Surgery of the aortic arch is one of the most challenging procedures in cardiac surgery requiring mastery of temporary manipulation of cerebral and systemic circulation. The purpose of this article is to describe the evolution of the approach to open repair of the aortic arch and its technical peculiarities.
ResultsDifferent cannulation and perfusion strategies may have a different effect on postoperative outcomes. Classically, organ protection was achieved by drastically reducing oxygen demand through hypothermia; currently, this is combined with antegrade cerebral perfusion to increase the safe time of interruption of the aortic arch without increasing neurological risk. Axillary artery cannulation in combination with other sources of cerebral perfusion is a proven and reproducible strategy.
Continuous selective cerebral perfusion throughout surgery has allowed surgery to be performed at more moderate temperatures, which has been shown to reduce surgical times, postoperative bleeding and the rate of neurological complications compared to deep hypothermia.
Despite these improvements, surgical times and systemic circulatory arrest remain the main determinants of perioperative morbi-mortality. Recently, the strategy of normothermic circulation without interruption of perfusion of the lower body – in selected cases – has been adopted for a more physiological repair of the aortic arch.
ConclusionsOverall, arch surgery requires extensive planning based on the aortic arch, supra-aortic vessels and cerebral anatomy. Therefore, the choice of arterial cannulation, organ perfusion strategy and the temperature at which the repair is completed should be individualized to the patient.
La cirugía del arco aórtico es uno de los procedimientos más complejos en cirugía cardiovascular, ya que requiere el dominio de la manipulación temporal de la circulación cerebral y sistémica. El objetivo de este artículo es describir la evolución del abordaje y las técnicas de la reparación abierta del arco aórtico.
ResultadosLas distintas estrategias de canulación y perfusión tienen distinto impacto en los resultados postoperatorios. Clásicamente, la protección de los órganos se conseguía reduciendo drásticamente la demanda de oxígeno mediante hipotermia, combinándola actualmente con perfusión cerebral anterógrada para aumentar el tiempo disponible de parada sin aumentar el riesgo neurológico. La canulación de la arteria axilar en combinación con otras fuentes de perfusión cerebral es una estratégia testada y reproducible.
La perfusión cerebral selectiva continua durante toda la cirugía ha permitido mantenerse a temperaturas moderadas, lo que disminuye los tiempos quirúrgicos, la coagulopatía y las tasas de complicaciones neurológicas, en comparación con la hipotermia profunda.
A pesar de estas mejoras, los tiempos quirúrgicos y la parada circulatoria sistémica siguen siendo los principales factores determinantes de la morbimortalidad perioperatoria. Recientemente se ha adoptado la estratégia de circulación normotérmica y sin interrupción de la perfusión del hemicuerpo inferior – en casos seleccionados – para una reparación más fisiológica del arco aórtico.
ConclusionesEn general, la cirugía del arco requiere una planificación extensa, basada en la anatomia aórtica, de troncos supraaórticos y cerebral. Por ello, la selección de la canulación arterial, la estrategia de perfusión orgánica y la temperatura en la que se completa la reparación deben individualizarse en cada paciente.
The approach to aortic arch pathologies remains a challenging task for cardiovascular surgeons as it demands the ability to artificially manipulate both visceral and neurologic perfusion.
Aortic arch operative management has been continuously evolving since its birth was described by its pioneers.1,2 Advances in surgical techniques and cerebral perfusion have significantly reduced mortality and stroke rates to less than 10% and 5%, respectively, in recent publications.3,4 Mastering the ability to safely interrupt or modify the cerebral circulation has been one of the key factors in the evolution of this surgery and outcome improvement.
Indications for open aortic arch interventionDifferent aortic arch pathologies including aortic arch aneurysms, aortic dissection, penetrating ulcers, intramural hematomas or some congenital abnormalities of the arch usually require an open surgical approach.
