The aim of this study was to evaluate the effects of alginate entrapment on fermentation metabolites of Kluyveromyces marxianus grown in agrowastes that served as the liquid culture media. K. marxianus cells entrapped in Na-alginate were prepared using the traditional liquid-droplet-forming method. Whey and pomaces from processed tomatoes, peppers, and grapes were used as the culture media. The changes in the concentrations of sugar, alcohol, organic acids, and flavor compounds were analyzed using gas chromatography–mass spectrometry (GC–MS) and high pressure liquid chromatography (HPLC). Both free and entrapped, K. marxianus were used individually to metabolize sugars, organic acids, alcohols, and flavor compounds in the tomato, pepper, grape, and acid whey based media. Marked changes in the fermentation behaviors of entrapped and free K. marxianus were observed in each culture. A 1.45-log increase was observed in the cell numbers of free K. marxianus during fermentation. On the contrary, the cell numbers of entrapped K. marxianus remained the same. Both free and entrapped K. marxianus brought about the fermentation of sugars such as glucose, fructose, and lactose in the agrowaste cultures. The highest volume of ethanol was produced by K. marxianus in the whey based media. The concentrations of flavor compounds such as ethyl acetate, isoamyl alcohol, isoamyl acetate, 2-phenylethyl isobutyrate, phenylethyl acetate, and phenylethyl alcohol were higher in fermented agrowaste based media compared to the control.
At present, there is a great deal of interest in exploring the potential of agrowastes to replace synthetic media in the microbial fermentation of high-value commercial bio-products. The advantages of using wastes from the food industry include their availability, low cost, and the presence of high levels of compounds that could be utilized by microorganisms.1,2 In a recent study by Guneser,3Trichoderma atroviride grown in a liquid fermentation culture containing tomato and pepper pomaces was shown to produce eight-carbon volatiles, including 1-octen-3-ol (a mushroom aroma). Göğüş et al.4 reported that apple pomace and orange peel may be used as culture media for the production of a polygalacturonase enzyme that serves as a pectolytic enzyme in the industrial production of wine and fruit juices.
While the use of agrowastes as raw material reduces the process costs of microbial fermentation, the microbial immobilization technique increases fermentation efficiency. Microbial cells entrapped in various substrates have been used extensively in the commercial bioreactor fermentation for decades, owing to improved stability and quality of products,5 and the economic advantages that can be accrued via the reutilization of microbial cells such as higher productivity, increased product yield, and easier isolation of the desired metabolites from the culture broths.6,7 For instance, the use of immobilized cell reactor not only increased the ethanol yield from glucose by approximately 27% but also reduced the fermentation time from 72h to 24h.8 In another study, Djordjevic et al. reported that immobilized Saccharomyces cerevisiae used in raspberry wine fermentation had a shorter lag phase, utilized sugars more rapidly, and produced ethanol more quickly than suspended free cells.9 Researchers also found that the concentrations of 3-methylbutyl acetate and 2-phenylethyl acetate were higher in raspberry wine fermented with immobilized S. cerevisiae than in that fermented with suspended free cells.
Although several studies have discussed the immobilization techniques used in alcoholic fermentation,8–10 there is a dearth of research into the potential use of immobilized cells in agrowaste fermentation processes to produce specific bio-products such as enzymes, microbial pigments, and volatile compounds. This study therefore sets out to evaluate the effects of microbial entrapment on fermentation metabolites of K. marxianus in liquid media composed of agrowaste materials; in particular, an attention has been given to the effect on volatile metabolites. K. marxianus possesses great biotechnological potential due to its metabolic properties such as high specific growth rate, high ethanol tolerance, and the ability to utilize a wide spectrum of substrate.11
Materials and methodsPreparation of agrowastesThe plant waste materials were prepared from Polish fruits and vegetables obtained from a Polish market. The fresh material was frozen (−20°C), then thawed, mashed, and heated to around 60°C. The juice was separated from the mash by subsequent pressing and decanting. After the decanting procedure, pomaces were obtained. The pomaces were pasteurized (15min at 85°C), bottled, and stored at 4°C until the fermentation experiments. Acid whey was prepared from Polish milk (3% fat) by lactic acid fermentation with commercial yoghurt starter cultures (24h at 44°C). Deproteinized whey was obtained by heating (60°C) followed by filtration. The basic composition of the materials was determined using the methods of analysis as recommended by AOAC.12
Yeast strain and culture conditionsKluyveromyces marxianus LOCK0024 strain obtained from the LOCK105 Culture Collection of Lodz University of Technology, Poland was used in this study. The yeast strains were maintained on Wort Agar slants (Merck-Millipore, Germany). In order to adapt the yeast strains to the specific environmental conditions, the yeast cells were cultivated in 100mL of minimal media (NH4)2SO4 (3.0g/L), KH2PO4 (1.0g/L), K2HPO4 (1.0g/L), MgSO4 (0.5g/L), yeast extract (Merck-Millipore) (0.5g/L), and glucose (1.0g/L) supplemented with 10% (w/v) of the agrowastes in 500mL round bottom flasks. The cultures were incubated at 25°C on a rotary shaker (Heidolph, Germany) at 220rpm for 5 days. The concentrations of yeast cells were determined using a hemocytometer, Olympus BX41 (Olympus, Japan) microscope, and a digital camera.
