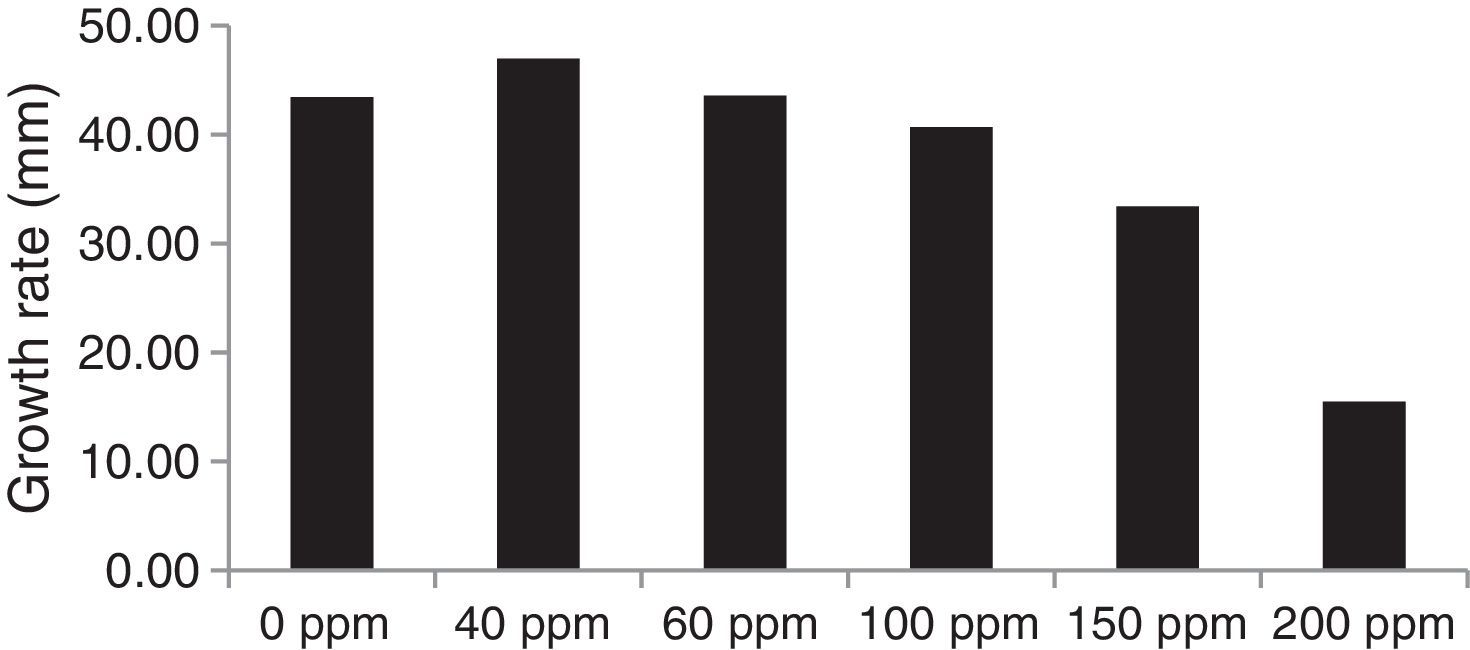
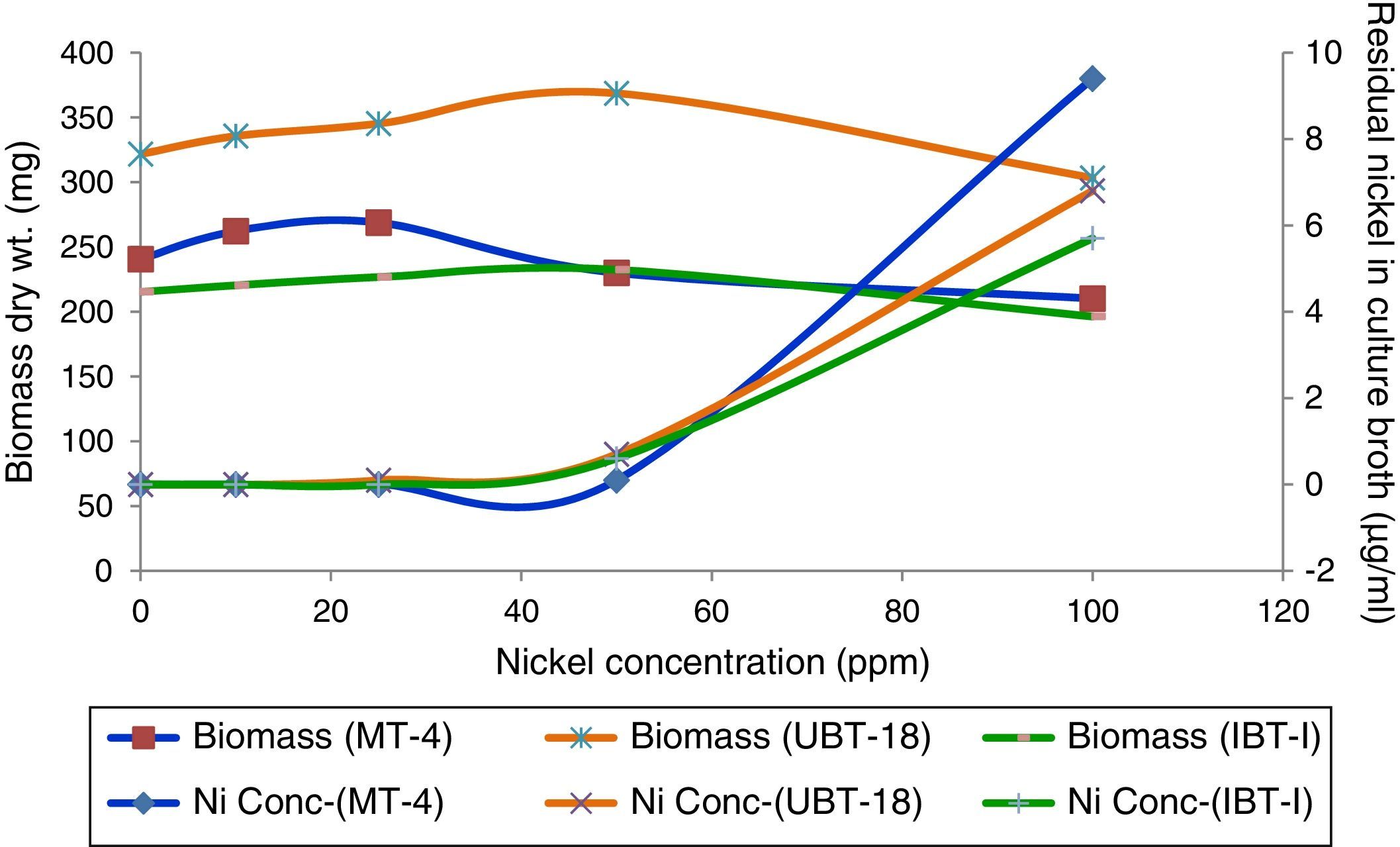
The effects of nickel on radial growth of the 14 Trichoderma isolates are presented in Figs. 1 and 2. It was noticed that the growth of the Trichoderma isolates was significantly influenced by the heavy metal; in particular, at 40ppm of Ni in the amended medium the growth was even higher than that observed in the non-amended medium. At higher concentrations of the heavy metal, from 60 to 200mg/L, there was a significant reduction in radial mycelial growth of all the Trichoderma