Elective open repair of arch aneurysms greater than 55mm in diameter is indicated (Class IIa for both European5 and American6 Guidelines) or in the presence of symptoms attributable to the aneurysm (hoarseness, dyspnoea and dysphagia may be caused by the mass effect of the aneurysm on the nerves, trachea or oesophagus, respectively, in low operative risk patients (Class I)). For patients with connective tissue disorders, the surgical size threshold is lowered to ≥50mm for Marfan syndrome (Class 2a) and ≥45mm for Loeys-Dietz syndrome (Class 2b). These guidelines incorporate recommendations on arch involvement in the setting of acute aortic syndromes, and arch replacement is indicated in the presence tear or significant aneurysm in this location at the time of ascending aorta repair. Urgent arch repair is also indicated in the presence of a focal intimal ulcer-like disruption involving the arch in acute type A intramural haematoma and in the presence of aortic arch penetrating aortic ulcer with associated intramural haematoma (Class IIA).
Strategies for neurological protectionPhysiologic cerebral and spinal cord perfusion is inevitably interrupted during aortic arch reconstruction with reimplantation of epiaortic vessels. Both sectors are the most vulnerable to tissue hypoxia, therefore, it is crucial to safely protect them during arch surgery with available perfusion strategies and temperature management. The quality of the central nervous system protection remains key for aortic arch operatie outcomes.
Deep hypothermia with complete circulatory arrest (DHCA) was the initial gold standard strategy for arch surgery.7 The principal element of brain protection in this method relies on profound hypothermia to reduce drastically oxygen demands in the absence of flow, however there are important limitations well described: offers a limited safe period of 30min of cold ischaemia for the brain, with an exponentially increased risk after 40–60min,8 and deep hypothermia and prolonged cardiopulmonary bypass (CPB) times required for cooling and rewarming produce deleterious systemic effects. It is, however, accepted that DHCA alone as means of cerebral protection is no longer an accepted isolated strategy for brain protection in aortic arch surgery.
In early 90's, Ueda et al.9 proposed the use of retrograde cerebral perfusion during DHCA. It consisted of providing continuous retrograde cerebral flow (100–500mL/min) by connecting the arterial line of the CPB circuit into the superior vena cava cannula, monitored with the jugular vein pressure (20mmHg). Its main benefit is the maintenance of brain hypothermia, along with a reduced risk of debris embolization accomplish by minimizing the dissection and the manipulation of the supra-aortic vessels with clamps and its capacity of flushing air and debris out of the cerebral circulation. On the other hand, this technique has not shown accurate flow and metabolism dynamics of the brain, which is a problem in prolonged aorta surgeries, reason why it has been superseded by anterograde cerebral perfusion techniques.