Entrapment of K. marxianusThe K. marxianus cells were entrapped using a traditional liquid-droplet-forming method from a foamed alginate solution.13 The resulting beads consisted of micro-spheres approximately 50–300μm in diameter. The foamed beads were kept in a sterile syringe under a mild vacuum at 10°C for 24h in a liquid minimal medium of glucose (0.05%, w/v).
Yeast fermentation trialsYeast fermentations using both entrapped and free cells were performed in 50mL minimal medium supplemented with 10% (w/v) of the agrowastes and incubated at 120rpm for 120h at 25°C. The same inoculum was used in both the free and entrapped cells (1.1×109cells/50mL of medium) and was determined beforehand using the microscopic method.
Immobilization process controlThe alginate beads were cut into slices (1mm thick), and the bead structure and colonization were observed using a light microscope (Olympus BX41). The alginate beads were dissolved in 0.2M Na2HPO4 with a ratio of 1bead/mL. The concentration of yeast cells per bead was determined using a hemocytometer and the free cells were counted via microscopic method.13
HPLC analysisThe quantities of sugars, alcohols, and organic acids were measured using high performance liquid chromatography (HPLC) with a Finnigan Surveyor chromatograph (Thermo Scientific, Waltham, MA, USA) equipped with an auto sampler system, a refractive index detector (Finnigan Surveyor-RI Plus detector), a diode array detector (Finnigan Surveyor-PDA Plus detector), and a 300mm×7.8mm Aminex HPX-87H column (BioRad, Hercules, CA, USA). HPLC was performed at 60°C using sulfuric acid (5mmol/L) as the eluent at a flow rate of 0.6mL/min and with a sample volume of 10μL.14
Extraction and analysis of volatile compoundsThe volatile compounds were extracted using solid-phase micro extraction (SPME).15 The sample (3g) was weighed in a 40mL amber colored screw top vial with a PTFE/silicon septum hole cap (Supelco, Bellafonte, USA), and 1g of NaCl was added. The vial was incubated at 40°C in a water bath (GFL, Grossburgwedel, Germany) for 20min to equilibrate the volatiles in the headspace. An SPME (2cm to 50/30μm DVB/Carboxen/PDMS, Supelco, Bellafonte) needle was then inserted into the vial. The SPME fiber was held in the headspace of the vial at a depth of 2cm for 20min at 40°C. The flavor compounds were identified using gas chromatography–mass spectrometry (GC–MS).3 A nonpolar HP5 MS column (30m×0.25mm ID×0.25μm film thickness, J&W Scientific, Folsom, CA) was used to separate the flavor compounds. The GC–MS system consisted of an HP 6890 GC and 7895C mass selective detector (Agilent Technologies, Wilmington, DE, USA). The temperature of the oven was increased from 40°C to 230°C at a rate of 10°C/min with initial and final hold times of 5 and 20min, respectively. Helium was used as the carrier gas with a constant flow of 1.2mL/min. The mass spectrometry detector (MSD) conditions were as follows: capillary direct interface temperature 280°C; ionization energy 70eV; mass range 35–350amu; scan rate 4.45scans/s. The identification of the flavor compounds was based on a comparison of the mass spectra of unknown compounds with the mass spectral databases in the National Institute of Standards and Technology16 and Wiley Registry of Mass Spectral Data.17 The volatiles were quantified based on the relative abundances of the compounds.18 The retention indices were calculated following the method developed by Van den Dool and Kratz.19 2-methyl-pentanoic acid and 2-methyl-3-heptanone were used as internal standards (IS) for acidic and neutral-basic compounds, respectively.
Statistical analysisThe analysis of variance (one way-ANOVA) was conducted to determine the differences between the pomaces with respect to the quantity of flavor compounds and metabolites produced.20 Tukey's Honestly Significant Differences (HSD) test was used to calculate the mean separations. The SPSS for Windows (version 17.0, SPSS Institute Inc., Chicago, IL, USA) and Minitab 16.1 (Minitab Inc. State College, PA, USA) were used for all statistical analyses.
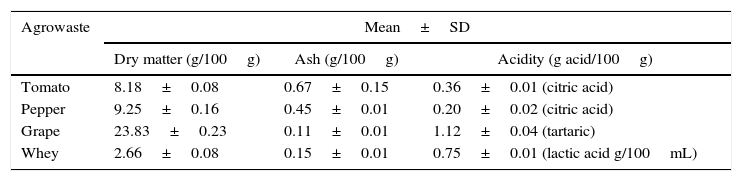
Results and discussionThe basic chemical composition of the agrowastes including dry matter, ash, and acidity are given in Table 1.
Characterization of agrowastes used in fermentation experiments.
| Agrowaste | Mean±SD | ||
|---|---|---|---|
| Dry matter (g/100g) | Ash (g/100g) | Acidity (g acid/100g) | |
| Tomato | 8.18±0.08 | 0.67±0.15 | 0.36±0.01 (citric acid) |
| Pepper | 9.25±0.16 | 0.45±0.01 | 0.20±0.02 (citric acid) |
| Grape | 23.83±0.23 | 0.11±0.01 | 1.12±0.04 (tartaric) |
| Whey | 2.66±0.08 | 0.15±0.01 | 0.75±0.01 (lactic acid g/100mL) |
SD: standard deviation.