isolates. Isolate MT-4 showed the highest tolerance to nickel. Taken together, isolates MT-4, IBT-I, and UBT-18 were considered to be resistant since their radial growth decreased at a slower rate than that of the other isolates, while isolates MT-7, MT-18, MT-11, MT-13, and MT-25 were most sensitive and no mycelial growth was detected at 200mg/L. Hence, three isolates, namely, MT-4, IBT-I and UBT-18, were finally screened due to their high Ni tolerance.
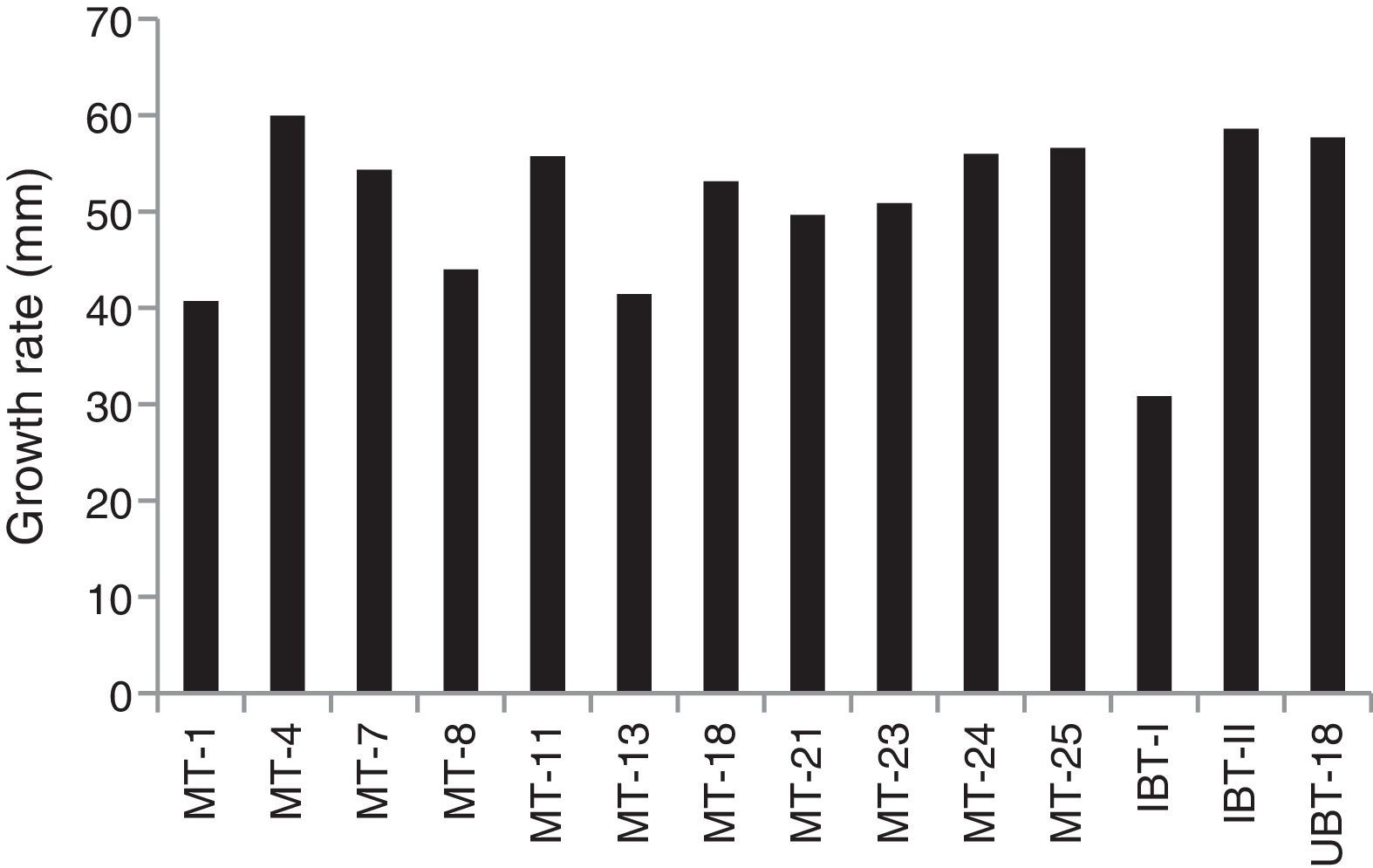
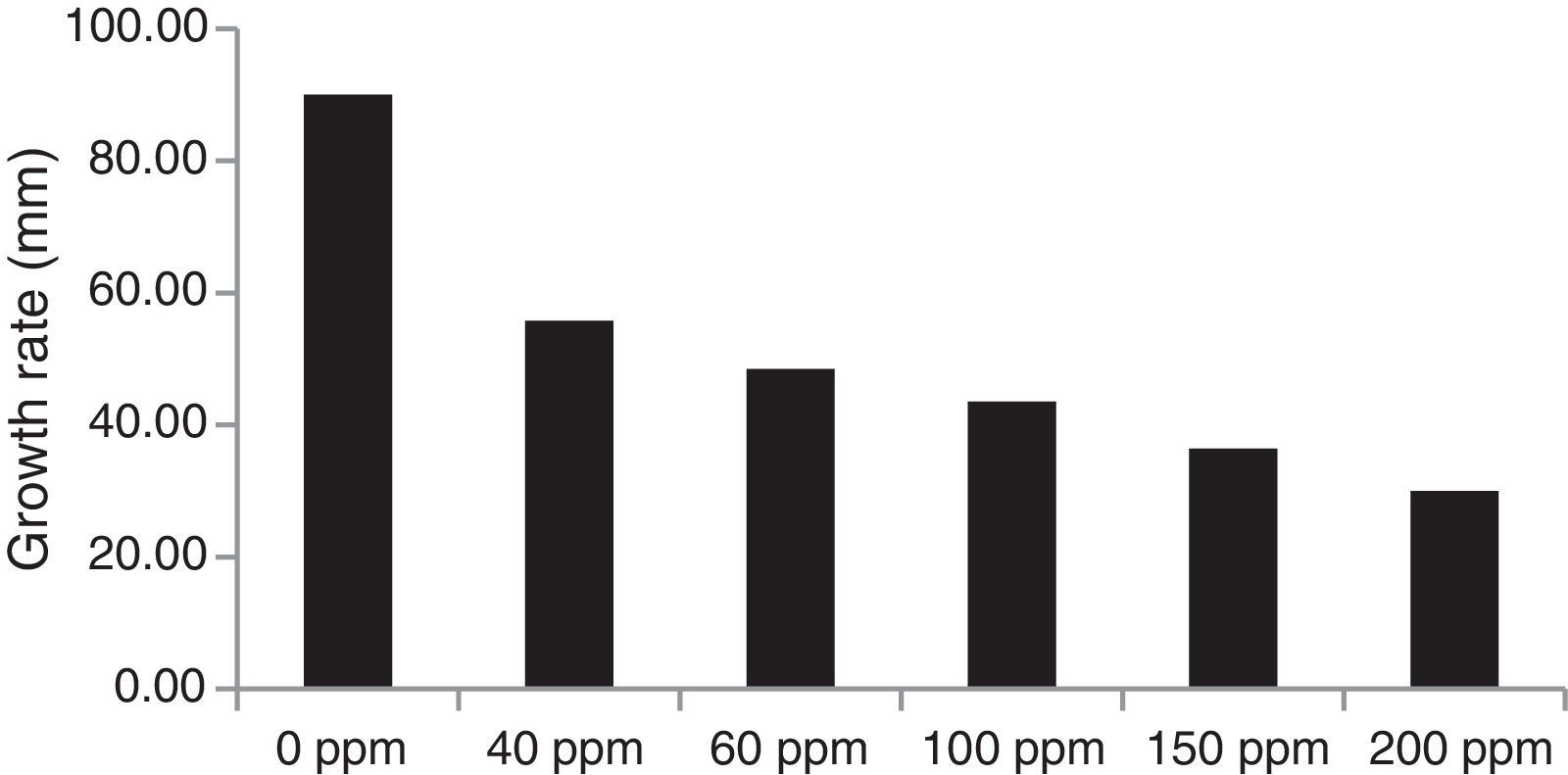