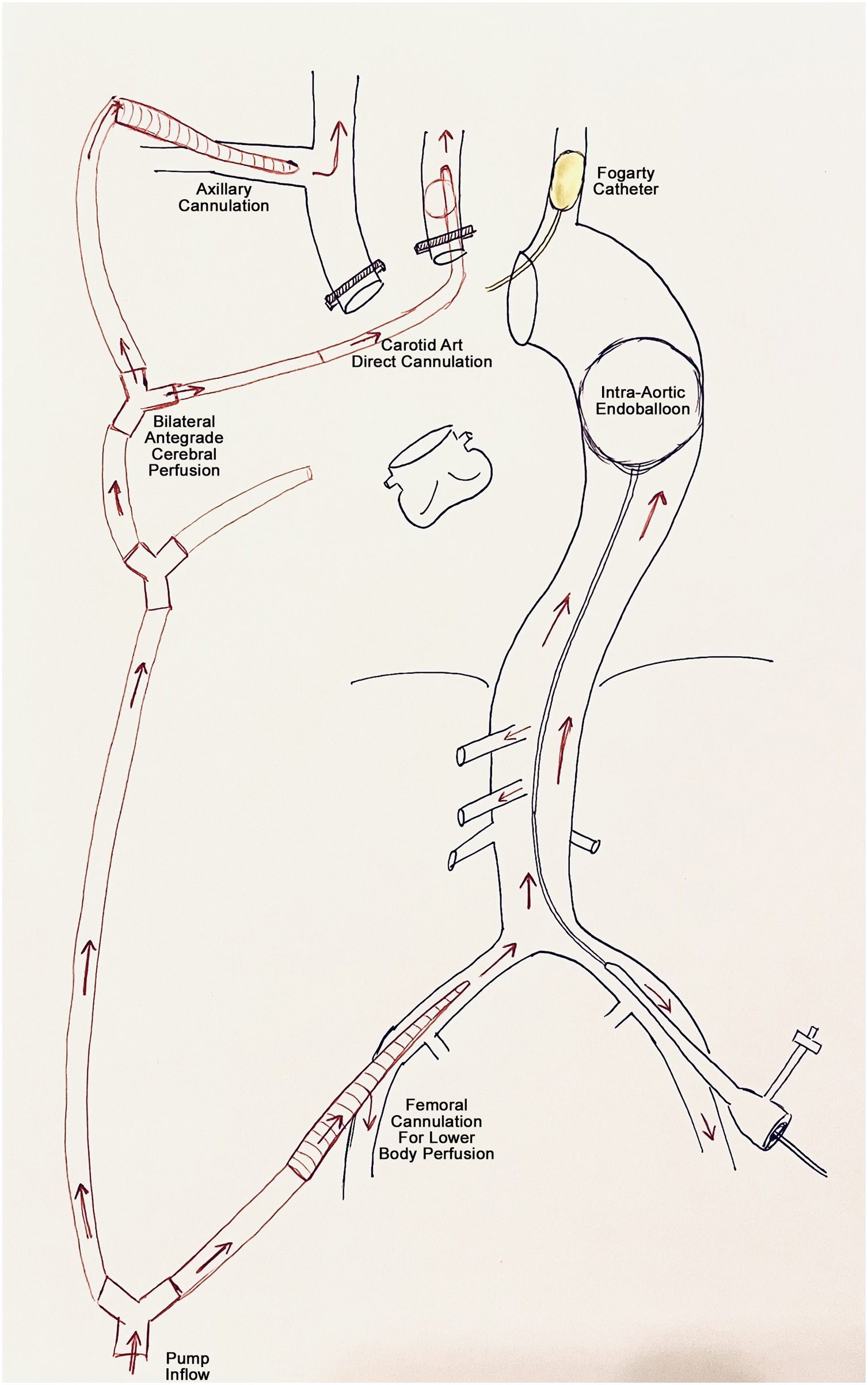
Antegrade cerebral perfusionAny segment of the aorta or its branches can be cannulated for CPB circuit inflow, directly or through a graft. The most common sites used during decades in all kinds of aorta procedures were direct ascending aorta/arch o femoral cannulation, but these two sites have several associated downsides: risk of embolization in atheromatous or thrombotic aortas, risk of malperfusion in type A aortic dissection and absence of antegrade cerebral perfusion (ACP) due to the cannulation itself, which requires the addition of cerebral perfusion cannulas. Therefore, in recent years there has been a trend to cannulate the axillary or innominate artery, as a proximal site that facilitates both antegrade full body blood flow and selective cerebral perfusion. Both accesses have proven similar safety profiles with no differences in the presence of new severe ischaemic lesions on diffusion-weighted magnetic resonance imaging and stroke/transient ischaemic attack rates.10
The axillary artery is the preferred cannulation site in most of the aortic centres of reference. However, accessing the axillary artery requires an additional incision, partial division of the pectoralis major and minor muscles and risk of brachial plexus injury. Innominate artery cannulation with a side graft has the potential to reduce the above complications. However, the presence of a graft/cannula into the body of the innominate artery may load the operative field in complex aortic arch surgeries and it should not be used for total arch reconstructions and in the presence of atherosclerotic or severe tortuous arteries.11 Finally, we must not forget that cerebral protection can also be achieved with selective ACP via direct cannulation of arch vessels with specific cerebral perfusion cannulas. This approach can be used alone to provide ASCP or can be used in combination with any other of the above mentioned in other to provide bilateral perfusion by direct left carotid artery cannulation. Manipulation of arch vessels with moderate/severe atherosclerotic plaques or dissection for selective perfusion must be individually assessed.
Once the cannulation site has been selected in order to provide antegrade cerebral perfusion, it is important to note that the ideal temperature and optimal flow rate of cerebral perfusion are not yet definitively established. A growing body of literature supports the safety of moderate hypothermic circulatory arrest (MHCA) always combined with antegrade cerebral perfusion. This strategy has proven to offer sufficient neurological and visceral organ protection.12,13 A randomized controlled trial by Sun et al.14 comparing the effects of moderate (25°C nasopharyngeal/28°C rectal temp) vs. deep hypothermia (20°C nasopharyngeal/25°C rectal temp), showed no differences in mortality between both groups and favourable short-term outcomes for the MHCA+ACP group, reporting less neurologic complications, faster recovery and more rapid extubation in those patients.