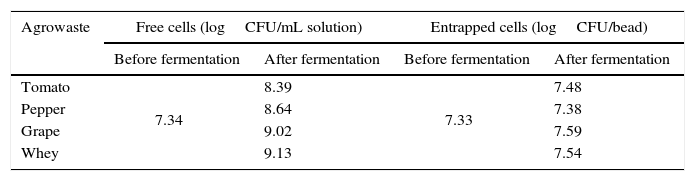
The grape pomace recorded the highest dry matter and acidity, while the tomato and pepper pomaces had higher ash content than the others. While grape pomace had 23.83% dry matter, tomato and pepper pomaces had approximately 8.0–9.0% dry matter. Whey had lower dry matter (2.66%) and ash (0.15%) contents. Similar agrowaste compositions have been reported by other researchers.3,21,22 Guneser3 reported higher dry matter for tomato (14%) and pepper pomaces (20%). Blaschek et al.21 found that Cheddar cheese sweet and salty whey, respectively, had 6.6% and 12.4% dry matter. Table 2 shows the growth of free and entrapped yeast cells over 5 days of fermentation. The number of entrapped cells remained stable between 7.38 and 7.59logCFU/bead, while the number of free cells increased by 1.05–1.79logCFU/mL, depending on the type of agrowaste employed. Similar growth behavior was observed with entrapped cell fermentation as had been described by Wilkowska et al.,23 for K. marxianus in the production of esters and alcohols – typical flavor compounds – from apple/chokeberry and apple/cranberry pomaces. In the case of free cell fermentation, it was observed that the increase in cell number was also higher with whey and grape pomaces than with pepper and tomato pomaces (Table 2).
Growth of free and entrapped yeast over the five-day fermentation.
| Agrowaste | Free cells (logCFU/mL solution) | Entrapped cells (logCFU/bead) | ||
|---|---|---|---|---|
| Before fermentation | After fermentation | Before fermentation | After fermentation | |
| Tomato | 7.34 | 8.39 | 7.33 | 7.48 |
| Pepper | 8.64 | 7.38 | ||
| Grape | 9.02 | 7.59 | ||
| Whey | 9.13 | 7.54 | ||
CFU: colony forming unit.
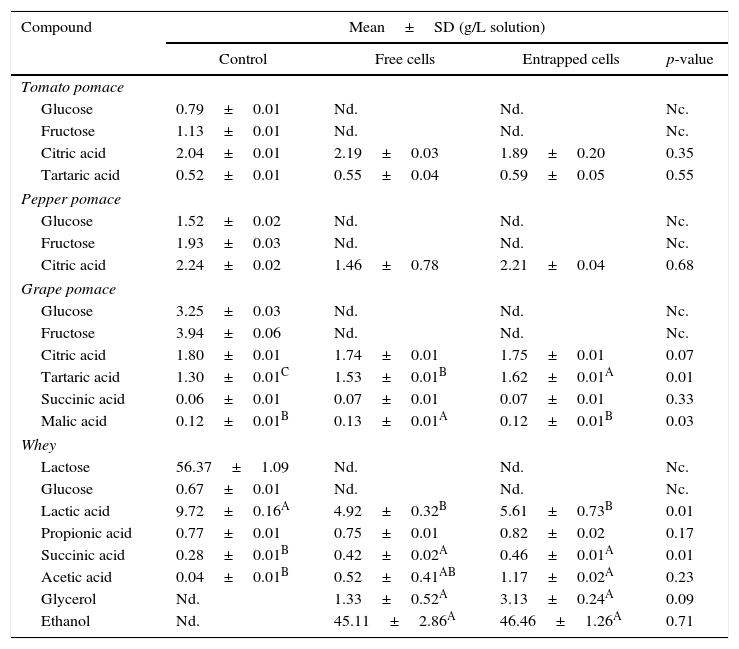
The changes in the composition of sugar, acid and ethanol of the agrowastes are shown in Table 4. The sugars (glucose, fructose, and lactose) were only measured in the control samples (non-fermented sample). All sugars were utilized by both free and entrapped yeasts during fermentation. A higher tartaric acid concentration was found in grape pomace fermented by entrapped yeast, while grape pomaces fermented by free yeast had higher levels of malic acid than the other samples (Table 3). No significant differences were observed between fermented and non-fermented tomato and pepper pomaces with respect to the concentrations of other acids (p>0.05). Ethanol production was only observed in whey. Ethanol fermentation was achieved by both free and entrapped yeast with higher proofs of 45.11g/L and 46.46g/L, respectively. Moreover, the control (non-fermented) whey sample had a significantly higher amount of lactic acid than the other samples, although there were no significant differences between the lactic acid contents of whey samples fermented with free and entrapped cells (p>0.05).
