Open surgery for thoracoabdominal aortic aneurysms remains one of the most aggressive procedures, with a risk of paraplegia reaching up to 20% in some series. Preconditioning of the spinal circulation through prior embolization has been postulated to improve outcomes. We evaluate our initial experience with six patients.
Materials and methodsFrom May 2022 to June 2023, we operated on six patients with type II thoracoabdominal aortic aneurysms after prior embolization of the majority of their intercostal and lumbar arteries in 2–3 sessions separated by one month, prior to open surgery. Ethical considerations: All patients give their informed consent for the procedure and the publication of the results. Disclosures: None.
ResultsWe encountered no technical or neurological issues during the embolizations. The surgery was notably simpler due to reduced thoracic bleeding. Upon awakening, neurological assessment in all patients revealed no deficits. However, one patient, who required vasoconstrictors for sepsis postoperatively, subsequently developed paraplegia (6 days after the surgery).
ConclusionsStepwise prior embolization of a significant portion of intercostal and lumbar arteries proves to be of great assistance during surgery. However, we believe it is necessary to reimplant some intercostal arteries to favor spinal cord irrigation. We do not consider indiscriminate embolization of all intercostal arteries advisable, as it significantly reduces irrigation, which can be further compromised by postoperative vasoconstrictors.
La cirugía abierta de los aneurismas de aorta tóraco-abdominales sigue siendo una de las cirugías más agresivas con un riesgo de paraplejia que en algunas series alcanza el 20%. El preacondicionamiento de la circulación medular mediante embolización previa se ha postulado para mejorar resultados. Valoramos nuestra experiencia inicial con seis pacientes.
Material y metodosDesde Mayo 2022 a Junio de 2023 hemos operado a 6 pacientes de aneurisma tóraco-abdominal tipo II, tras embolización previa de la mayor parte de sus arterias intercostales y lumbares en 2-3 sesiones separadas por un mes, previamente a la cirugía abierta.
ResultadosNo tuvimos ningún problema durante las embolizaciones, técnico ni neurológico. La cirugía fue claramente más sencilla dado el menor sangrado durante el tiempo torácico. La valoración neurológica al despertar en todos los pacientes no mostró déficit neurológico alguno, sin embargo un paciente con necesidades posteriores de vasoconstrictor por sepsis presentó posteriormente paraplejia (6 días después de la cirugía).
ConclusionesLa embolización previa escalonada de gran parte de intercostales y lumbares resulta de gran ayuda durante la cirugía, no obstante creemos necesaria la reimplantación de alguna intercostal a fin de favorecer la irrigación medular. No creemos prudente la embolización indiscriminada de todas las arterias intercostales, al reducirse drásticamente la irrigación que puede ser empeorada por vasoconstrictores en el postoperatorio. Resumen secundario.
The open repair of thoracoabdominal aortic aneurysms requires an aggressive and multidisciplinary approach.1–4 Despite a notable mortality rate of 5–20%,2 it remains the primary alternative when endovascular treatment is not a therapeutic option, either due to anatomy or the patient's underlying aortic pathology.3 Despite improvements in outcomes, rates of paraplegia and renal insufficiency continue to be a challenge, impacting patient survival.4
Since the early strategies described by DeBakey and Crawford, the incorporation of techniques such as left bypass, sequential clamping, cerebrospinal fluid drainage, and even epidural cooling or moderate hypothermia have aimed to reduce the probability of spinal cord ischemia.
Traditionally, the preoperative description and localization of the Adamkiewicz artery (or rather the Adamkiewicz anastomotic system) have influenced spinal cord management, leading to the partial reimplantation of intercostal arteries (from T8 to T12), based on the results of the study published by Crawford,4–8 which demonstrated a 1.3 times reduction in the paraplegia rate.8
In 2007, Griepp et al., through resin injection in cadavers, described the existence of an extensive arterial and capillary anastomotic network beyond a single main radicular artery, which was named “The Collateral Network.” This discovery became the new cornerstone of studies aimed at spinal cord protection.
Recently, there has been consideration of “preconditioning” the Adamkiewicz anastomotic network through prior embolization of intercostal arteries.9
We present an observational study based on the results of preconditioning in our patients scheduled for open thoracoabdominal aortic surgery.
Materials and methodsFrom May 2022 to June 2023, for all patients scheduled for open surgery for type II chronic aneurysmatic thoracoabdominal aortic dissection, we decided to proceed with spinal cord preconditioning through embolization of a maximum number of intercostal and lumbar arteries, following informed consent from the patients.
Prior to this, all patients had undergone aortic arch replacement with an elephant trunk graft (using Siena or Thoraflex grafts, both from Terumo Aortic) without any complications.
The embolization protocol was carried out by the interventional radiology department of our multidisciplinary aortic team. It involved multiple sessions, depending on the patient's specific anatomy, to assess and occlude as many intercostal and lumbar arteries as possible, provided they were not identified as critical for feeding the Adamkiewicz system.10–12 All procedures were performed percutaneously via femoral access (5F). Various 5F catheters were used, typically CobraII, Simmons 1, Multipurpose (Cordis Corporation, Miami Lakes, USA), sometimes requiring manual or steam catheter preforming for optimal ostium anchoring to advance a microcatheter (Progreat 2.7/2.8F, Terumo, Japan).