The results of a recent propensity score-matched analysis using data from the International Registry of Acute Aortic Dissection (IRAD)3 were less promising but showed no detrimental effects of MHCA (20–28°C) compared with deep hypothermia (<20°C). In-hospital mortality and 30-day survival were similar, with a trend towards lower rates of stroke and bleeding requiring reoperation in the MHCA group. This is consistent with other studies,15,16 all of which showed reduced CBP times in the MCHA group, one of the strong negative effects of DHCA.
Cerebral neuro-monitoring toolsDespite continuous cerebral perfusion and hypothermia, adverse neurological events still occur. Routine neurophysiologic intraoperative monitoring is recommended, when available, to provide real-time assessment of the cerebral status and incorporate immediate intraoperative changes when these events are detected in order to improve the outcomes. The most commonly used modalities are near-infrared spectroscopy (NIRS) cerebral oximetry and bispectral index (BIS), two non-invasive, easy to handle, and easy to interpret methods for intraoperative monitoring of the neurological status.
Near-infrared spectroscopy (NIRS) is a non-invasive, widely accepted, optical measurement tool used to estimate the regional haemoglobin oxygen saturation of blood in the brain (rSO2), allowing recognition of the adequacy of cerebral oxygenation and perfusion during cardiac surgery. NIRS oximetry is primarily applied to the upper forehead, giving information limited to the anterior area of the brain. There is no consensus on normal and abnormal values, but less than 50% or a reduction of more than 20% from baseline are accepted as pathological desaturation17 and thus, it has been shown to correlate significantly with neurocognitive status and mortality outcome.
The BIS algorithm initially processes the frontal electroencephalogram (EEG) to detect presence of cerebral suppression, with 0 indicating complete cerebral suppression and 90–100 consistent with the awake state. It is not validated for ischaemia detection, but helps to determine when circulatory arrest can be safely initiated and may be suggestive of ischaemia if a sudden drop in BIS occurs in the absence of a recent change in anaesthetic drug dosage. EEG is more reliable than BIS as it covers the cerebral metabolic activity from more brain areas, but on the other hand it is a more complicated technique that requires a clinical neurophysiologist in the operating room to interpret EEG changes during surgery and to identify and reduce artefacts. Both modalities are strongly influenced by anaesthetics, which are often discontinued during cooling to avoid misinterpretation,18 and deep hypothermic circulatory arrest suppresses EEG and BIS, which rise on rewarming, however NIRS values do not vary much and provide adequate continuous information on cerebral perfusion.
Lastly, transcranial colour Doppler (TCD) is another available tool consisting of real-time assessment of blood flow and distribution in large intracranial vessels. TCD is very useful for rapid detection of micro/macro emboli or inadequate cerebral perfusion, allowing rapid optimization of the perfusion strategy. However, TCD is time-consuming, sensitive to displacement and requires a high level of expertise.
The common goal of all these brain monitoring techniques is to quickly detect changes in cerebral perfusion and to be able to take immediate action to improve patient outcomes: intraoperatively, we can increase cerebral perfusion by optimizing PaCO2 or hematocrit, adding additional cannulas, evaluating the anastomosis for possible kinking, increasing pulsatility, and even immediate computed tomography to determine if neurointervention is indicated.
Normothermic arch replacementResearch has continued on how to further reduce the deleterious effects of systemic circulatory arrest and prolonged CBP times. ACP allows continuous perfusion of the brain, but visceral organs can critically suffer from prolonged arrest time at moderate hypothermia. If the time of arrest exceeds 50min, there is an increased risk of spinal cord injury and paraplegia. Damage from prolonged distal hypothermic visceral perfusion interruption may also translate in renal and hepatic failure.