Changes in sugar, acid and ethanol composition of agrowastes.
| Compound | Mean±SD (g/L solution) | |||
|---|---|---|---|---|
| Control | Free cells | Entrapped cells | p-value | |
| Tomato pomace | ||||
| Glucose | 0.79±0.01 | Nd. | Nd. | Nc. |
| Fructose | 1.13±0.01 | Nd. | Nd. | Nc. |
| Citric acid | 2.04±0.01 | 2.19±0.03 | 1.89±0.20 | 0.35 |
| Tartaric acid | 0.52±0.01 | 0.55±0.04 | 0.59±0.05 | 0.55 |
| Pepper pomace | ||||
| Glucose | 1.52±0.02 | Nd. | Nd. | Nc. |
| Fructose | 1.93±0.03 | Nd. | Nd. | Nc. |
| Citric acid | 2.24±0.02 | 1.46±0.78 | 2.21±0.04 | 0.68 |
| Grape pomace | ||||
| Glucose | 3.25±0.03 | Nd. | Nd. | Nc. |
| Fructose | 3.94±0.06 | Nd. | Nd. | Nc. |
| Citric acid | 1.80±0.01 | 1.74±0.01 | 1.75±0.01 | 0.07 |
| Tartaric acid | 1.30±0.01C | 1.53±0.01B | 1.62±0.01A | 0.01 |
| Succinic acid | 0.06±0.01 | 0.07±0.01 | 0.07±0.01 | 0.33 |
| Malic acid | 0.12±0.01B | 0.13±0.01A | 0.12±0.01B | 0.03 |
| Whey | ||||
| Lactose | 56.37±1.09 | Nd. | Nd. | Nc. |
| Glucose | 0.67±0.01 | Nd. | Nd. | Nc. |
| Lactic acid | 9.72±0.16A | 4.92±0.32B | 5.61±0.73B | 0.01 |
| Propionic acid | 0.77±0.01 | 0.75±0.01 | 0.82±0.02 | 0.17 |
| Succinic acid | 0.28±0.01B | 0.42±0.02A | 0.46±0.01A | 0.01 |
| Acetic acid | 0.04±0.01B | 0.52±0.41AB | 1.17±0.02A | 0.23 |
| Glycerol | Nd. | 1.33±0.52A | 3.13±0.24A | 0.09 |
| Ethanol | Nd. | 45.11±2.86A | 46.46±1.26A | 0.71 |
A,B Means followed by different superscript letters represent significant differences for the same compounds in each pomace solution (p<0.05). Nd: not detected; Nc: not calculated.
There have been numerous studies on the production of organic acids and ethanol using yeast fermentation of various agrowastes.24–26 Nzelibe and Okafoagu24 reported that ethanol could be produced from Garcinia kola (bitter kola) pulp using baker's yeast (S. cerevisiae). A maximum of 70.7g of ethanol per liter was produced by the acid hydrolysis of Garcinia kola pulp at 30°C for 120h. Musatto et al.,25 also produced ethanol with S. cerevisiae from coffee industry waste hydrolysates with a 50.2% efficiency (11.7gethanol/L). In a study by Imandi et al.,27 it was found that pineapple waste could be used as a substrate for the production of citric acid using solid state fermentation using the yeast, Yarrowia lipolytica. Similar findings were observed in the present research.
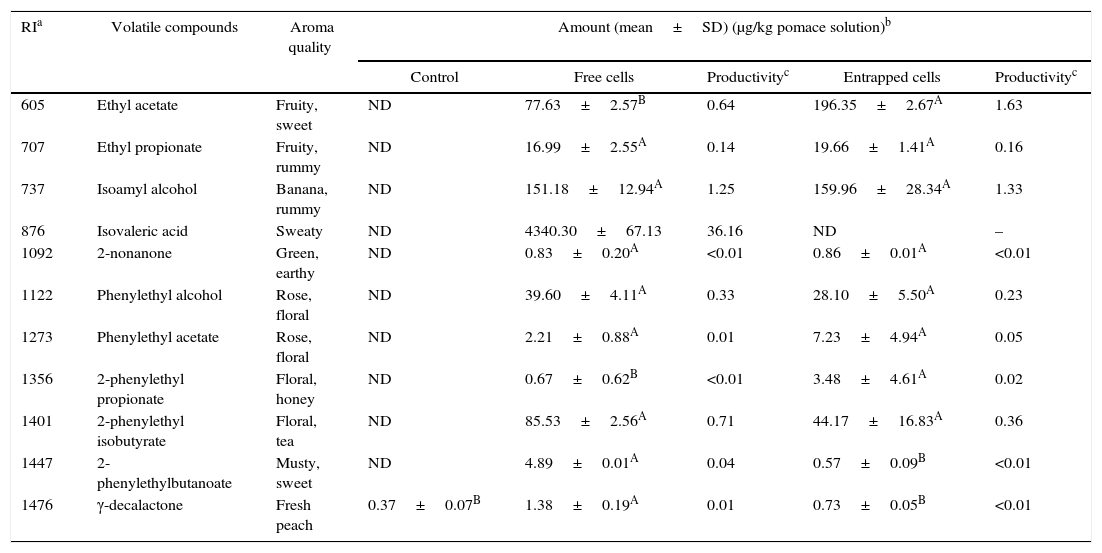
Tables 4–7 show the list of different volatile compounds produced by the fermentation of agrowastes using K. marxianus. Significant differences were observed between unfermented and fermented agrowastes in terms of the quantity of volatile compounds (p<0.05). High levels of esters and alcohols with fruity and floral notes were detected in agrowastes fermented with free and entrapped K. marxianus cells. These flavor compounds were identified as isoamyl alcohol (banana), isoamyl acetate (fruity, banana), phenylethyl alcohol (rose), phenylethyl acetate (floral), phenylethyl butyrate (floral musty), 2-phenyl ethyl propionate (floral), and phenylethyl isobutyrate (balsam). The amount of volatile compounds produced varied depending on whether the K. marxianus was free or entrapped and on the type of agrowaste used. The amounts of ethyl acetate and 2-phenethyl propionate produced were higher in tomato pomace fermented with entrapped cells (Table 4). The 2-phenylethylbutanoate and γ-decalactone contents (Table 4) were higher in tomato samples fermented with free cells.
