The Spitzenkörper is a dynamic and specialized multicomponent cell complex present in the tips of hyphal cells. The amphiphilic styryl dye FM4-64 was found to be ideal for imaging the dynamic changes of the apical vesicle cluster within growing hyphal tips. It is widely used as a marker of endocytosis and to visualize vacuolar membranes. Here we performed uptake experiments using FM4-64 to study the dynamic of the Spitzenkörper in Trichosporon asahii. We observed that Spitzenkörpers were present at the tip of the budding site of the spore, blastospore, and the germ tube of T. asahii. We also found that Spitzenkörpers were present at the tip of the hyphae as well as the subapical regions. Cytochalasin D, an inhibitor of actin polymerization, leads to abnormal Spitzenkörper formation and loss of cell polarity.
Studies have shown that vesicle trafficking is fundamental to numerous activities in eukaryotic organisms, and is involved in cell growth and differentiation. The vesicle trafficking network includes exocytosis and endocytosis.1 In filamentous fungi, vesicle trafficking is concerned with the polarized tip growth.2,3 Vesicle trafficking to the apex during tip growth is highly organized and involves the activity of a specific, multicomponent organelle complex which, in most cases, is called the Spitzenkörper. The Spitzenkörper is a vesicle-organizing center that is intimately involved in fungal cell morphogenesis, and it spatially and temporally coincides with polarity-related components. The Spitzenkörper was originally recognized as a dark region in phase-contrast microscopy at the tip of actively growing hyphae.4 Subsequently, electron microscopy revealed that this structure is rich in secretory vesicles.5 During tip growth, the dynamic behavior of the Spitzenkörper is predominated by secretory vesicles that form an “apical vesicle cluster,” which is intimately associated with the precise growth pattern of the hyphal apex.6
The Spitzenkörper regulates hyphal apical growth primarily via exocytosis. The role of Spitzenkörper in endocytosis and recycling is currently not clear. Here we report that the Spitzenkörper comprises early compartments of the endocytic pathway in Trichosporon asahii. We performed uptake experiments using FM4-64, a widely used dye for endocytosis, to study the vesicle trafficking.7 We observed that cytochalasin D, an inhibitor of actin polymerization, leads to abnormal Spitzenkörper formation and loss of cell polarity.
MethodsStrains and growth conditionsThe type strain of T. asahii, strain CBS 2479, was purchased from Centraalbureau voor Schimmelcultures (Utrecht, Netherlands) and used in this study. Cells were propagated by incubation in YPD (1% yeast extract, 2% peptone, 2% dextrose) medium at 37°C. For FM4-64 (Biotium) staining, 5μL of the stock solution (1.64mmol/L in dimethyl sulfoxide (DMSO)) was added directly to 20mL of culture, followed by incubation for an additional 10min. The cells were harvested by brief centrifugation and washed with phosphate-buffered saline (PBS, pH 7.4).
Spitzenkörper inhibitionCytochalasin D (Fermentek Ltd.) was dissolved in DMSO to obtain a concentration of 2mmol/L, and this was added to culture to a final concentration of 0.2, 0.5, 1, 2, or 5μmol/L. Same amount of DMSO without Cytochalasin D was used as a control. The effects of the various concentrations of inhibitor were tested by pretreating the samples with the indicated compound for 15min prior to the addition of FM4-64. In some experiments, the cells were treated with the inhibitor for 2h, after which the inhibitor solution was removed, and the cells were washed once and then resuspended in fresh medium without inhibitor or dye. The cells were then allowed to recover for up to 4h, after which the dye was added to the cells for analysis.
Fluorescence microscopyMounted slides were photographed using a Leica DM 3000 fluorescence microscope with a Leica DFC300FX camera and an LP560 filter (Ex543/Em560). The viability of the single-celled propagules was confirmed by taking aliquots and determining the percentage that germinated after overnight incubation in the medium. Viability of hyphae was confirmed by their general appearance, presence of cytoplasmic streaming, and observation of continued tip elongation and growth. Images were recorded using a real-time digital imaging setup. At least 30 individual cells were examined for each experiment, and all the experiments were performed using at least three independent batches of cells.
ResultsUptake of FM4-64 at different stages of cell growth and developmentFM4-64 is a lipophilic probe that fluoresces strongly after binding to the outer plasma membrane. Ten minutes after FM4-64 was added to the culture medium, fluorescence was observed in both apical and subapical hyphal compartments. To determine the kinetics of FM4-64 staining, cells were cultured at room temperature in the presence of FM4-64 and samples were examined at various time-points.