A new technique has been proposed to avoid distal body circulatory arrest with or without the addition of protective hypothermia, thereby improving organ protection during surgery and reducing coagulopathy. This can be achieved with the use of an endoballoon to completely occlude the descending thoracic aorta, allowing perfusion of the lower half of the body through femoral artery cannulation, combined with ACP.19 The endoballoon can be inserted antegrade through the arch opening or retrograde from the femoral artery, and be placed into the native descending aorta if its free from thrombus or into the stented part of the frozen elephant trunk (FET) graft20 (Fig. 1).
Total operation, cardiopulmonary bypass and cardiac arrest time have been reported to be significantly shorter compared to patients operated on with MHCA, resulting in less renal and hepatic impairment and avoidance of coagulopathy and other adverse effects of DHCA.21,22
The use of endoballoon occlusion has emerged in recent years as a safe and effective strategy to complete aortic arch surgery in normothermia with uninterrupted systemic organ perfusion.
Proposed surgical strategyOpen aortic arch repair, including elective hemiarch, total arch with or without elephant trunk (ET), and dissection repair, requires thoughtful preoperative and intraoperative planning.
Depending on the extent of arch pathology, different surgical approaches are used. The most commonly used is the standard median sternotomy. Alternative options include: sternotomy with left lateral thoracotomy (hemi-Clamshell), bilateral sterno-thoracotomy (Clamshell) or an anteroposterolateral thoracotomy may be required. These options should be in the operative armamentarium. The preferred cannulation site for most groups, including our group, is the right axillary artery followed by the central aortic cannultion or multiarterial cannulation. Unilateral, bilateral or trilateral hypothermic ACP is applied by addition of dedicated inflatable perfusion cannulas in the supra-aortic vessels, targeting a flow of 0.7–1.0L/min, resulting in a pressure-controlled flow of 50–80mmHg to achieve appropriate cerebral protection.23 During ACP, the left subclavian artery is perfused or blocked using a clamp or a Fogarty catheter catheter to protect posterior brain perfusion. NIRS and arterial pressure monitoring is used for neuro-monitoring and moderate hypothermia (24–28°C) for organ protection. Control of perfusion pressure, ideally with double site pressure control with bilateral arterial radial lines to assess appropriateness of perfusion and to avoid cerebral oedema.24 Selection of the monitoring site should take the vessel pathology into account. Of note, axillary perfusion can lead to misleadingly high right radial readings. Nasopharyngeal and tympanic temperatures are recommended to ensure adequate cerebral cooling and urinary bladder probe to target core temperature of organs and spinal cord.
Open surgical techniquesDifferent techniques for replacement of the aortic arch are available. The choose of one or other will depend on the location, type and cause of arch disease, insitutional routinnes and the experience developed.
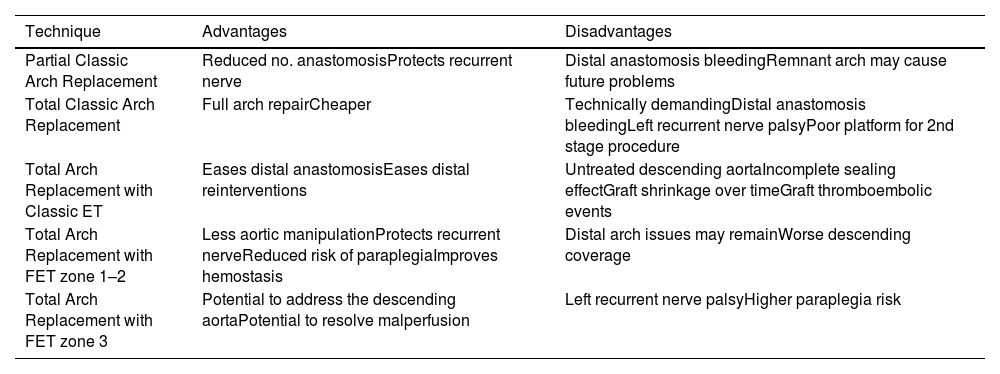
“Classic” arch replacement is performed with a direct anastomosis of a Dacron graft to the desired arch zone, depending on the extent of surgical resection required (partial or total arch replacement). The used of the 4-branch graft or the trifurcated arch graft (Vascutek, Inchinnan, UK) facilitates the selectively reimplantation of the supra-aortic vessels. The island reimplantation technique, almost obsolete nowadays, should be avoided on patients with known genetic disorders. This surgical approach focuses on addressing the critical part of the arch with reliable supra-aortic vessels perfusion, but there is a higher risk of bleeding from the distal anastomosis as becomes a more technically demanding anastomosis and it is a poor platform for potential secondary interventions.
