Libman-Sacks endocarditis (LSE) is a cardiac manifestation of systemic lupus erythematosus and antiphospholipid syndrome characterized by non-bacterial verrucous vegetations with thrombogenic potential, causing valvular disfunction in left heart predominantly, we present a case of Libman-Sacks endocarditis with aortic and tricuspid valve location, which is very rare.
MethodsWe present a case of aortic and tricuspid vegetation due to Libman-Sacks endocarditis in a 40-year-old male patient with antiphospholipid syndrome requiring surgical treatment by aortic and tricuspid valve replacement.
ResultsAfter procedure, the patient responded satisfactorily and got hospital discharged at 8 days following surgery with postoperative control after 1 week and 3 months later.
ConclusionReported cases on tricuspid LSE are scarce, because of its low prevalence, a standard surgical decision based on morphologic damage to the valve is yet to be established. Additional research is needed to reach a consensus on the best treatment approach for valve repair tricuspid versus valve replacement in this pathology.
La endocarditis de Libman-Sacks (LSE) es una manifestación cardíaca del lupus eritematoso sistémico y síndrome antifosfolípidos que se caracteriza por vegetaciones no bacterianas con potencial trombogénico, causando disfunción valvular en las cavidades izquierdas predominantemente. Presentamos un caso de endocarditis de Libman-Sacks con localización valvular aórtica y tricuspídea la cual es muy poco frecuente.
MétodosPresentamos un caso de vegetación valvular aórtica y tricúspide por LSE en un paciente masculino de 40 años con síndrome antifosfolípidos que requirió tratamiento quirúrgico mediante cambio valvular aórtico y tricuspídeo.
ResultadosTras el procedimiento, el paciente respondió satisfactoriamente y recibió egreso hospitalario a los 8 días de la cirugía con control postoperatorio a la semana y 3 meses después.
