In this work, four isolates of endophytic fungi (Alternaria alternata, Colletotrichum gloesporioides, Glomerella cingulata and Nigrospora sphaerica), deposited in the culture collection ‘University Recife Mycologia’ (URM) at the Universidade Federal de Pernambuco, were characterized for the genes ITS 1 and 4 (region 5.8 S) and evaluated for taxol production.
Endophytic microorganisms are those that live inside plants, inhabiting the aerial parts, such as the leaves and stems, without causing any damage to their hosts, unlike pathogens.1,2 However, an endophytic microorganism may become pathogenic, if there is an imbalance between their virulence and the plants defense.3 Endophytic fungi have biotechnological relevance for many applications, such as for use in bioremediation processes4 and the production of compounds with antimicrobial, antioxidant or antitumor activities.5–7 However, endophytic fungi have still been poorly explored industrially.8
According to the World Health Organization (WHO), 8.8 million deaths occurred in 2015, due to cancer, and it is estimated that 12.6 million deaths will occur per year by 2030.9 Taxol (paclitaxel) is a potent drug used in the treatment of some neoplasms, as both a first – and second – line of treatment.10 It acts by inhibiting cell replication through binding to the beta-tubulin subunit of microtubules and induces apoptosis by inactivating the apoptosis inhibitory protein Bcl-2.11 This compound is naturally produced by the bark of yew plants (Taxus species). However, new methods to obtain it are being explored, because its extraction or semi-synthesis of sufficient quantities to supply the current demand would imply devastation, as the removal of the bark results in the death of the Tree.12,13
Taxol can also be produced by some fungi such as Colletotrichum gloeosporioides, Glomerella cingulata, Nigrospora sphaerica, Pestalotiopsis guepinii, Alternaria alternata, and Fusarium solani.14 Genetic screening for taxol production is currently used to identify microorganisms that produce it, and three key genes are usually investigated: ts (encoding taxadiene synthase), dbat (encoding 10-deacetylbaccatin III-10-O-acetyltransferase), and bapt (encoding C-13 phenylpropanoyl side chain-CoA acyltransferase).15
Due to the great importance of taxol in anti-cancer therapy, there is a constant search for new production methods. In this work, four isolates of endophytic fungi, deposited in a culture collection from the Universidade Federal de Pernambuco, were evaluated for their ability to produce taxol through a search of the genes involved in the taxol metabolic pathway. Next, the presence of taxol in the cell mass and metabolic liquid of fungal cultures was investigated by LC/MS. In addition, the isolates were molecularly characterized for the ITS 1 and 4 genes (region 5.8 S).
The study was performed with four isolates of endophytic fungi that, belong to different genera and were, previously deposited in the culture collection ‘University Recife Mycologia (URM)’ of the Departamento de Micologia at the Universidade Federal de Pernambuco. The place of collection and the plant from which they were isolated are described in Table 1. All of the samples were stored lyophilized and/or in mineral oil.
Fungal isolates used in this work, with respective place and plant of collection.
| Isolate | City of collection | Year | Plant |
|---|---|---|---|
| Nigrospora sphaerica | Recife | 2007 | Indigofera suffruticosa |
| Alternaria alternata | Recife | 2007 | Lippia sidoides (stem) |
| Colletotrichum gloesporioides | Jaboatão dos Guararapes | 2012 | Plantago major (leaf) |
| Glomerella cingulata | Arcoverde | 2008 | Bean leaves |
All cities are located at Pernambuco state, northeastern Brazil.
The isolates were reactivated in Potato Dextrose Agar (PDA) medium. The standard cetyl trimethylammonium bromide (CTAB) method was used for the extraction of total DNA. The genes ITS 1 and 4 (ITS1-5.8S-ITS2 rDNA) were amplified using the oligonucleotide primers ITS1 and ITS4. The rDNA gene sequences of the samples were compared with others deposited in the NCBI Genbank database using the BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST/). The quality of the sequencing was analyzed using the Pregap4 4.0 and Gap4 4.0 programs of the Staden package. Screening of the genes in the taxol etabolic pathway was performed using three conserved sequences of the main genes involved in taxol biosynthesis: ts, dbat and bapt (Table 2).
