The Blalock–Taussig–Thomas Shunt (BTTS) procedure was first introduced by Alfred Blalock, Hellen B. Taussig, and Vivien Thomas in 1944. The procedure is performed by making a connection between the subclavian artery to the pulmonary artery to augment pulmonary blood flow. BTTS is used to palliate neonates born with restrictive pulmonary blood flow disorders (cyanotic congenital heart disease). The modified BTTS (MBTTS) procedure is still seen as high risk and has quite high morbidity and mortality rates, especially in the neonatal group. Recent studies suggest a mortality of up to 15% in the single ventricle group and 3% in the biventricular group. Based on a literature search, several risk factors such as patient weight, shunt size/body weight ratio, underlying cardiac malformations, use of mechanical ventilator support, sternotomy approach and use of CPB machines have been shown to increase mortality. Current perioperative risk assessment methods and recommendations are also covered. In addition, our review article specifically discusses perioperative strategies including strategies for surgeons using the MBTTS procedure (surgical approach, determining shunt size and anastomosis techniques) and postoperative strategies (anticoagulation management and strategy to deal with undershunting/overshunting problems).
El procedimiento de derivación de Blalock-Taussig-Thomas fue introducido por primera vez por Alfred Blalock, Hellen B. Taussig y Vivien Thomas en 1944. El procedimiento se realiza al hacer una conexión entre la arteria subclavia y la arteria pulmonar para aumentar el flujo sanguíneo pulmonar. El procedimiento de derivación de Blalock-Taussig-Thomas se utiliza para paliar a los recién nacidos que nacen con trastornos restrictivos del flujo sanguíneo pulmonar (cardiopatía congénita cianótica). El procedimiento MBTTS todavía se considera un procedimiento de alto riesgo y tiene tasas de morbilidad y mortalidad bastante altas, especialmente en el grupo neonatal. Estudios recientes sugieren que hay una mortalidad de hasta el 15% en el grupo de un solo ventrículo y del 3% en el grupo de anomalías cardíacas biventrículos. Según la búsqueda bibliográfica, se ha demostrado que varios factores de riesgo, como el peso del paciente, la relación tamaño de la derivación/peso corporal, malformaciones cardíacas subyacentes, uso de asistencia respiratoria mecánica, abordaje de esternotomía y uso de máquinas de CEC, aumentan la mortalidad. También se tratan los métodos y recomendaciones actuales de evaluación de riesgos perioperatorios. Además, nuestro artículo de revisión analiza específicamente las estrategias perioperatorias, incluidas las estrategias para los cirujanos en el procedimiento MBTTS (abordaje quirúrgico, determinación del tamaño de la derivación y técnicas de anastomosis) y las estrategias postoperatorias (manejo de anticoagulación y estrategia para lidiar con problemas de sobrecapacidad/sobrecadencia).
The Blalock–Taussig–Thomas Shunt (BTTS) was first introduced by Alfred Blalock, Hellen B. Taussig and Vivien Thomas in 1944, using a connection of subclavian artery to pulmonary artery as a way to augment the pulmonary blood flow. BTTS is utilized to palliate neonates born with restrictive pulmonary blood flow including those with single ventricle (SV) or biventricle (BV) cardiac anomalies. In 1975, this technique was modified by Marc R. de Leval and colleagues using a polytetrafuoroethylene (PTFE) graft interposed between the subclavian artery and the ipsilateral pulmonary artery. Since the modification, this procedure popularly known as modified Blalock–Taussig–Thomas Shunt (MBTTS).1 MBTTS is still categorized as a procedure with relatively high morbidity and mortality for neonatal age group despite the advancement of diagnostic, intraoperative technique, and perioperative management in intensive care unit.2,3 Recent data by Alsoufi et al. show that overall hospital mortality of neonatal MBTTS is 9%, which include 15% in patients with SV cardiac anomalies and 3% in patients with BV cardiac anomalies.2 The purpose of this literature review is to discuss several risk factors related to post-procedure mortality and perioperative management strategies for surgeons to minimize mortality.