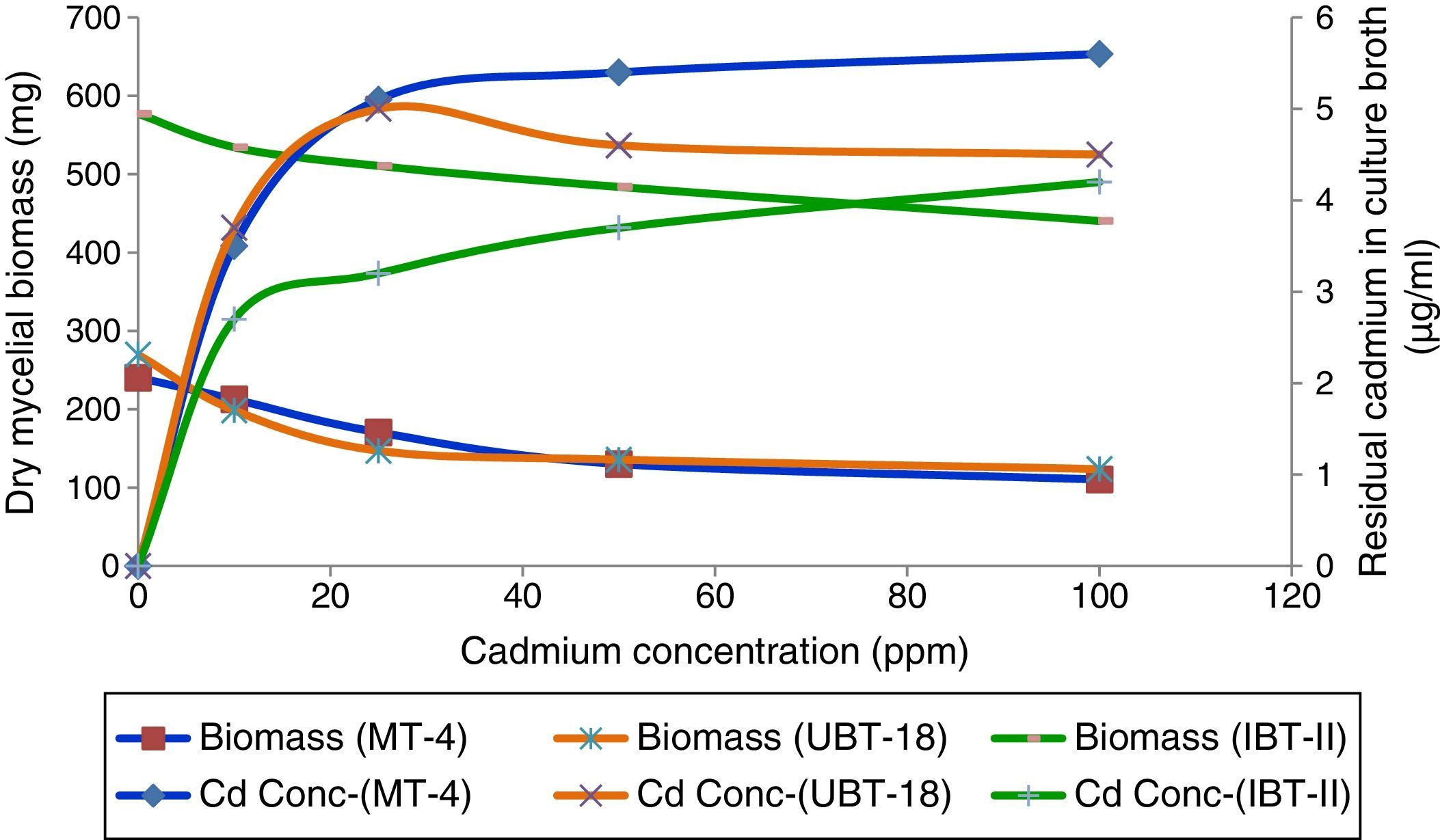
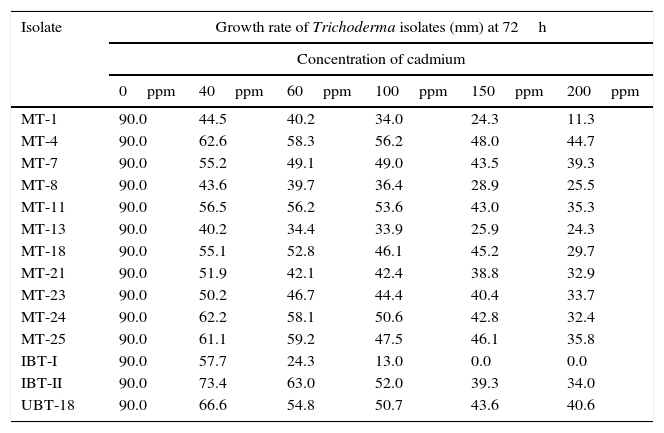
Screening of the Trichoderma isolates for their cadmium tolerance revealed that the isolates varied significantly in their levels of tolerance, irrespective of the cadmium concentrations tested (Table 3). There was a significant reduction in the mycelial growth of the Trichoderma isolates upon exposure to cadmium (Fig. 3). At a cadmium concentration of 40ppm, the maximum mycelial growth was shown by IBT-II (73.4mm), which was significantly higher than that of UBT-18 (66.6mm), followed by MT-4 and MT-24 (62.6 and 62.2mm, respectively). The degree of tolerance of the Trichoderma isolates, irrespective of the cadmium level, is presented in Fig. 3. The data indicated that MT-4, UBT-18, and IBT-II were tolerant, IBT-I, MT-7, MT-11, MT-18, MT-21, and MT-23 were moderately tolerant, and MT-1, MT-8, and MT-13 were susceptible to cadmium toxicity. In Fig. 4, the effects of cadmium concentrations on the Trichoderma isolates are depicted, and a trend of a decreasing growth rate of the isolates was observed with increasing concentrations of cadmium.