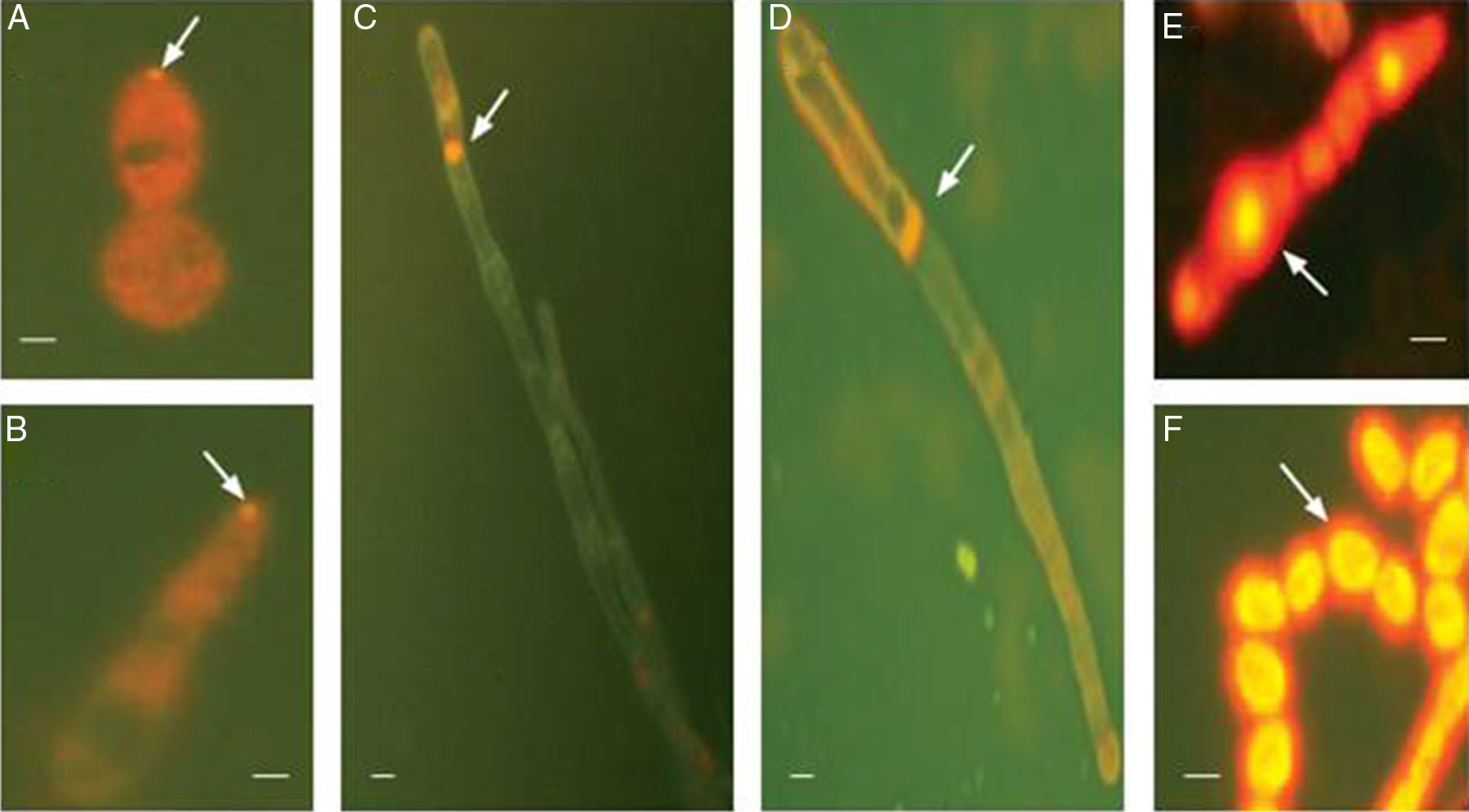
At 8h, a single fluorescent spot with a diameter of 0.5–1μm was observed at the tip of the budding site of spores (Fig. 1A). Similar fluorescent spots were observed at the tip of the germ tube (Fig. 1B) at 16h. Dim background staining was observed in the cytoplasm (Fig. 1A and B). After 24h of culture, a larger and brighter fluorescent spot (1–2μm in diameter) was observed at the apical or subapical hyphae (Fig. 1C). This fluorescent spot resembles the Spitzenkörper structure.8 Fluorescent staining was also observed at septins (Fig. 1D), which could be the sites for branch projection. In addition, strong fluorescence was observed in the elongated spores of the pseudohyphae (Fig. 1E), as well as the chain-like spores (Fig. 1F). No Spitzenkörpers were noted in these structures.