Volatile compounds in tomato pomace.
| RIa | Volatile compounds | Aroma quality | Amount (mean±SD) (μg/kg pomace solution)b | ||||
|---|---|---|---|---|---|---|---|
| Control | Free cells | Productivityc | Entrapped cells | Productivityc | |||
| 605 | Ethyl acetate | Fruity, sweet | ND | 77.63±2.57B | 0.64 | 196.35±2.67A | 1.63 |
| 707 | Ethyl propionate | Fruity, rummy | ND | 16.99±2.55A | 0.14 | 19.66±1.41A | 0.16 |
| 737 | Isoamyl alcohol | Banana, rummy | ND | 151.18±12.94A | 1.25 | 159.96±28.34A | 1.33 |
| 876 | Isovaleric acid | Sweaty | ND | 4340.30±67.13 | 36.16 | ND | – |
| 1092 | 2-nonanone | Green, earthy | ND | 0.83±0.20A | <0.01 | 0.86±0.01A | <0.01 |
| 1122 | Phenylethyl alcohol | Rose, floral | ND | 39.60±4.11A | 0.33 | 28.10±5.50A | 0.23 |
| 1273 | Phenylethyl acetate | Rose, floral | ND | 2.21±0.88A | 0.01 | 7.23±4.94A | 0.05 |
| 1356 | 2-phenylethyl propionate | Floral, honey | ND | 0.67±0.62B | <0.01 | 3.48±4.61A | 0.02 |
| 1401 | 2-phenylethyl isobutyrate | Floral, tea | ND | 85.53±2.56A | 0.71 | 44.17±16.83A | 0.36 |
| 1447 | 2-phenylethylbutanoate | Musty, sweet | ND | 4.89±0.01A | 0.04 | 0.57±0.09B | <0.01 |
| 1476 | γ-decalactone | Fresh peach | 0.37±0.07B | 1.38±0.19A | 0.01 | 0.73±0.05B | <0.01 |
Mean relative abundance=(concentration of internal standard×peak area of compound)/(peak area of the internal standard).
Productivity was calculated based on the maximum concentration of each volatile compounds observed over 120h fermentation (μg/kg tomato pomace solution/hour).
A,B Means followed by different superscript letters represent significant differences for the same compounds in each fermentation type (p<0.05).
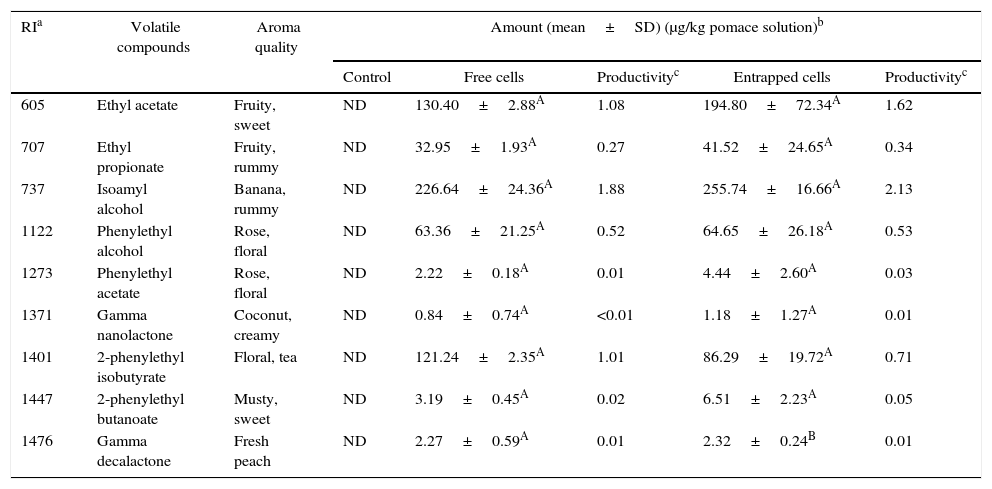
Volatile compounds in pepper pomace.