Growth rate of the Trichoderma isolates at different concentrations of cadmium in PDA.
| Isolate | Growth rate of Trichoderma isolates (mm) at 72h | |||||
|---|---|---|---|---|---|---|
| Concentration of cadmium | ||||||
| 0ppm | 40ppm | 60ppm | 100ppm | 150ppm | 200ppm | |
| MT-1 | 90.0 | 44.5 | 40.2 | 34.0 | 24.3 | 11.3 |
| MT-4 | 90.0 | 62.6 | 58.3 | 56.2 | 48.0 | 44.7 |
| MT-7 | 90.0 | 55.2 | 49.1 | 49.0 | 43.5 | 39.3 |
| MT-8 | 90.0 | 43.6 | 39.7 | 36.4 | 28.9 | 25.5 |
| MT-11 | 90.0 | 56.5 | 56.2 | 53.6 | 43.0 | 35.3 |
| MT-13 | 90.0 | 40.2 | 34.4 | 33.9 | 25.9 | 24.3 |
| MT-18 | 90.0 | 55.1 | 52.8 | 46.1 | 45.2 | 29.7 |
| MT-21 | 90.0 | 51.9 | 42.1 | 42.4 | 38.8 | 32.9 |
| MT-23 | 90.0 | 50.2 | 46.7 | 44.4 | 40.4 | 33.7 |
| MT-24 | 90.0 | 62.2 | 58.1 | 50.6 | 42.8 | 32.4 |
| MT-25 | 90.0 | 61.1 | 59.2 | 47.5 | 46.1 | 35.8 |
| IBT-I | 90.0 | 57.7 | 24.3 | 13.0 | 0.0 | 0.0 |
| IBT-II | 90.0 | 73.4 | 63.0 | 52.0 | 39.3 | 34.0 |
| UBT-18 | 90.0 | 66.6 | 54.8 | 50.7 | 43.6 | 40.6 |
SEM±1.13; LSD (p=0.05) 3.10.
PDA, potato dextrose agar.
In the absence of a rational method for an a priori prediction of a biosorption potential of a microorganism, the only method for identifying and developing newer and efficient biosorbents is sustained screening of microbes.24 Variations in metal tolerance among different species of a genus or within the same species might be due to the presence of one or more resistance mechanisms exhibited by different fungi.25 Sarkar et al.26 reported Trichoderma harzianum to be moderately tolerant to up to 60ppm of Ni, at that concentration the level of inhibition of mycelial growth was 33.3%. A further increase in the Ni concentration reduced the growth, and total inhibition was observed at 200mg/L. Lima et al.27 also observed influence of cadmium on radial growth of T. harzianum. The results of the present investigation are in line with these earlier observations.
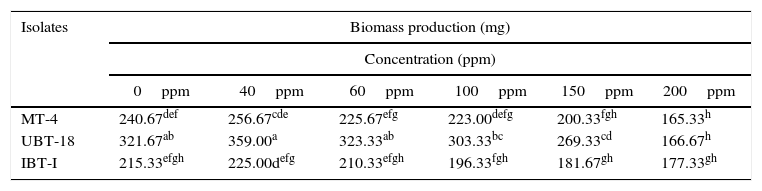
When the effects of different nickel concentrations on biomass production of the three promising Trichoderma isolates (in order of ranking) were studied, the maximum biomass weight was recorded for UBT-18 (359mg), which was significantly higher than the values obtained for MT-4 (256.67mg) and IBT-I (225mg), at a Ni concentration of 40ppm. With a further increase of the nickel concentration in the medium to 60ppm, biomass production by all the isolates screened was insignificant compared with their respective controls. At higher concentrations of nickel, from 100 to 200ppm, there was a significant reduction in biomass of all three isolates. Although isolate UBT-18 showed significantly higher biomass production at Ni concentrations of up to 150ppm, IBT-I showed somewhat higher biomass production compared to the other two isolates at 200ppm (Table 4).
Biomass production of the Trichoderma isolates under different concentration of nickel.
| Isolates | Biomass production (mg) | |||||
|---|---|---|---|---|---|---|
| Concentration (ppm) | ||||||
| 0ppm | 40ppm | 60ppm | 100ppm | 150ppm | 200ppm | |
| MT-4 | 240.67def | 256.67cde | 225.67efg | 223.00defg | 200.33fgh | 165.33h |
| UBT-18 | 321.67ab | 359.00a | 323.33ab | 303.33bc | 269.33cd | 166.67h |
| IBT-I | 215.33efgh | 225.00defg | 210.33efgh | 196.33fgh | 181.67gh | 177.33gh |
* Values followed by different letters differ significantly according to Duncan's multiple range test at p=0.05.