Oligonucleotide primers used in the screening of taxol biosynthesis genes by PCR.
| Gene | Primers | Sequences (5′–3′) | Size of amplification |
|---|---|---|---|
| ts | ts – F ts – R | CAAACCCATGTCGAATTGAGAAG CAAGTTTGCATACACTCTGGAATCT | 631 bp |
| dbat | dbat – F dbat – R | GGGAGGGTGCTCTGTTTG GTTACCTGAACCACCAGAGG | 153 bp |
| bapt | bapt – F bapt - R | CCTCTCTCCGCCATTGACAA TCGCCATCTCTGCCATACTT | 453 bp |
To obtain the pre-inoculum, fungi were cultivated in Petri dishes in PDA medium for 7 days at 28°C. Next, blocks (6mm diameter) were made, and six of them were transferred to 2-L Erlenmeyers flasks, with one quarter of their capacity filled with Potato Dextrose medium (24g/L), supplemented with chloramphenicol (0.1g/L), phenylalanine (0.04g/L), magnesium sulfate heptahydrate (2g/L), and ammonium sulphate (12g/L). Three flasks were prepared for each fungus. The fungi were cultivated at 28°C under agitation (160rpm) and every 7 days an erlenmeyer of each species was used for extraction.
The cell mass was treated with ethyl acetate in order to extract intracellular secondary metabolites. For this, 10mL of the solvent was added to 1g of the wet weight of each cell mass, and the mixture was subjected to agitation (180rpm) for 20min. For extraction of the compounds present in the metabolic liquid, ethyl acetate was added to the liquid at a ratio of 2:1 (v/v) and subjected to stirring at 180rpm for 30min.
To verify taxane production, the extracts were evaluated by thin layer chromatography (TLC) in a saturated chamber, using 60 F254 silica gel plates with 0.25mm thickness (Macherey-Nagel, Germany). The standard, paclitaxel (Cayman Chemical, MI, USA), was dissolved in methanol, and the Liebermann-Burchard reagent, anisaldehyde-H2SO4, and sulfuric vanillin were used as chemical developers. Chloroform–acetonitrile (7:3 v/v) was used as the elution system.
High performance liquid chromatography (HPLC) was performed using a Prominence LC-20AT liquid chromatograph (Shimadzu, Kyoto, Japan), consisting of an LC-20AT quaternary pump, DGU-20As degasser, CTO-20AC column oven, SPDM-20A diode array detector (DAD), SIL-20A autoinjector and CBM-20A communication module, controlled by the LcSolution software. The extracts were passed through 0.45μm membrane filters (Supelco, Sigma–Aldrich, MO, USA). A flow rate of 1.0mL/min was employed. The solvents used were methanol, acetonitrile (Merck, Darmstadt, Germany), and ultrapure water at a ratio of 25:35:40 (v/v/v). The taxol content was estimated using a standard curve (Y=5189.7X+1083.3), with a limit of detection of 0.0473μg/mL and a limit of quantitation of 0.1435μg/mL.
Liquid chromatography coupled with mass spectrometry (LC/MS) was performed with an LC/MS ACQUITY UPLC H-Class-SQ Detector 2 column (Waters), with a dimension of 2.1×100mm, pore size 130Å, and particle size 1.7μm. The mobile phase was acetonitrile/MilliQ water/methanol at flow rate of 0.610mL/min.
In the present work, we evaluated four isolates from this collection, obtained from different plants collected in different regions of Pernambuco, in regard to their ability to produce taxol.