FM4-64 staining of T. asahii cells at different time points. Cells were cultured at room temperature in the presence of FM4-64 and were imaged after 8h (A), 16h (B) and 24h (C–F). (A) Fluorescence spot at the tip of the budding site of a spore (arrow). Background staining was seen in cytoplasm (orange). (B) Fluorescence spot at the tip of a germ tube (arrow). Background staining was seen in cytoplasm (orange). (C) Staining of Spitzenkörper structure at the subapical hyphae (arrow). (D) Staining of septins (arrow). (E and F) Staining of elongated spores of the pseudohyphae and chain-like spores (F). Scale bar: 1μm.
FM4-64 uptake was assessed in the presence of Cytochalasin D, an inhibitor of filamentous actin. T. asahii cells were treated with different concentration of Cytochalasin D (0.2, 0.5, 1, 2, or 5μmol/L) for 15min prior to the addition of the FM4-64, and the inhibitor was kept in the solution throughout the course of the experiment.
Light microscopy observation revealed that as the increase of Cytochalasin D concentration (from 0.2 to 5μmol/L), the inhibition effects also increased. Hyphal growth became slower and hyphae became shorter but wider (Fig. 2).
When treated with a low dose of Cytochalasin D (0.2μmol/L), the fluorescence was scattered in the central portion of the hyphae, but no obvious fluorescence was observed at the top of the hyphae (Fig. 3B). As the concentration increased, fluorescence was scattered further into the entire cell body (Fig. 3C–E). At 5μmol/L, the hyphal growth was completely blocked and only a low background fluorescence was observed (Fig. 3F). No Spitzenkörper was observed when cells were treated with any concentration of Cytochalasin D. In the absence of Cytochalasin D, FM4-64-stained apical vesicle clusters at the tip of hyphae (Fig. 3A).
Cytochalasin D treatment blocked the formation of Spitzenkörper. Cells were treated with various concentration of Cytochalasin D (0, 0.2, 0.5, 1, 2, or 5μmol/L) then added FM4-64 and grew for 72h. Cells were imaged with a fluorescence microscope. The 0μmol/L Cytochalasin D (A) was used as a control. Scale bar: 1μm.
The Spitzenkörper is a dynamic structure and closely related to polarized growth. It is continuously present at the hyphal tip at all stages of the cell cycle. In this study, we revealed the presence of Spitzenkörpers at the tip of the budding site of the spore, blastospore, and the germ tube of T. asahii. We also found that Spitzenkörpers were present at the tip of the hyphae as well as the subapical regions. In addition, the spores at the tip of the hyphae showed FM4-64 fluorescence, suggesting that the spore may have produced germ tubes in the opposite direction. Interestingly, some other structures of T. asahii, such as hyphae and pseudohyphae were also presented at both ends of the Spitzenkörper (Fig. 1E), suggesting that pseudohyphae could grow bi-directionally and continue to be regulated by the Spitzenkörper.
Using transmission electron microscopy, we showed previously that pseudohyphae produced germ tubes and hyphae.9 The strong fluorescence at the elongated spores of the pseudohyphae further suggests that T. asahii pseudohyphae could form true hyphae. The phenomenon confirmed that T. asahii pseudohyphae did not lose polar growth momentum, and they continued the budding transition to hyphae.
Polarized growth, which enables cells to assume a shape typical for the species, is a fundamental feature for all fungal cells. In Saccharomyces cerevisiae, growth is confined to the daughter buds, which initially grow in a polarized fashion and then switch to isotropic growth.10–14 In pseudohyphal cells, polarized growth persists, resulting in longer buds.15 Polarized growth depends on the actin cytoskeleton, which consists of cortical actin patches and actin cables.14 In both Candida albicans and T. asahii, disruption of actin cables by cytochalasin A (for C. albicans) or cytochalasin D (for T. asahii) causes the hyphal tips swell and the growth mode switches from polarized to isotropic. Consistent to these reports, our current study showed that Cytochalasin D treatment caused the loss of polarity (Fig. 3), which could be caused, at least part, the diminishing of the Spitzenkörper.16
Conflicts of interestThe authors declare no conflicts of interest.
We thank the staff of the Dermatology and Venereology Department, Beijing Military Command General Hospital of PLA for their assistance. This project was supported by the National Natural Science Foundation of China (No. 81271764) and the 11th Plan Project of PLA of China (No. 06MB078).