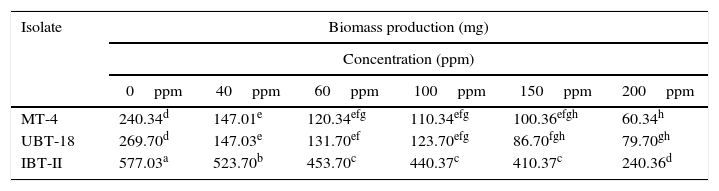
Effects of different cadmium concentrations on biomass production were studied using the three most tolerant Trichoderma isolates. The maximum biomass value was recorded for IBT-II (523.70mg), and it was significantly higher than the values obtained for UBT-18 (147.03mg) and MT-4 (147.01mg) at a Cd concentration of 40ppm (Table 5). With a further increase in the cadmium concentration, biomass production was significantly reduced, except that IBT-II showed insignificant variations in biomass production at cadmium concentrations of up to 150ppm. Isolate MT-4 (165.33mg) and UBT-18 (166.67mg) exhibited insignificant variations between each other in biomass production upon exposure to cadmium toxicity (up to 200ppm).
Biomass production of the Trichoderma isolates under different concentration of cadmium.
| Isolate | Biomass production (mg) | |||||
|---|---|---|---|---|---|---|
| Concentration (ppm) | ||||||
| 0ppm | 40ppm | 60ppm | 100ppm | 150ppm | 200ppm | |
| MT-4 | 240.34d | 147.01e | 120.34efg | 110.34efg | 100.36efgh | 60.34h |
| UBT-18 | 269.70d | 147.03e | 131.70ef | 123.70efg | 86.70fgh | 79.70gh |
| IBT-II | 577.03a | 523.70b | 453.70c | 440.37c | 410.37c | 240.36d |
*Values followed by different letters differ significantly according to Duncan's multiple range test at p=0.05.
Significant reductions in microbial biomass and soil respiration have been found in metal-contaminated soils compared to uncontaminated ones.28–30 Optimum biosorption conditions depend on pH, biomass of the microorganism, contact time and temperature. The Langmuir, Freundlich and Dubinin–Radushkevich model, which describes the biosorption isotherm of a metal ion, has indicated that biosorption of cadmium by Hylocomium splendens biomass occurs through chemical ion exchange.31 The main functional groups responsible for a biosorption process are hydroxyls, carbonyls, carboxyls, sulfonates, amides, imidazoles, phosphonates, and phosphodiester groups as established by Pradhan et al.32 and Volesky.33 Some of these groups are present in Trichoderma sp. biomass and may interact with the metal ions. It has also been reported that binding of Ni(II) to biopolymers occurs mainly in the peptidoglycan layer of the cell surface.34
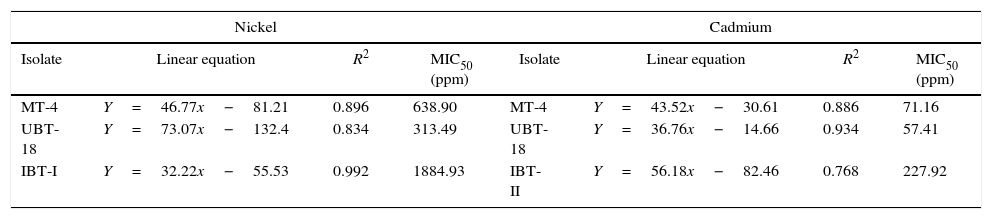
The minimum inhibitory concentrations of nickel and cadmium required for 50% of growth inhibition (MIC50) of the isolates were computed, and the results are presented in Table 6. The highest minimum inhibitory concentration of nickel was calculated for IBT-I (1884.93ppm), followed by MT-4 (638.90ppm), whereas the highest MIC50 of cadmium was calculated for IBT-II (227.92ppm), followed by MT-4 (71.16ppm).
Minimum inhibitory concentrations of nickel and cadmium for the Trichoderma isolates.
| Nickel | Cadmium | ||||||
|---|---|---|---|---|---|---|---|
| Isolate | Linear equation | R2 | MIC50 (ppm) | Isolate | Linear equation | R2 | MIC50 (ppm) |
| MT-4 | Y=46.77x−81.21 | 0.896 | 638.90 | MT-4 | Y=43.52x−30.61 | 0.886 | 71.16 |
| UBT-18 | Y=73.07x−132.4 | 0.834 | 313.49 | UBT-18 | Y=36.76x−14.66 | 0.934 | 57.41 |
| IBT-I | Y=32.22x−55.53 | 0.992 | 1884.93 | IBT-II | Y=56.18x−82.46 | 0.768 | 227.92 |
Determination of residual nickel in the culture broth after harvesting mycelial biomass from the metal-amended media revealed total removal of nickel by the increased biomass of the Trichoderma isolates at Ni concentrations of up to 40ppm, followed by an increase in residual nickel and a decrease in biomass production at higher nickel concentrations in the medium. Among the isolates, IBT-I was most potent in biosorption of nickel, followed by UBT-18 and MT-4 (Fig. 5). The results are in agreement with the findings of Sarkar et al.,26 who recorded 90.2% removal of Ni from a 50ppm-amended culture broth by T. harzianum after seven days of growth, beyond that, there was no increase in metal uptake. The use of a solid-phase extraction process to determine biosorption of heavy metals showed that 0.59μg of nickel could be removed by Aspergillus fumigatus from a liter of polluted water.35
The trend of cadmium biosorption was quite different, so that the increasing cadmium concentrations resulted in decreasing biomass production by all of the test isolates and positively correlated with increased residual cadmium in the culture broth (Fig. 6).