Since the introduction of the elephant trunk technique by Borst et al.,25 this technique has been widely adopted as it simplifies the cons of the classic technique. This technique has been widely adopted as it simplifies the disadvantages of the classic technique. The distal anastomosis is simpler, reinforced and reduces bleeding risk; it can be performed more proximally resulting in less arch dissection and recurrent nerve injury.26 Another clear benefit of ET technique is the facilitation of a second stage procedure as serves as a platform for open or endovascular approaches. Classic ET by leaving a self-made free floating segment of graft or using the Sienna (TM) collared graft (Vascutek, Inchinnan, UK) is useful for mega-aortas, with no arch landing zone. This approach is also very valuable in chronic type B dissections in view need for a distal thoracoabdominal operation. Shrinkage of the classic elephant trunk over time and difficulties in endovascular navigation through this flexible part are downsides of this approach.
On the other hand, the FET procedure with the collared stented-graft prosthesis available permits the simplified hybrid single-stage repair of complex concomitant aortic arch and descending aortic disease. This technique is indicated in acute aortic dissection, particularly in association with malperfusion syndromes where it facilitates true lumen expansion and false lumen obliteration, in addition to chronic degenerative aneurysmal disease of the aortic arch and descending aorta. All the mentioned collared grafts facilitates the distal anastomosis by compensating the mismatch between the graft and the aorta and reducing the tension of the anastomosis.27 Nevertheless, there are potential complications secondary to the use of FET, including spinal cord injury, tromboembolic events and new distal entry tears.28 To improve outcomes and avoid these complications, there are several recommendations: the stented FET graft should be no longer than 10mm whenever possible and the left subclavian artery should be preserved by reimplantation during the same procedure or by an extra-anatomic graft in a first stage.29 All pros and cons of the mentioned techniques are shown in Table 1.
Sumarizes the advantages and disadvantages of the different open surgical techniques available to replace the aortic arch.
| Technique | Advantages | Disadvantages |
|---|---|---|
| Partial Classic Arch Replacement | Reduced no. anastomosisProtects recurrent nerve | Distal anastomosis bleedingRemnant arch may cause future problems |
| Total Classic Arch Replacement | Full arch repairCheaper | Technically demandingDistal anastomosis bleedingLeft recurrent nerve palsyPoor platform for 2nd stage procedure |
| Total Arch Replacement with Classic ET | Eases distal anastomosisEases distal reinterventions | Untreated descending aortaIncomplete sealing effectGraft shrinkage over timeGraft thromboembolic events |
| Total Arch Replacement with FET zone 1–2 | Less aortic manipulationProtects recurrent nerveReduced risk of paraplegiaImproves hemostasis | Distal arch issues may remainWorse descending coverage |
| Total Arch Replacement with FET zone 3 | Potential to address the descending aortaPotential to resolve malperfusion | Left recurrent nerve palsyHigher paraplegia risk |
Many options are available and aortic surgeons need to know and individualize which type of repair is best and most feasible for each arch pathology and patient. Finally, it is important to perform a technically appropriate operation and to ensure secure anastomoses prior to weaning from cardiopulmonary bypass, as this is critical to achieving good haemostasis. Bleeding remains an issue in arch surgery and must be properly managed at all stages of the procedure. Each anastomosis should be carefully assessed immediately after completion and reinforced if necessary, together with active homeostasis support both in the operating room and in the postoperative care unit.
ConclusionsAortic arch surgery requires critical planning based on the extent of repair with an adequate selection of arterial inflow site to provide systemic perfusion and facilitation of cerebral protection. An appopriately selected technique of organic protection and vascular reconstruction translates into excellent perioperative outcomes and unmatched durable repair.
Ethical considerationsThe authors assure that this is an original work and that ethical approval is not required as this study retrieves and synthesizes data from previously published studies.
Conflict of interestThe authors declare that they have no conflict of interest.