Systemic sclerosis (SSc) is a rare connective tissue disease (estimated prevalence: 0.5—2/10,000), and is characterized by diffuse vascular lesions and fibrosis of the skin and major organs, including lungs, kidneys, and heart.1
Cardiac involvement may include the endocardium, myocardium, and pericardium. It is often asymptomatic, but is recognized as an ominous prognostic factor, because it is one of the leading causes of mortality in these patients.2
Primary myocardial involvement (without systemic or pulmonary hypertension, and without significant renal involvement) may be a consequence of the typical vascular lesions of this disease, including abnormal vasoreactivity and impairment of microcirculation resulting in myocardial fibrosis.2,3
Myocardial fibrosis can lead to systolic dysfunction and heart failure in its course. The prevalence of left ventricle (LV) systolic dysfunction appears to be low (1.5-5%).1,4
We report the case of a young woman, newly diagnosed with SSc, who had primary myocardial disease leading to severe biventricular systolic dysfunction.
A 39-year old woman with a recent diagnosis of SSc with severe cutaneous, musculoskeletal, and gastrointestinal involvement, was referred to our center with a two months history of progressive exertional dyspnea and signs of systemic congestion: bilateral coarse crackles, abdominal distension, and lower limb edema. A previous 6-month echocardiogram, however, showed preserved LV function.
ECG showed a sinus rhythm, signs of left atrial enlargement, and an incomplete right bundle branch block. Signs of alveolar edema, without cardiomegaly, were found via chest X-ray. Laboratory tests revealed mild anemia, with ferritin levels of 44ng/ml, and elevated brain natriuretic peptide levels of 492pg/ml.
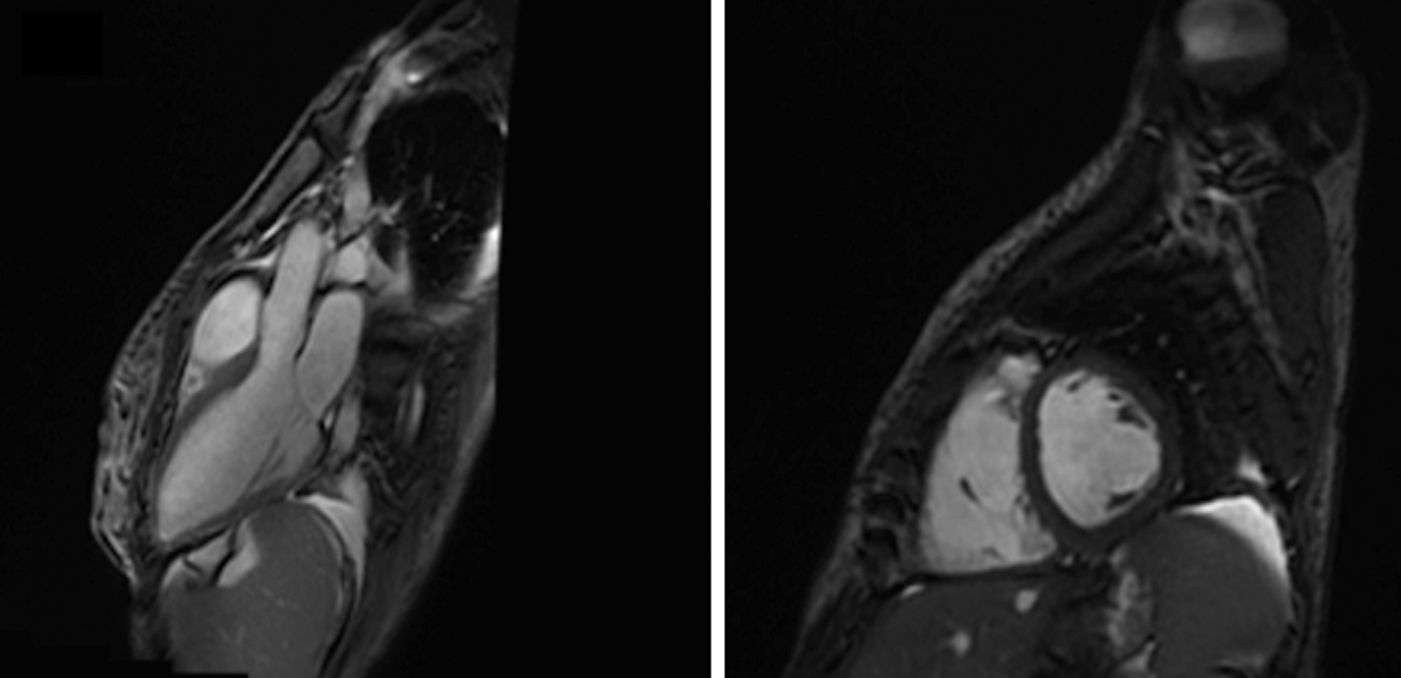
A transthoracic echocardiogram showed severely hypokinetic right and LV, without dilatation or hypertrophy, and severe tricuspid regurgitation with no evidence of pulmonary hypertension. Cardiac magnetic resonance imaging (MRI) confirmed biventricular systolic dysfunction but no abnormalities in delayed myocardial enhancement were detected. (Fig. 1)
The diagnostic work-up was completed with right cardiac catheterization, which showed no pulmonary hypertension (with a mean pulmonary arterial pressure of 22mmHg, and a pulmonary capillary wedge pressure of 16mmHg). An endomiocardial biopsy was not performed as the patient rejected this possibility. In addition, a coronary CT angiography showed no artery stenosis.