It has been suggested that metal uptake by T. harzianum is highly pH- and temperature-dependent and the maximum metal uptake takes place at pH 4.36,37 At pH values above 7, metal uptake is reduced as metals exist as hydroxide colloids and precipitate at alkaline pH due to osmotic changes and a hydrolyzing effect,38,39 thus resulting in a decrease in the sorption rate.40 As biomass of T. harzianum increased, the pH of the medium was shown to become more acidic41; however, below pH 4 biomass production was reduced and subsequently the residual metal concentration increased. Low absorption of heavy metals at low pH is attributed to the competition between the hydrogen ion and the metal ion at the sorption site.42
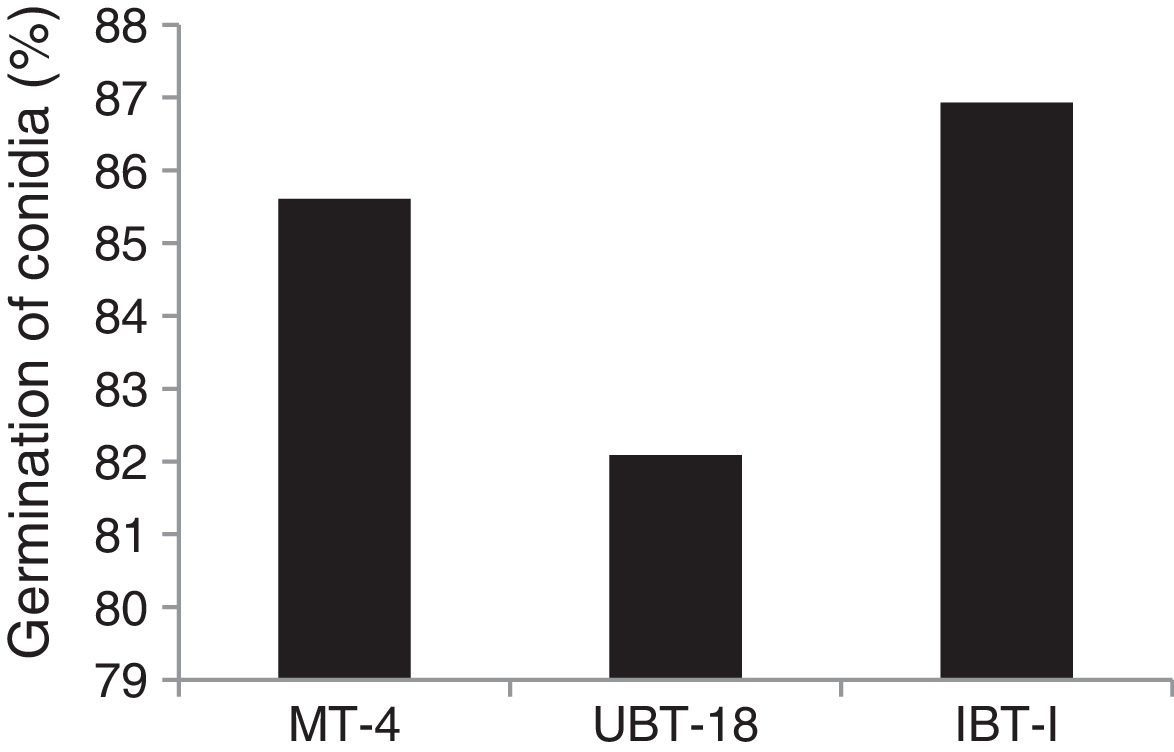
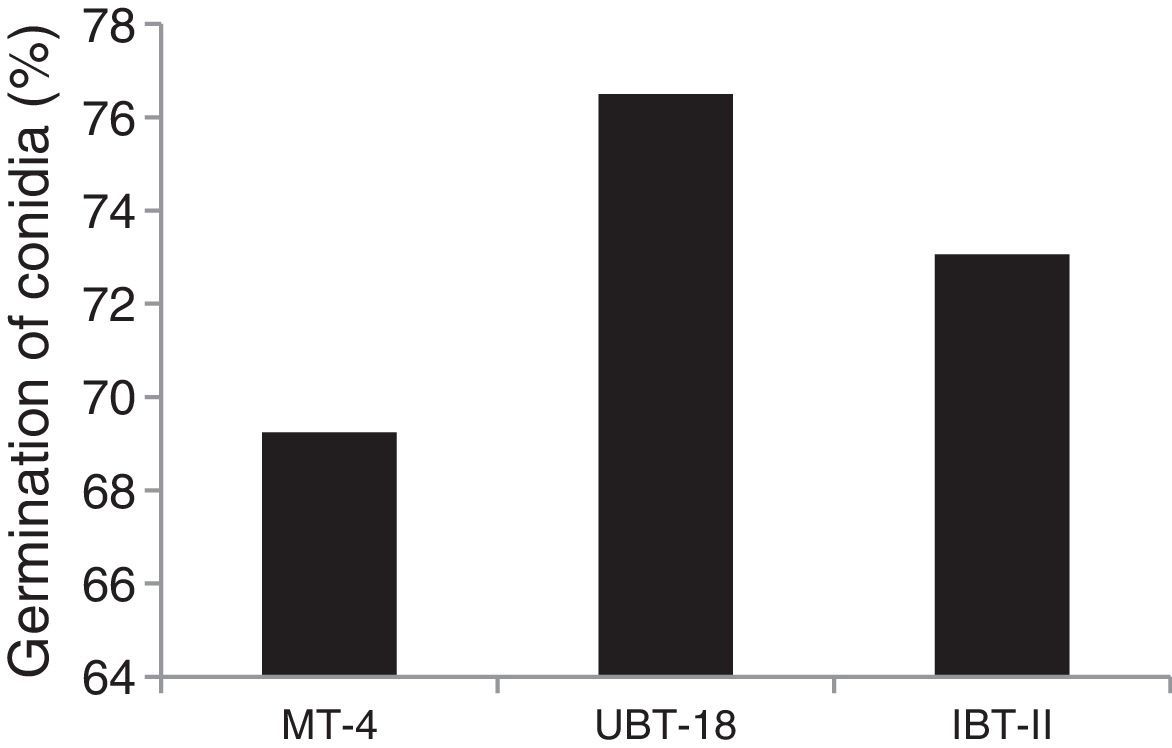
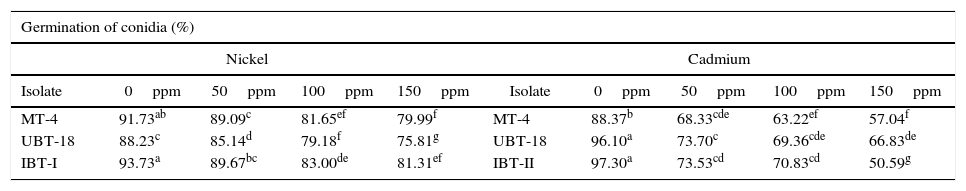
The results obtained while studying germination of conidia of the Trichoderma isolates under nickel and cadmium stress conditions revealed that the isolates differed significantly in their responses to fungistatic effects (Table 7). Under non-amended conditions, a significantly lower germination rate was shown by UBT-18 (88.23%) compared to IBT-I and MT-4 (93.73 and 91.73%, respectively). The different levels of nickel stress also had significant effects on spore germination; however, the variation in the interaction effect with the Trichoderma isolates was non-significant. Irrespective of the nickel concentration, IBT-I was most resistant to fungistatic effects (86.93% conidial germination), followed by MT-4 and UBT-18 (85.61 and 82.09% conidial germination, respectively) (Fig. 7). In the absence of cadmium, IBT-II exhibited the highest germination rate (97.30%), which significantly differed from that of MT-4 (88.37%). With the increase in the cadmium concentration, germination was significantly affected, particularly in the case of IBT-II, indicating that