Risk factors for mortality after the neonatal Blalock–Taussig Shunt ProcedureAnalysis of several studies recognizes several risk factors that have been shown to increase post-procedure mortality in neonatal MBTTS.3,4
WeightPetrucci et al. presented their multivariate analysis that the patient's weight is a significant risk factor for mortality after the MBTTS procedure.3 Patients weighing less than 3kg have a mortality rate of 8–15.63%, almost twice to five times than patients weighing more than 3kg, having a mortality rate around 3.77%. These findings are consistent with Singh et al., who concluded that body weight less than 3kg is a risk factor for mortality.5,6 Based on the study of these data, we can conclude that low body weight (less than 3kg) is an independent risk factor for post-procedure mortality.
Shunt size/body weight ratioDetermination of the ideal shunt size is still a matter of debate by experts. In general, the shunt size is based on the patient's body weight. Incorrect shunt size may result in morbidity and even mortality for the patient. If the shunt size is too small, it would lead to a shunt thrombosis, whereas if the shunt size is too large, it would result in a state of pulmonary overflow, a decrease in diastolic pressure, and a decrease in systemic perfusion.
Alsoufi et al. explained the ratio of shunt size to body weight >1 in SV cardiac anomalies has been shown to increase the risk of mortality in neonates undergoing MBTTS, the survival in single-ventricle physiology with shunt size/kg of <1 and body weight >2.5kg is highest when compared with patients with shunt size/kg of >1 and body weight of <2.5kg.2
The latest research in 2019 by Sisli et al. shows that a shunt/body weight ratio >1.3 is significantly associated with the incidence of shunt thrombosis.7 This is in line with the research of Bove et al., which states that there is a shunt complication along with increase in the size-to-weight shunt ratio.8 Dirk et al. also presented that mortality is also associated with an increase in shunt/body weight ratio (median of shunt size-to-body weight ratio of survivors 1.19 vs non-survivors 1.59).9 In conclusion of these several studies, there was a strong association between shunt size/weight ratio and patient survival in neonatal MBTTS.
Underlying cardiac malformationsThe risk of mortality in the MBTTS procedure is also reflected on the type of underlying congenital heart disease suffered. The relationship between diagnosis and mortality is illustrated in the data that patients with SV and pulmonary atresia with intact ventricular septum (PA-IVS) anomalies have higher mortality rate after MBTTS. However, patients with PA-IVS have the highest mortality (15.6%) compared to other types of SV cardiac anomalies.
The mortality outcome of PA-IVS is associated with the presence of ventricular-coronary artery fistulas or sinusoids. However, the author explains that there is a possibility that the diagnostic modality could not identify these sinusoids. It is also elaborated that the poor outcome is due to poor judgment to proceed to MBTTS.3 Alsoufi et al. explained that patients with univentricular heart have higher needs of extracorporeal membrane oxygenation (ECMO) support than patients with biventricular heart.2
Pre operative mechanical ventilatory supportPreoperative mechanical ventilatory support is also an independent risk factor for death and morbidity of patients. It is suspected that the need for ventilator support reflects the preoperative condition which is quite severe. In addition, there is a suspicion that the need for a ventilator worsens the neonatal circulation system and makes it difficult to manage the pulmonary-to-systemic blood flow ratio.3
Cardiopulmonary bypass in MBTTSThe use of cardiopulmonary bypass machines is also associated with in hospital mortality, especially in the first 48h.3,10 However, researchers have not been able to conclude that the use of CPB machines as predictors of death in patients. It can be surmised that the use of CPB machines was carried out due to the condition of patients who could not tolerate the MBTS procedure because of other concomitant risk factor, or on the other hand the use of CPB machines might exaggerate inflammatory cascade and coagulopathy.
Sternotomy approachIn a multivariate analysis of large central study conducted by Mckenzie et al., the sternotomy approach was closely related to in hospital mortality, with hazard ratio of 3.2.4 This should be suspected that the sternotomy approach is indeed indicated in patients with previously more severe conditions. The author also believes that the sternotomy approach may have a tendency to the creation of larger shunts.