ConclusiónLos casos reportados sobre LSE tricúspide son escasos por lo que, debido a su baja prevalencia, aún no se ha establecido una decisión quirúrgica estándar basada en el daño morfológico de la válvula. Se necesita investigación adicional para llegar a un consenso sobre el enfoque de tratamiento óptimo para la reparación valvular vs. el reemplazo valvular en esta patología.
Libman-Sacks endocarditis, is a well-recognized cardiac manifestation of systemic lupus erythematosus and antiphospholipid syndrome. These vegetations are sterile, with abnormal growth around the valves, and with thrombotic and inflammatory pathogenesis mediated by autoimmune mechanisms, they appear predominantly in left side valves and are detected by echocardiography in 11% of patients with SLE, most of the cases are asymptomatic, however the clinical presentation can mimic infective endocarditis, making differential diagnosis and treatment very difficult.
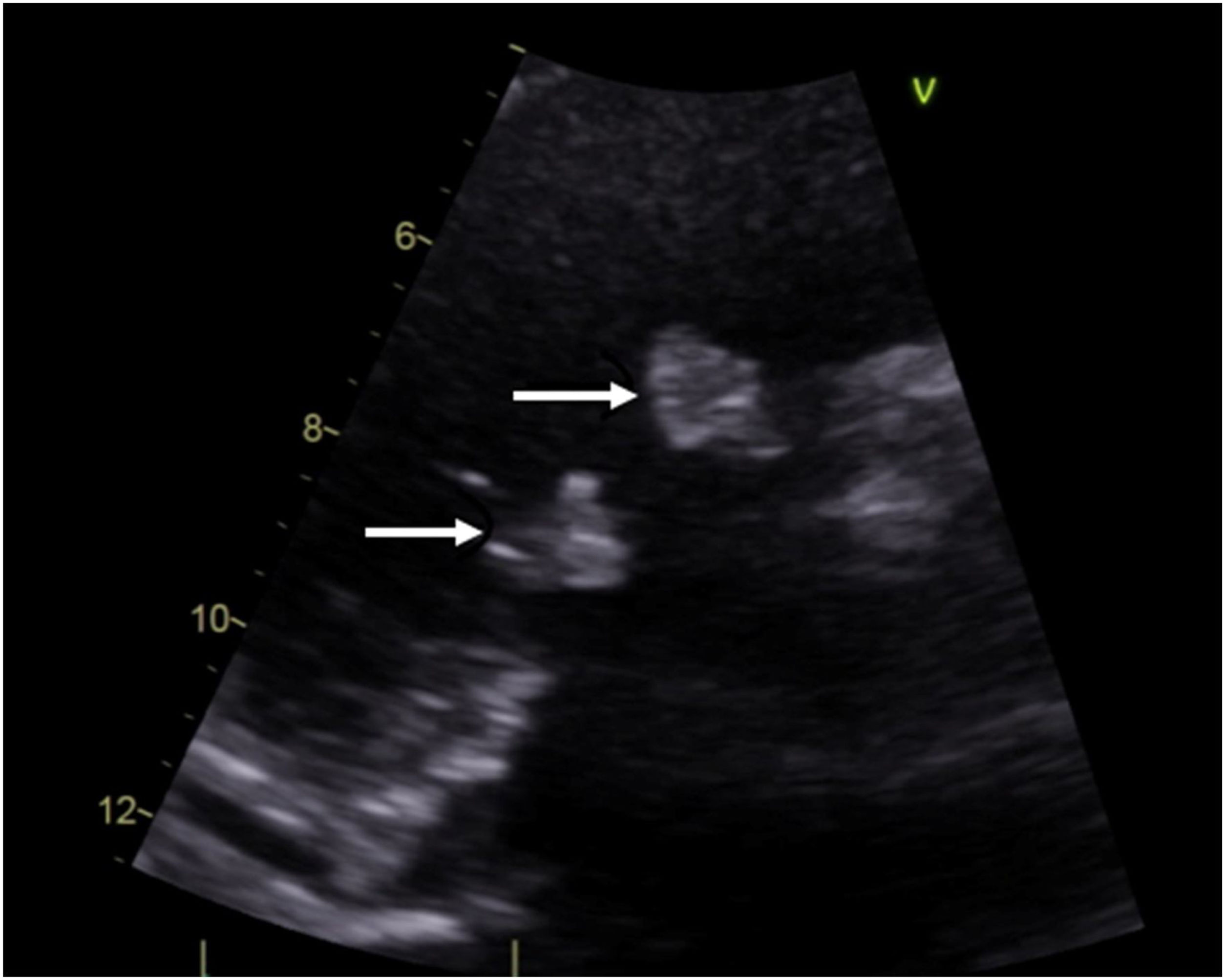
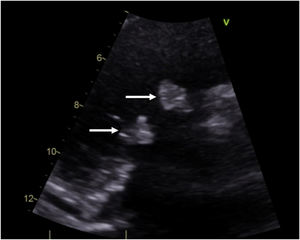
Case descriptionA 40-year-old male patient with a clinical history of antiphospholipid syndrome and chronic pneumonitis, presented with complaints of sudden severe chest pain predominantly in left hemithorax, cough and dyspnea, ongoing pulmonary embolism was ruled out by a computed thoracic angiography which also revealed bilateral pleural effusion, blood cultures and biomarkers reported negative and coronary angiography showed no significant lesions in the coronary tree. An echocardiogram confirmed severe aortic and tricuspid regurgitation and preserved left ventricular function with vegetations in aortic and tricuspid valves of 15mm×13mm and 17mm×15mm, respectively so considering LSE with vegetation of those size which had not improved with previous medical therapy the patient was managed by surgical valves replacement (Fig. 1).