| RIa | Volatile compounds | Aroma quality | Amount (mean±SD) (μg/kg pomace solution)b | ||||
|---|---|---|---|---|---|---|---|
| Control | Free cells | Productivityc | Entrapped cells | Productivityc | |||
| 605 | Ethyl acetate | Fruity, sweet | ND | 130.40±2.88A | 1.08 | 194.80±72.34A | 1.62 |
| 707 | Ethyl propionate | Fruity, rummy | ND | 32.95±1.93A | 0.27 | 41.52±24.65A | 0.34 |
| 737 | Isoamyl alcohol | Banana, rummy | ND | 226.64±24.36A | 1.88 | 255.74±16.66A | 2.13 |
| 1122 | Phenylethyl alcohol | Rose, floral | ND | 63.36±21.25A | 0.52 | 64.65±26.18A | 0.53 |
| 1273 | Phenylethyl acetate | Rose, floral | ND | 2.22±0.18A | 0.01 | 4.44±2.60A | 0.03 |
| 1371 | Gamma nanolactone | Coconut, creamy | ND | 0.84±0.74A | <0.01 | 1.18±1.27A | 0.01 |
| 1401 | 2-phenylethyl isobutyrate | Floral, tea | ND | 121.24±2.35A | 1.01 | 86.29±19.72A | 0.71 |
| 1447 | 2-phenylethyl butanoate | Musty, sweet | ND | 3.19±0.45A | 0.02 | 6.51±2.23A | 0.05 |
| 1476 | Gamma decalactone | Fresh peach | ND | 2.27±0.59A | 0.01 | 2.32±0.24B | 0.01 |
Mean relative abundance=(concentration of internal standard×peak area of compound)/(peak area of the internal standard).
Productivity was calculated based on the maximum concentration of each volatile compounds observed over 120h fermentation period (μg/kg pepper pomace solution/hour).
A,B Means followed by different superscript letters represent significant differences for the same compounds in each fermentation type (p<0.05).
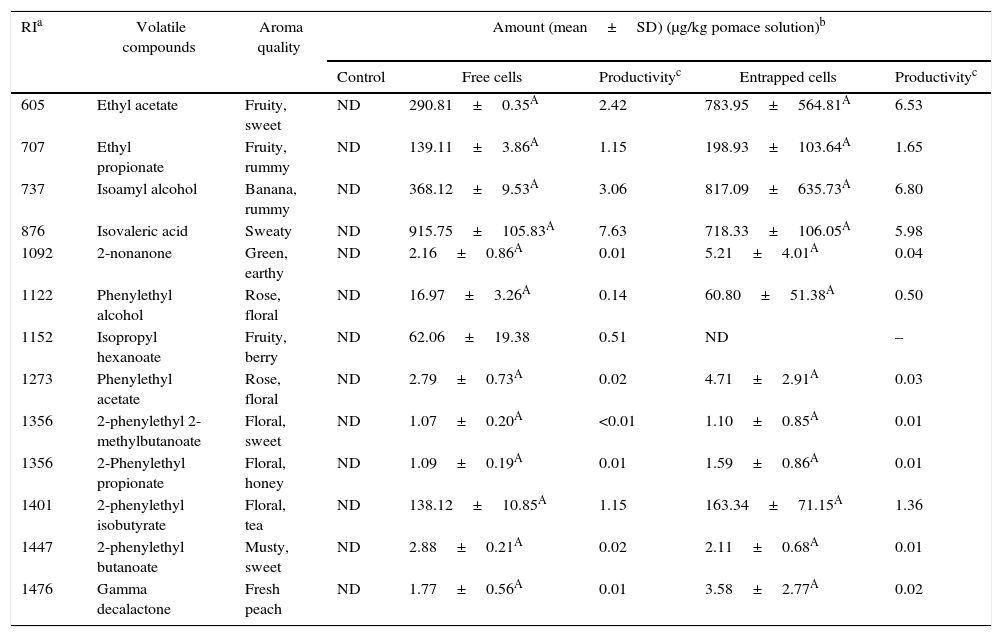
Volatile compounds in grape pomace.
| RIa | Volatile compounds | Aroma quality | Amount (mean±SD) (μg/kg pomace solution)b | ||||
|---|---|---|---|---|---|---|---|
| Control | Free cells | Productivityc | Entrapped cells | Productivityc | |||
| 605 | Ethyl acetate | Fruity, sweet | ND | 290.81±0.35A | 2.42 | 783.95±564.81A | 6.53 |
| 707 | Ethyl propionate | Fruity, rummy | ND | 139.11±3.86A | 1.15 | 198.93±103.64A | 1.65 |
| 737 | Isoamyl alcohol | Banana, rummy | ND | 368.12±9.53A | 3.06 | 817.09±635.73A | 6.80 |
| 876 | Isovaleric acid | Sweaty | ND | 915.75±105.83A | 7.63 | 718.33±106.05A | 5.98 |
| 1092 | 2-nonanone | Green, earthy | ND | 2.16±0.86A | 0.01 | 5.21±4.01A | 0.04 |
| 1122 | Phenylethyl alcohol | Rose, floral | ND | 16.97±3.26A | 0.14 | 60.80±51.38A | 0.50 |
| 1152 | Isopropyl hexanoate | Fruity, berry | ND | 62.06±19.38 | 0.51 | ND | – |
| 1273 | Phenylethyl acetate | Rose, floral | ND | 2.79±0.73A | 0.02 | 4.71±2.91A | 0.03 |
| 1356 | 2-phenylethyl 2-methylbutanoate | Floral, sweet | ND | 1.07±0.20A | <0.01 | 1.10±0.85A | 0.01 |
| 1356 | 2-Phenylethyl propionate | Floral, honey | ND | 1.09±0.19A | 0.01 | 1.59±0.86A | 0.01 |
| 1401 | 2-phenylethyl isobutyrate | Floral, tea | ND | 138.12±10.85A | 1.15 | 163.34±71.15A | 1.36 |
| 1447 | 2-phenylethyl butanoate | Musty, sweet | ND | 2.88±0.21A | 0.02 | 2.11±0.68A | 0.01 |
| 1476 | Gamma decalactone | Fresh peach | ND | 1.77±0.56A | 0.01 | 3.58±2.77A | 0.02 |
Mean relative abundance=(concentration of internal standard×peak area of compound)/(peak area of the internal standard).