Perioperative risk assessmentThere are currently several scoring systems available to predict the outcome of congenital heart surgery, including the Aristotle Comprehensive Complexity (ACC), the Risk Adjustment in Congenital Heart Surgery (RACHS-1), and Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STS-EACTS/STAT) mortality score.11,12
In the ACC score, the MBTS is categorized in Category 2 out of 4 categories. In the RACHS-1 score, the MBTS is categorized in Category 3 (systemic to pulmonary artery shunt) out of 6 categories. In the STATmortality score, it is categorized in Category 4 out of 5 categories. All of the three scores have been proven to be able to provide significant differences in mortality between categories, but institutional based study is recommended to determine which score could best predict the mortality for each institution.12,13
RecommendationsCurrent guidelines for several cyanotic anomalies have incorporated MBTS as part of the therapy, although most recommendations are not first class recommendations. There are several recommendations regarding MBTS in the latest European Association for Cardiothoracic Surgery (EACTS) guideline for the management of neonates and infants with hypoplastic left heart syndrome. MBTS is recommended to facilitate regulation of pulmonary blood flow, including titration of excessive pulmonary blood flow (Class IIa, level C). However, it is stated that the use of MBTS is more difficult with aberrant subclavian artery (Class II, level C). In the case of shunt stenosis, catheter intervention could be effective (Class IIa, level C). Moreover, ECMO could be a useful adjunct in managing acute shunt failure (Class IIa, level C).14 Among patients with transposition of the great arteries (TGA), MBTS with a size 3.0–3.5mm PTFE shunt (according to the patient's weight) is also recommended to train the left ventricle. However, only a moderate degree of volume overload should be considered as the target (Class IIa, level C).15
There is currently no clear guideline on when to use the MTBS as part of univentricular palliation for patients with pulmonary atresia. In general, univentricular palliation is commonly performed in children not suitable for biventricular repair (i.e. those with TV Z-score of <−4 or those with right ventricular-dependant coronary circulation/RVDCC).16