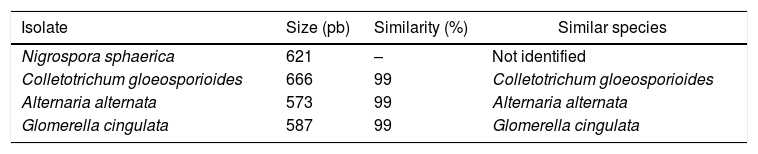
The isolates were previously identified by Stackebrant and Goebel.16 In the present work, we performed a molecular characterization of the isolates based on the ITS genes. For all the isolates, the sequences of the ITS rDNA genes obtained, showed a homology of greater than or equal to 99% (Table 3) with the previously determined species, except for N. sphaerica. All four isolates had the ts and dbat genes, and only two presented the bapt gene. Extracts from the cell mass and metabolic liquid were then evaluated by TLC, but taxol was not identified in any sample. This result may be associated with interfering substances present in the samples. On the other hand, HPLC analysis showed that the extracts from the metabolic liquid produced by C. gloeosporioides (14th and 21st days) exhibited a peak with a retention time close to that of paclitaxel, and the UV spectrum was also similar to this standard, confirming taxol production. The standard paclitaxel showed a retention time of 2.71, min and the sample presented a peak at 2.62±0.02min (Fig. 1). In addition, the molecular mass detected for the compound, produced by C. gloeosporioides, was 852.32g/mol, which is close to the molecular mass of taxol (853.906g/mol). The taxol content was estimated at 5.24μg/mL in the sample collected on the 14th day and 4.4μg/mL in the sample from the 21st day.
Molecular characterization of the isolates by analysis of ITS rDNA genes.
| Isolate | Size (pb) | Similarity (%) | Similar species |
|---|---|---|---|
| Nigrospora sphaerica | 621 | – | Not identified |
| Colletotrichum gloeosporioides | 666 | 99 | Colletotrichum gloeosporioides |
| Alternaria alternata | 573 | 99 | Alternaria alternata |
| Glomerella cingulata | 587 | 99 | Glomerella cingulata |
The search for new strategies to obtain taxol is of utmost importance, as about 10,000kg of yew leaves and bark are needed to isolate 1kg of this substance.12 In addition, the semi-synthesis methods consume a large amount of trees and are not enough to meet the demand.8 Alternative strategies, such as the optimization of Taxus cell cultures and production using microbial sources, have gained increasing attention. The URM culture collection possesses a large collection of endophytic fungi that have still not been screened for their biotechnological potential. This is the first report of an evaluation of the biotechnological potential of some of the isolates in the collection for taxol production.
In addition, molecular analysis was performed to confirm the identity of the isolates. According to Nilsson et al.,17 a margin of 2% is acceptable for intraspecific divergence in the ITS region sequences. The ts gene has been proposed as a primary screening method to identify taxol-producing fungi whereas dbat has been reported to be more diagnostic as a molecular marker.18 However, it is common to only detect one or two of the genes associated with taxol production in microorganisms. For example, endophytic fungi from different genera, isolated from Salacia oblonga bark, were screened for the presence of two genes; seven had the dbat gene, and one contained bapt.19 Despite the detection of the genes, the effective production of the compound must also be confirmed. According to Xiong et al.,15 the ts and dbat genes are essential for taxol biosynthesis, but are not diagnostic, because the bpat gene encodes the enzyme required to convert the precursor baccatin III to taxol. In the present study, all of the isolates presented ts and dbat. However, our data showed that only the C. gloeosporioides isolate is a promising producer of paclitaxel, although it was negative for the bpat gene.
The other isolates may also be able to produce taxol, but under conditions different from those used here. It has been reported that stress conditions can be favorable for the production of this compound. Somjaipeng et al.20 reported that stress, associated with environmental factors (water activity or pH), and the presence of elicitors (ammonium acetate, jasmonic acid, phenyl-alanine, salicylic acid, serine, silver nitrate and sodium acetate) induced the production of taxol by the endophytic fungi Paraconiothyrium variabile and Epicoccum nigrum, isolated from Taxus baccata. In addition, the production of metabolites by endophytes may depend on others factors, such as and multipartite interactions with host plants, as well as the selection pressures of biotic (such as pathogens and predators) and abiotic (such as precursors of metabolites and environmental conditions) factors.8 Many studies have been conducted searching for taxol-producing microorganisms among the endophytes found in Taxus plants.15,19,20 In this study, the isolate from endophytic C. gloeosporioides possessed two genes involved in taxol biosynthesis, and the presence of this compound was confirmed in the metabolic liquid it produced. This study shows the biotechnological potential of an isolate stored at the URM collection as a producer of a substance with high pharmacological relevance. However, future studies using polymer nanoparticles may facilitate the industrial use of taxol in antitumor activities.
The authors express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for fellowships (PMGP, THN and MTSC) as well as to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are also grateful for the Plataforma de Sequenciamento (LABCEN-CB) of the Universidade Federal de Pernambuco.