Productivity was calculated based on the maximum concentration of each volatile compounds observed over a 120-h fermentation period (μg/kg grape pomace solution/hour).
A,B Means followed by different superscript letters represent significant differences for the same compounds in each fermentation type (p<0.05).
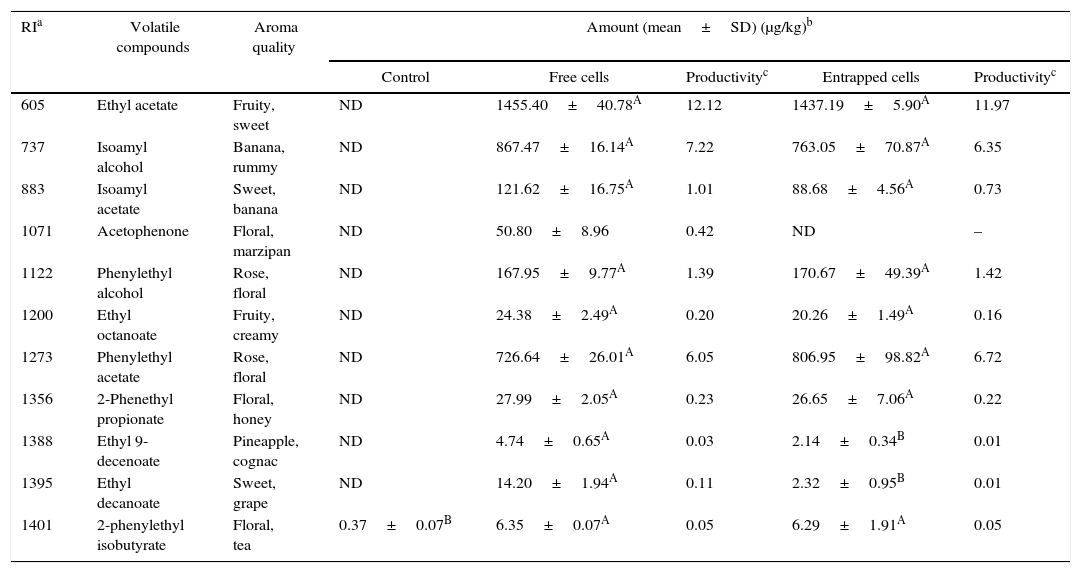
Volatile compounds in acid whey.
| RIa | Volatile compounds | Aroma quality | Amount (mean±SD) (μg/kg)b | ||||
|---|---|---|---|---|---|---|---|
| Control | Free cells | Productivityc | Entrapped cells | Productivityc | |||
| 605 | Ethyl acetate | Fruity, sweet | ND | 1455.40±40.78A | 12.12 | 1437.19±5.90A | 11.97 |
| 737 | Isoamyl alcohol | Banana, rummy | ND | 867.47±16.14A | 7.22 | 763.05±70.87A | 6.35 |
| 883 | Isoamyl acetate | Sweet, banana | ND | 121.62±16.75A | 1.01 | 88.68±4.56A | 0.73 |
| 1071 | Acetophenone | Floral, marzipan | ND | 50.80±8.96 | 0.42 | ND | – |
| 1122 | Phenylethyl alcohol | Rose, floral | ND | 167.95±9.77A | 1.39 | 170.67±49.39A | 1.42 |
| 1200 | Ethyl octanoate | Fruity, creamy | ND | 24.38±2.49A | 0.20 | 20.26±1.49A | 0.16 |
| 1273 | Phenylethyl acetate | Rose, floral | ND | 726.64±26.01A | 6.05 | 806.95±98.82A | 6.72 |
| 1356 | 2-Phenethyl propionate | Floral, honey | ND | 27.99±2.05A | 0.23 | 26.65±7.06A | 0.22 |
| 1388 | Ethyl 9-decenoate | Pineapple, cognac | ND | 4.74±0.65A | 0.03 | 2.14±0.34B | 0.01 |
| 1395 | Ethyl decanoate | Sweet, grape | ND | 14.20±1.94A | 0.11 | 2.32±0.95B | 0.01 |
| 1401 | 2-phenylethyl isobutyrate | Floral, tea | 0.37±0.07B | 6.35±0.07A | 0.05 | 6.29±1.91A | 0.05 |
Mean relative abundance=(concentration of internal standard×peak area of compound)/(peak area of the internal standard).
Productivity was calculated based on the maximum concentration of each volatile compounds observed over a 120-h fermentation period (μg/kg acid whey solution/hour).
A,B Means followed by different superscript letters represent significant differences for the same compounds in each fermentation type (p<0.05).
In pepper and tomato pomaces, no significant differences in terms of the concentrations of volatile compounds (except for γ-decalactone) were observed when fermented with either entrapped or free cells. The concentration of γ-decalactone in tomato pomaces fermented with free cells was higher than in tomato pomaces fermented with entrapped cells (Table 5).