Perioperative and operative management strategyIn an effort to maximize postoperative outcomes in patients, we have summarized some of the best strategies or schemes at each step. Some of the surgical considerations include the size and length of the graft, the location of the shunt, and the surgical approach. We also summarized some steps of post operative ICU management and its algorithm to deal with complications (pulmonary overflow/shunt thrombosis).
Surgical approach consideration (thoracotomy vs sternotomy)The MBTTS approach through median sternotomy gives the surgeon a wider operative view and allows the surgeon to initiate cardiopulmonary bypass if needed.
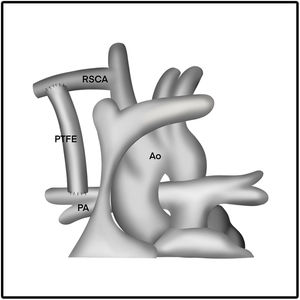
The exposure to the right subclavian artery (RSCA) via median sternotomy will appear wider, allowing for a more proximal anastomosis of the systemic vascular tree so that the graft placed can be shorter and smaller for pulmonary flow control. The approach through sternotomy is thought to be related to longer use of mechanical ventilatory support, length of stay in ICU and hospital, and higher mortality rate.4
Meanwhile, the thoracotomy approach provides for the creation of a proximal anastomosis beyond the bifurcation of innominate artery. This will lead to the need for a longer graft. Graft diameter size becomes less important in estimating shunt resistance, so the flow can be a problem. Some complications related to thoracotomy include chylothorax, Horner's syndrome, distortion of the pulmonary arteries, a tendency for blood to flow to one of the lungs, and additional surgical scars on the chest.4
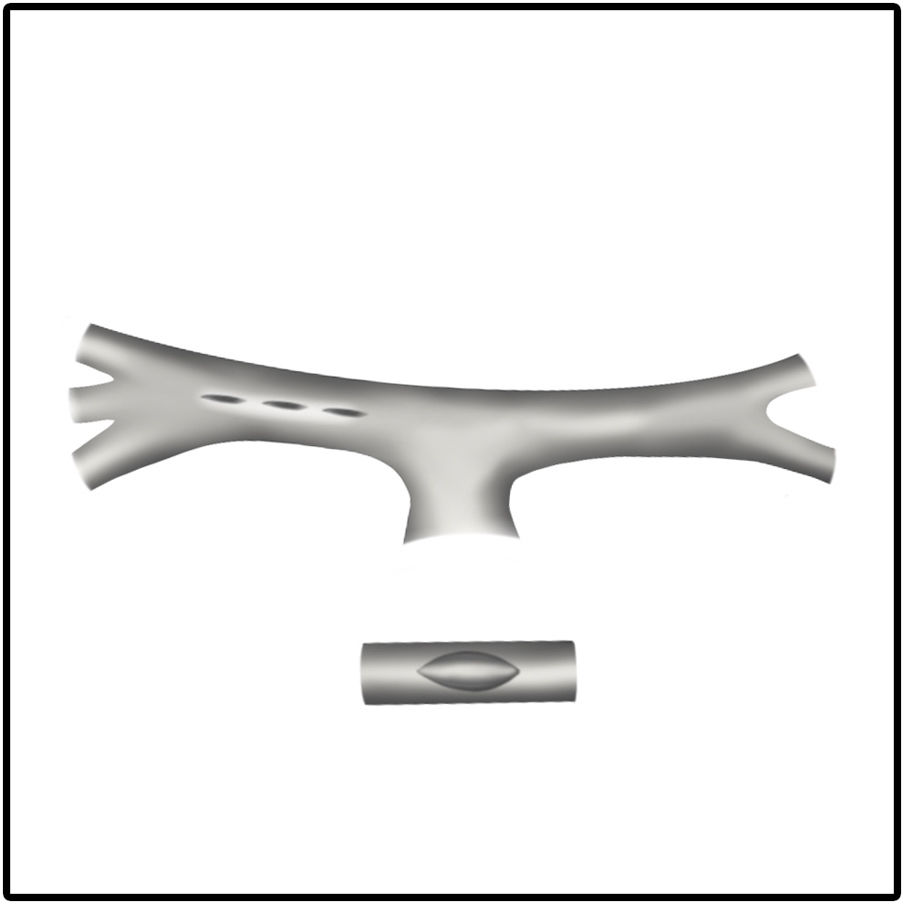
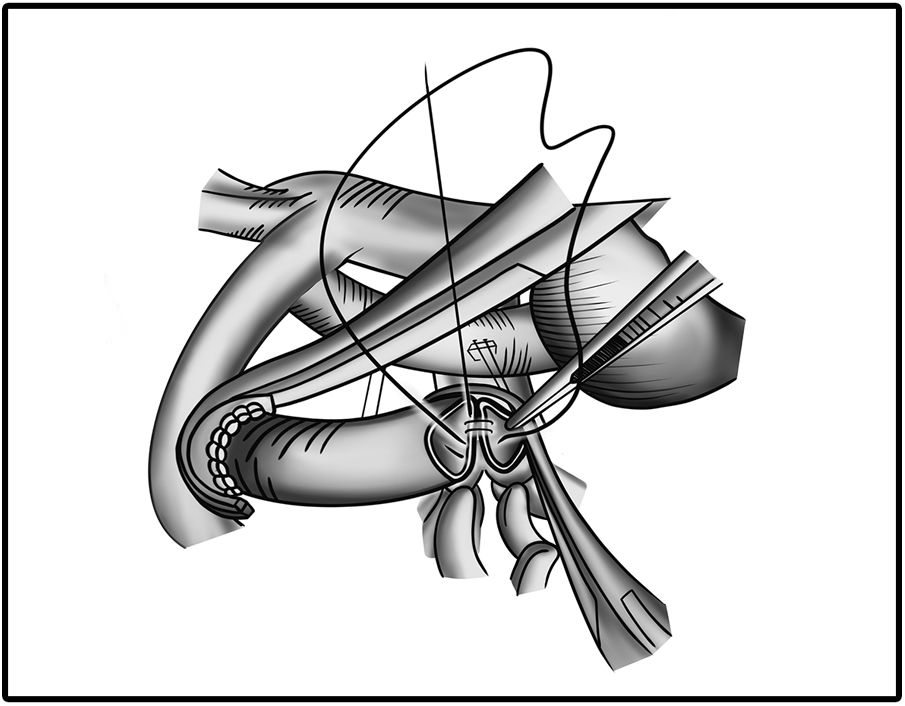
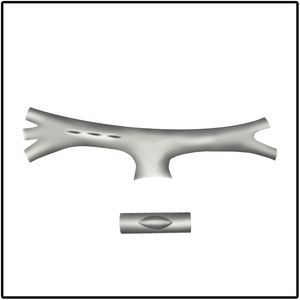
Surgical incision and anastomosis techniquesA longitudinal arteriotomy incision both in the subclavian and pulmonary arteries provides a wider circumferential area of the anastomosis for a larger shunt size. This technique proves more beneficial for hypoplastic PA. Pulmonary artery distortion at the anastomotic site is also less common with the longitudinal incision technique (Fig. 1). In the transverse arteriotomy technique, it is not possible to place a larger shunt size on the hypoplastic PA because of the smaller circumferential area.17