In addition to her routine SSc treatment, she was placed on heart failure guideline recommended medical treatment, including diuretics, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, aldosterone receptor antagonists, and intravenous iron.5
Medical discharge occurred after clinical stability was achieved. A diagnosis of SSc-related congestive heart failure was assumed, after excluding other potential causes of miocardial dysfuntion, such as coronary disease, hypertension, or a family history of cardiomyopathy. As mentioned before, the patient rejected the possibility of endomiocardial biopsy, so a definitive diagnosis based on histological criteria was not possible. However, cardiac involvement in previously diagnosed SSc patients is actually confirmed by standard diagnostic techniques (electrocardiography, echocardiography, cardiac catheterization, cardiac MRI) and myocardial biopsy is not commonly used.1 At 1-month follow-up, the patient remained symptomatic with NYHA III/IV for dyspnea despite optimal medical treatment, and unfortunately she died 3 months later because of a septic schock at another center.
This case-report illustrates primary myocardial involvement with severe biventricular dysfunction in a patient with SSc, an uncommon clinical scenario in the natural history of this disease.1,2
SSc vascular lesions result in major impairment of the cardiac microcirculation producing an abnormal vasoreactivity, even in the abscense of structural vascular disease. The potential role of coronary epicardial vasospasm, known as “coronary Raynaud's phenomenon”, has also been emphasized. General impairment of the coronary microcirculation produces sustained myocardial ischemia and results in fibrosis and ventricular dysfunction.3
Factors associated with LV dysfunction in SSc include diffuse cutaneous disease, disease duration, digital ulcerations, renal and muscle involvement, pulmonary fibrosis and pulmonary arterial hypertension. This may suggest that markers of severity of SSc, as well as markers of microvascular lesions (i.e., digital ulcerations), are associated with reduced LVEF, as seen in our case.3,4
The overall mortality in SSc resulting from cardiac complications is relatively low in comparison with manifestations such as interstitial lung disease and pulmonary arterial hypertension, however once cardiac dysfunction becomes apparent in these patients, it can severely increase morbidity and mortality.
When cardiac failure is observed, standard heart failure therapies should be used as for any other patient group with impaired cardiac function. At present, no treatments have been demonstrated to alter the natural history of primary cardiac involvement in patients with SSc. However, plausible treatment options exist; the ACE inhibitor captopril and the calcium channel blockers nifedipine and nicardipine have been shown to improve cardiac microcirculation for some patients. Nifedipine and bosentan may simultaneously increase myocardial perfusion and function, which are commonly impaired in patients with SSc. It remains to be shown whether early intervention with these agents can limit the progression of these life-threatening complications. Although definitive therapy for heart disease related to SSc has been elusive, novel approaches are being explored. Although still considered to be investigational and of uncertain efficacy, hematopoietic stem cell transplantation (HSCT) is being offered to an increasing number of patients with SSc. Burt and colleagues recently reported results from their 2-center experience, citing a 6% treatment-related mortality after HSCT and a significant improvement in skin disease and pulmonary forced vital capacity. It is hoped that HSCT might demonstrate improvement in heart disease related to SSc, but this has not been investigated to date. Cyclophosphamide and imatinib are other potential therapies for patients with SSc, but data regarding efficacy in heart disease associated with SSc are also lacking.6,7
The cardiac disease associated with SSc can present in many forms and is often clinically silent until significant organ dysfunction has ensued. Given the poor prognosis associated with cardiac involvement in SSc, awareness of heart disease, whether clinically evident or occult, is now an important part of the management. On the basis of available data, ideal screening includes annual comprehensive echocardiography (including tissue Doppler imaging) and natriuretic peptide testing. If present, symptoms and signs of possible cardiac involvement should be thoroughly investigated, thereby ensuring timely intervention in the at-risk patient.4