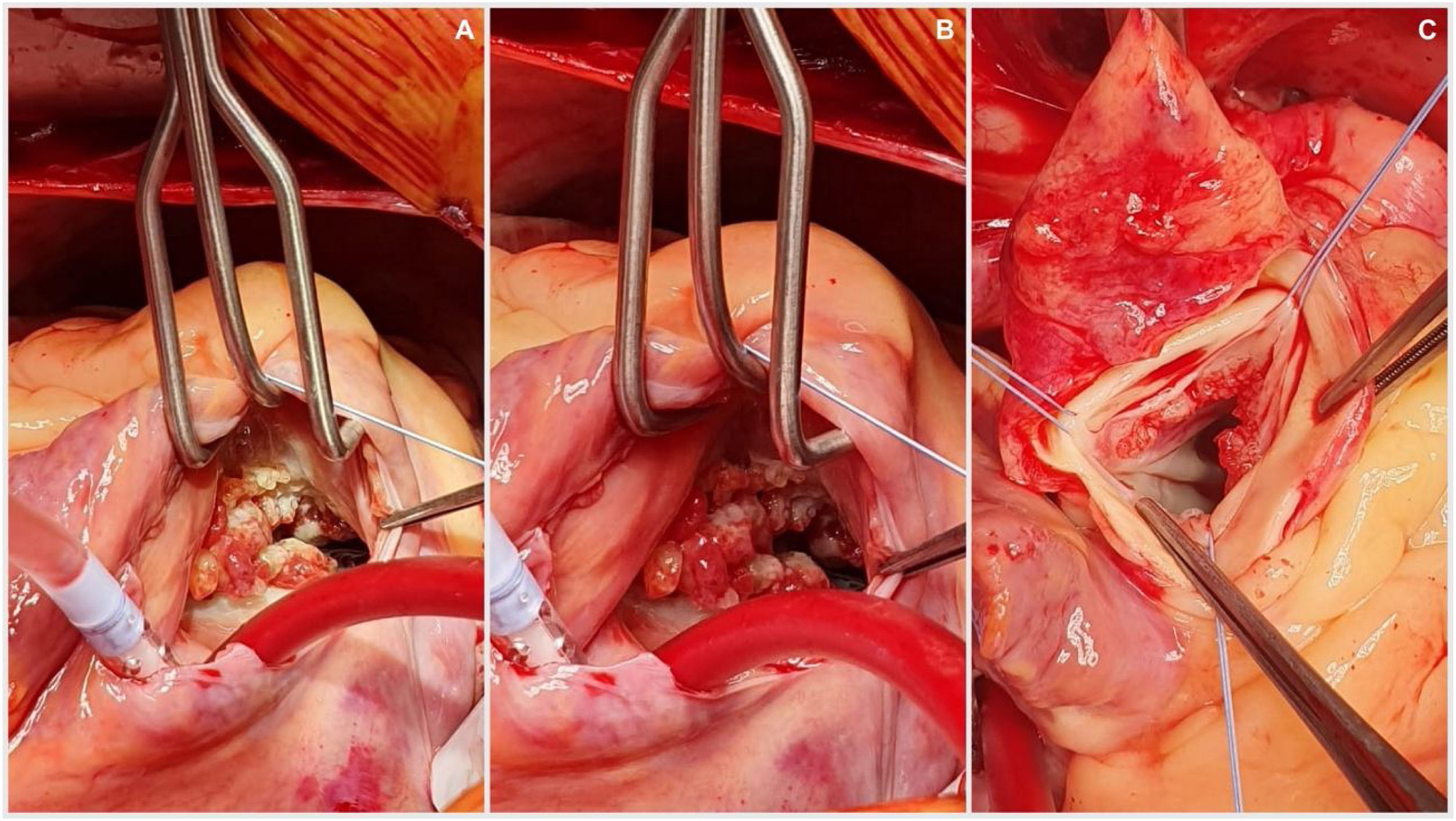
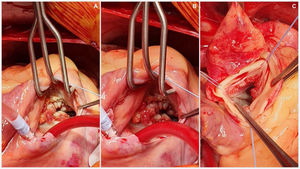
The procedure was performed starting with a median sternotomy and then upper and lower vena cava and aortic cannulation. The aorta was cross-clamped and cardioplegia was administered directly to the aortic root as well as through the coronary ostia. The aorta was then opened transversally enabling visualization of the aortic valve an its vegetation (Fig. 2), complete removal of the aortic valve and vegetation, placement of biological aortic valve Saint Jude Trifecta 23mm was performed and then the aorta was closed with 4-0 monofilament in two planes. The right atrium was opened and dissection and removal of tricuspid valve and the endocarditis mass was performed and the placement of tricuspid biological valve saint Jude EPIC 29mm, the reason why we used mechanic prosthesis in this patient was to avoid the anticoagulation program in a patient with cognitive impairment, complete dependence of his mother to take care and also the high risk of mechanic prosthesis thrombosis in tricuspid position, the right atrium was closed with 4-0 monofilament in two planes, aortic clamp is retired and deaeration by previous cannulation. The patient was taken off by-pass with no complications and returned to sinus rhythm. Total extra-corporeal circulation time was 153min, with 87min of cross clamping. This difference is because at our service we stablished that patients with active infectious process as endocarditis should have more perfusion time to have a better hemodynamic state at the withdraw of extracorporal perfusion. Postoperative trans-esophageal echocardiography showed a normal functioning of prosthetic valves. The patient was discharged 8 days following surgery with Warfarin anticoagulation therapy guided by INR only for the first three months to avoid prosthesis thrombosis After discharged, the patient was seen in consultation, with report of pathology confirming LSE.