In terms of determining the thread size, some studies have used 7/0 polypropylene for anastomoses.18 However, Swain et al. used a smaller needle size of 8/0 polypropylene which provided better anastomosis and less needle hole bleeding.17
Shunt size and length of tube graft considerationDetermination of the tube size/PTFE graft holds the most important aspect in the MBTTS procedure. Determination of the shunt size/diameter is generally based on the patient's body weight or pulmonary artery branch size. Alsoufi et al. determined the shunt size to around 1.16 (0.9–1.6)mm/kg body weight.2 The easiest way to determine the shunt size is to select shunt size of 3.0mm for a body weight up to 3.0kg and 3.5mm for body weight around 3.5kg.9,19
The ideal ratio of shunt size to body weight >1 especially in SV cardiac anomalies. Considering shunt size to pulmonary artery size (S/PA ratio) <0.9 especially in neonatal age, which has high pulmonary vascular resistance and cyanotic patient with polycythemia, decreases the incidence of shunt thrombosis.7
Other studies have shown that biventricle heart abnormalities (which have forward blood flow in the main pulmonary artery) can use a small diameter tube graft to reduce excessive pulmonary blood flow. They analyzed the relationship between shunt size and postoperative outcomes in patients undergoing biventricle repair. A small graft, 3.0mm in diameter, was concluded to be safe, providing adequate pulmonary flow and a significant increase in body weight (prior to definitive intra cardiac repair) in neonates with biventricular heart defects and low body weight. The authors conclude that a 3.0mm graft is considered feasible and safe for pulmonary stenosis patients weighing 3.5kg.20
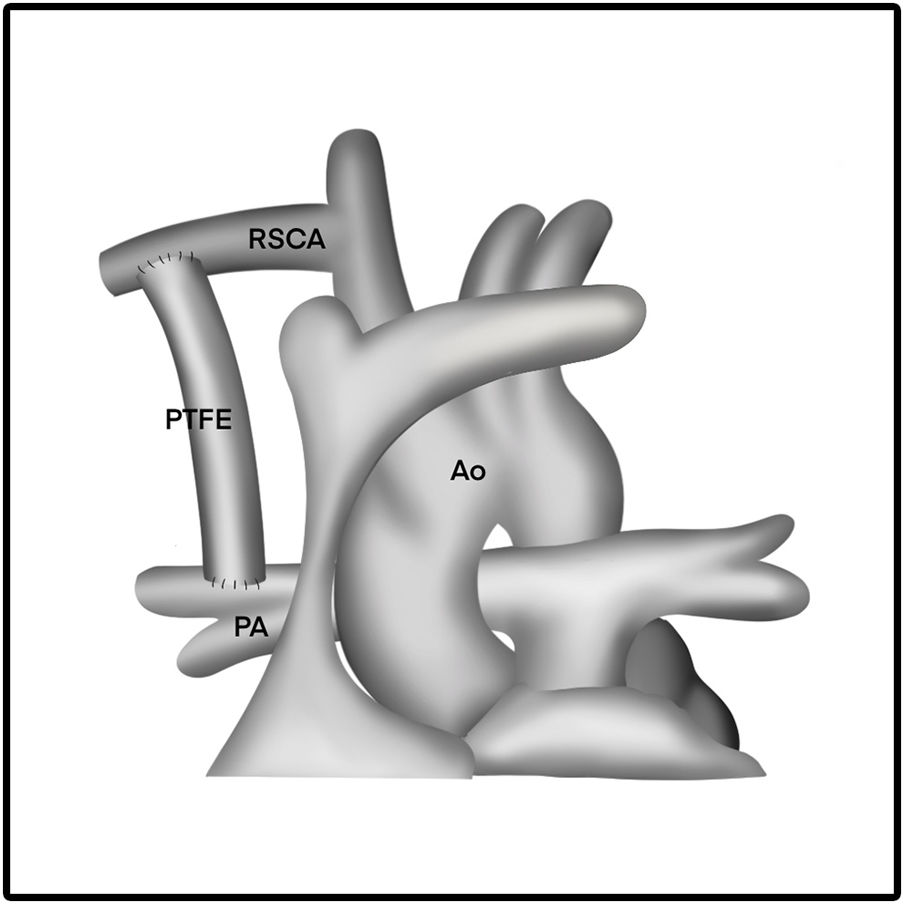
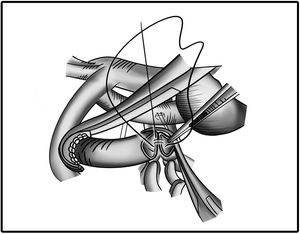
The length of the PTFE graft is determined before surgery or before the clamp is placed. The length of the graft is adjusted in such a way that the graft appears to be in a curved shape. In addition, the graft is also certainly not kinked or retracted in relation with pulmonary arteries. This procedure is best illustrated in Figs. 2 and 3.