The levels of volatile compounds produced in grape pomaces fermented by free and entrapped cells are shown in Table 6. The major volatiles in the samples were esters. Just as with pepper pomaces, there were no significant differences between the amounts of volatile esters produced by both free and entrapped cells.
The acid whey fermented by K. marxianus had a similar composition of volatiles to that produced with other agrowastes. Fermentations with both the entrapped and free K. marxianus cells showed higher amounts of ethyl acetate, isoamyl alcohol, and phenylethyl acetate (Table 7).
The levels of volatile compounds produced from the agrowastes were clearly dependent on the metabolism of sugar and amino acids in K. marxianus cells. The results indicate that not all fermentable sugars were detected in the fermented agrowastes. Esters and alcohols are the common volatiles produced by yeasts and have a fruity flavor. While alcohol volatiles can be produced by either the Genevois or Erhlich pathway in yeast metabolism, ester volatiles are formed by an enzyme-catalyzed condensation reaction between acyl-CoA and alcohols. In the Genevois pathway, the fermentable sugars (glucose, fructose, and lactose) are reduced to 2-oxo acids and then decarboxylated to form formaldehydes, which are reduced to the corresponding alcohols. Ehrlich pathways include biochemical reactions involving branched chain amino acids such as transamination, decarboxylation, oxidation, and reduction. Several enzymes play important roles in ester synthesis during yeast metabolism. The alcohol acetyl transferases I and II are well documented enzymes known to catalyze the production of esters in yeasts.7,28–30 Lactones were also produced by both entrapped and free K. marxianus cells in tomato, pepper, and grape pomaces (Tables 5–7). All of the pomaces contained seeds, skin, and seed oil, which could therefore have been related to fatty acid metabolism by K. marxianus. The production of lactone-type volatiles by yeasts is achieved by the β-oxidation of fatty acids, especially C18: Ricinoleic acid.31
Previous studies also reported esters, alcohol, acid, and lactone volatiles to be produced from different agrowastes using different yeast strains.32–35 Mantzouridou and Paraskevopoulou34 investigated the production of esters from orange pulp-containing medium using S. cerevisiae. S. cerevisiae produced isoamyl acetate, phenylethyl acetate, and various ethyl esters (e.g. ethyl hexanoate, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate) from orange pulp by de novo synthesis; this observation is in close agreement with the results obtained in this study. However, in contrast to our results, Lalou et al.33 reported that S. cerevisiae immobilized on Ca-alginate microbeads produces higher concentrations of phenylethyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate than do free cells with orange peel waste hydrolysate. The effect of immobilization on the alcoholic fermentation of whey was previously investigated by Kourkoutas et al.10 They observed that K. marxianus IMB3 yeast immobilized on delignified cellulosic material (DCM) produced propanol, isobutanol, and n-amyl alcohol at low concentrations, while a higher concentration of ethyl acetate was produced by the same immobilized yeast during fermentation. Mallouchos et al.36 indicated that a higher level of ester production could be achieved in wine fermentation using S. cerevisiae AXAZ-1 immobilized on DCM. Lee et al.37 also found that Na-alginate immobilized Sporoidiobolus salmonicolor produced high concentrations of γ-decalactone in a synthetic media.
The productivities of the volatiles depended on the types of agrowaste and fermentation employed (with free or entrapped cells). In general, the productivities of the volatile compounds produced from tomato and pepper pomaces were lower than that with grape and whey pomaces. The highest productivity of flavor in the tomato pomace was for isovaleric acid at 36.16μg/kg of tomato pomace solution per hour. The production of volatile compounds was also higher in grape pomace than with whey and pepper pomaces. In whey, ethyl acetate had a higher productivity than other volatile compounds in fermentations with both free and entrapped K. marxianus cells. The productivities of isoamyl alcohol and phenylethyl acetate in whey were comparable, and their productivities using free and entrapped K. marxianus cells ranged from 6.05 to 7.22μg/kg of tomato pomace solution per hour. Moreover, γ-decalactone, 2-phenylethyl isobutyrate, and 2-phenyl butanoate had lower productivities in all of the agrowastes used as the media (Tables 4–7). These aroma compounds can have a higher sensory impact on food. Therefore, further studies should be conducted to optimize the fermentation process of these compounds.
ConclusionsFree and entrapped K. marxianus can ferment tomato, pepper and grape pomaces, and acid whey. The results of this study show that alcohol, esters, and lactone volatiles, including ethyl acetate, isovaleric acid, isoamyl alcohol, isoamyl acetate, 2-phenylethyl isobutyrate, phenylethyl acetate, and phenyl ethyl alcohol can be produced in higher concentrations by entrapped K. marxianus. Because of the several advantages it offers, the entrapment of yeast cells can be an effective method of producing certain volatile compounds which are important in the flavor industry. Further studies should be conduct to evaluate biotechnological approaches in the production of natural flavors using microbial immobilization techniques.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was conducted by multidisciplinary partners from Lodz University of Technology (Poland) and Canakkale Onsekiz Mart University (Turkey) as the part of the BIOFLAVOUR COST Action FA0907. Support from The Scientific and Technological Council of Turkey (TUBITAK, Turkey Project No. 110O903 COST) is also gratefully acknowledged.