the isolate was very sensitive to the fungistatic effect. UBT-18 was found to be most tolerant to the fungistatic effect, even at a higher cadmium stress level (66.83% conidial germination), and its germination rate was significantly different from that of MT-4 (57.04%). Irrespective of the cadmium concentration, UBT-18 showed the highest resistance to the fungistatic effect (76.50% conidial germination), followed by IBT-II and MT-4 (73.06 and 69.24% conidial germination, respectively) (Fig. 8). Partial annulment of soil fungistasis has a significant impact on survival and population dynamics of Trichoderma and Gliocladium in soil.43 Roy and Pan44 reported that gamma irradiation significantly increased phialospore and chlamydospore germination rates in mutants of T. harzianum and Gliocladium virens compared to their wild types.
Effect of fungistasis on conidial germination of the Trichoderma isolates under nickel and cadmium stressed condition.
| Germination of conidia (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nickel | Cadmium | ||||||||
| Isolate | 0ppm | 50ppm | 100ppm | 150ppm | Isolate | 0ppm | 50ppm | 100ppm | 150ppm |
| MT-4 | 91.73ab | 89.09c | 81.65ef | 79.99f | MT-4 | 88.37b | 68.33cde | 63.22ef | 57.04f |
| UBT-18 | 88.23c | 85.14d | 79.18f | 75.81g | UBT-18 | 96.10a | 73.70c | 69.36cde | 66.83de |
| IBT-I | 93.73a | 89.67bc | 83.00de | 81.31ef | IBT-II | 97.30a | 73.53cd | 70.83cd | 50.59g |
Values followed by different letters differ significantly according to Duncan's multiple range test at p=0.05.
In conclusion, the present investigation highlights the significance of Trichoderma spp. as potential metal biosorbents. Four isolates obtained in this study, viz., MT-4, UBT-18, IBT-I, and IBT-II, can be exploited as potent bioremediation agents in nickel- and cadmium-polluted agricultural fields.
Conflicts of interestThe authors declare no conflicts of interest.