Anticoagulant managementSeveral central institutions have implemented protocols for administering anticoagulation (heparin and aspirin). Anticoagulant management has been shown to reduce the incidence of shunt thrombosis and mortality rates in patients undergoing the MBTS procedure. Ismail et al. carry out the heparin protocol 2–4h postoperatively if there is no evidence of bleeding (with reference to chest tube drainage of less than 2–4cm3/kg/h). Heparin is given at an initial dose of 15IU/kg/h and titration is carried out until the therapeutic level achieved (PTT 60–80s) and aspirin is usually given 24h after surgery.21,22 Data from 39 centers show that aspirin given earlier (initiation on day 2) after surgery can reduce the number of shunt thrombosis compared to centers that give aspirin later (initiation day 4).23 Initial administration of aspirin can be given starting from 3 to 5mg/kg/day, and continue this treatment until the shunt takedown.24 In our center, we start the heparin as soon as possible if there is no evidence of bleeding, heparin is administrated at an initial dose of 5–10IU/kg/h and titration is carried out until the therapeutic level is achieved (PTT 60–80s). We usually start aspirin dosage of 5mg/kg/day after extubation.
Strategy to deal with over shuntingPulmonary over circulation (over shunting) problems are mainly characterized by oxygen saturation (SaO2) >85% in room air, wide pulse pressure (decreased systemic diastolic pressure due “run off flow” to the lungs), low cardiac output syndrome, persistent metabolic acidosis, and pulmonary plethora on chest X-ray. Several causes of overshunting are the unnecessarily high FiO2 ventilation, the presence of PDAs and MAPCA as sources of additional blood flow to the lungs and the larger size of the shunt graft.21
If clinical signs of overshunting are known, we have to optimize the balance of Qp: Qs by decreasing systemic vascular resistances (SVR) and increasing pulmonary vascular resistances (PVR). We may increase the PVR by reducing fraction of inspired oxygen (FiO2) to 0.21 despite suctioning or nebulizing, avoiding hyperventilation with targeted permissive hypercapnea, consider administering high PEEP (PEEP 6–8) and maintaining blood pH of 7.35–7.40. We may evaluate lactic acid and arterial blood gas every 4–6h. Some inodilators is effective and can be used to decrease SVR. Dobutamine, milrinone or levosimendan may be considered while maintaining DBP >25mmHg and consider to start nitrogliserine if the blood pressure is still high after using inodilator.21
Strategy to deal with under shuntingPulmonary under circulation (under shunting) problems are marked with sudden desaturation of SaO2 <70% and the need to increase FiO2 >60%. Some risk factors of under shunting are thrombus which blocked the shunt circulation, smaller shunt size, inadequate flow due to systemic hypotension, and inadequate ventilation (pneumothorax, atelectasis, pneumonia, pleural effusion or displaced endo tracheal tube (ETT)).25
If the signs of under shunting are known (oxygen saturation <70%, despite given FiO2 >60%), we must first exclude the cause of under shunting, especially shunt thrombosis with urgent echocardiography evaluation for shunt patency, the respiratory system failure, such as displaced ETT, obstructed ETT, pneumothorax, and failure of hemodynamic support and equipment.21
If we already know that the shunt patency is good, the strategic management is to optimize the balance of Qp: Qs by increasing FiO2 to achieve SaO2 of 70–85%, avoid acidosis and keep blood pH 7.40–7.45. Adequate volume status must be ensured by titrating fluid slowly according to body response with 5ml 5% albumin. If the blood pressure is low, we may consider to start vasoconstrictors, such as norepinehrine or epinephrine. Pulmonary vasodilator (sildenafil and inhaled nitric oxide (iNO)) may be needed to decrease the pulmonary vascular resistance.21
Conflict of interestsThe authors declare that they have no conflict of interest.