DiscussionThe vegetations formation of LSE are seen as a cardiac manifestation of SLE, APS and tumors, aortic valve is the mostly affected in these cases, while tricuspid valve is less frequent like it was reported by Moyssakis with 38 LSE in 342 SLE patient which were diagnosed by echocardiography, among which there were 24 mitral and 13 aortic involvement with only one case of tricuspid LSE.1
It is suggested that the pathogenesis of the disease is secondary to the deposit of fibrin and platelet thrombi in the valves already affected by immunological damage mediated by immune complexes, which causes valve fibrosis, distortion and subsequent dysfunction.2,5 The prevalence of Libman-Sacks endocarditis in all SLE patients is reportedly approximately 30–50% and It is also reported that clinically significant hemodynamic dysfunction is detected in only 8% of patients.3,6
Doppler echocardiography can be considered as the diagnostic technique of choice, but sometimes it is very difficult to identify LSE and true infectious endocarditis, for the former may also have fever due to the original immunology diseases and the latter may also have vegetation.6 Two echocardiographic morphological patterns have been described: valve masses (vegetations) and valve thickening, vegetations appear as masses of different sizes, with irregular borders, firmly adhered to the valve surface and do not show independent movement of the leaflets, with a predominant location in the proximal and middle portion of the same ones.2
Treatment for LSE consists of drug treatment and surgical intervention. Corticosteroid and anticoagulation drugs are used for LSE drug treatment.4 Corticosteroids cannot prevent LSE, but they can help healing LSE lesions by lessening inflammation; However, they can also increase tissue fibrosis and scarring, finally worsening valvular damage and dysfunction.2,7
In the past decade, large studies on the surgical treatment of LSE were mostly focused on patients with mitral LSE, which is explained by the higher prevalence of endocarditis on the left side of the heart.1 This finding was reported by Bouma et al. who reviewed the English literatures of surgical treatment for mitral regurgitation caused by LSE and from 1974 to 2010 there were 41 patients with LSE undergoing mitral valve surgery, 13 patients underwent mitral valve repair while 28 underwent replacement, suggesting that for only localized abnormalities with otherwise relatively normal leaflets, repair rather than replacement was considered as a good surgical opinion.8
Bai and colleagues reported a case on isolated tricuspid vegetations describing a patient which exhibited vegetations obstructing valve orifice who they decides to repair rather than valve replacement.1 While cases with subvalvular apparatus involvement were mainly undergone valve replacement, there are other cases reported like that from Chan-Lam et al., with tricuspid valve repair by removing two masses from the valve.7
ConclusionReported cases on tricuspid LSE are scarce, because of its low prevalence, a standard surgical decision based on morphologic damage to the valve is yet to be established. Concerning reported papers about mitral LSE if there is sub valvular involvement valve replacement should be the treatment of choice. Additional research is needed to reach a consensus on the best treatment approach for tricuspid repair versus replacement in LSE.
Informed consentAlso, the patient provided informed consent for this submission. The article was approved by the institution's review board committee.
FundingThe authors have nothing to declare regarding financial support and followed ethics protocols.
Conflict of interestsNone.