Depending on the patient's anatomy, embolization was considered for arteries from T1 to L4 bilaterally. Patients underwent a minimum of one embolization session, with one patient requiring up to four procedures. In three cases, the Adamkiewicz artery was identified between T8 and L2. Occlusion testing was performed with a microcatheter-balloon (Scepter XC, Microvention, California, USA). After 30min of occlusion without clinical symptoms, embolization was performed in two of them. One patient experienced lumbar pain during the balloon occlusion test, leading to the interruption of occlusion, with the pain instantly disappearing. Furthermore, the artery was reimplanted during surgery.
An average of 4 arteries were embolized per procedure, and the average number of embolized arteries per patient was 11. Microcoils with controlled release, such as Optima (Bard, Irvine, USA), Concerto (Medtronic, Irvine, USA), and Microvascular plugs (MVP, Medtronic, Minneapolis, USA), were used as embolization materials according to each artery's caliber.
All patients were monitored for a few hours in the intensive care unit after the procedure and were discharged within 48h. The embolization sessions were spaced at least one month apart, and the surgery was performed approximately 8 weeks after the last embolization session. No repeat angiographic CT scans were performed after the sessions to avoid patient irradiation.
The surgery was conducted conventionally via thoracoabdominal laparotomy, extracorporeal circulation with a centrifuge, without a membrane, left atriofemoral bypass, and sequential aortic clamping with complete heparinization. The proximal anastomosis was made first to the previous elephant trunk, clamping the terminal arch and descending aorta at the T5 level and ligating any intercostals that were still patent. Subsequently, supraceliac clamping was performed, closely observing the condition of any existing intercostals in both lumens (in case of previous dissection) and deciding on a case-by-case basis. Intercostal arteries were reimplanted if the aorta's quality and their diameter were acceptable (greater than 2mm). Then, infrarenal clamping was performed, followed by continuous hematic perfusion of the celiac and mesenteric arteries, as well as the bilateral renal arteries using intermittent Custodiol® (500ml every 20min – 1.5ml/g of kidney, measured by CT scan). Finally, the four visceral trunks were reanastomosed to the four branches of the Coselli® graft we typically used.
During the intraoperative period, mean arterial pressure (radial and pedal) was maintained above 75mmHg through volume infusion, judicious use of the left bypass, maintaining hemoglobin levels above 10g/dl, and the use of vasoactive drugs if necessary (when clamping or unclamping the aorta).
For spinal cord protection, we used the automated controlled cerebrospinal fluid drainage system Licoguard (Möller Inc.), which monitored cerebrospinal fluid pressure and the volume of cerebrospinal fluid drained. Tissue perfusion in the cervical and lumbar regions was also monitored using tissue oxygen saturation sensors (INVOS, Medtronic Inc., USA).12 We did not use evoked potential monitoring, given its low therapeutic yield (it informs us of events that have already occurred).
In the immediate postoperative period, within the first 12h after arrival in the ICU (using a sedation window), the mobility of the lower limbs is assessed. Subsequently, after extubation, motor function of the lower limbs is evaluated according to the Tarlov scale13 to identify any temporary or permanent defects, as well as those with early or late onset.
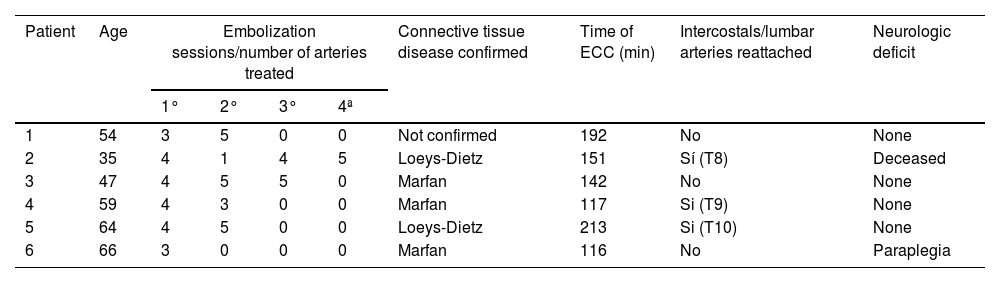
ResultsWe have operated on a total of 6 patients (Table 1). The average age was 60 years, with four of them being male. In terms of previous medical history, five of the patients had poorly controlled hypertension. Three of the operated patients had Marfan syndrome, two had Loeys-Dietz syndrome, and one presented Marfanoid features without confirmed genotypic confirmation.
Summary of patient characteristics, procedures performed and results.
| Patient | Age | Embolization sessions/number of arteries treated | Connective tissue disease confirmed | Time of ECC (min) | Intercostals/lumbar arteries reattached | Neurologic deficit | |||
|---|---|---|---|---|---|---|---|---|---|
| 1° | 2° | 3° | 4ª | ||||||
| 1 | 54 | 3 | 5 | 0 | 0 | Not confirmed | 192 | No | None |
| 2 | 35 | 4 | 1 | 4 | 5 | Loeys-Dietz | 151 | Sí (T8) | Deceased |
| 3 | 47 | 4 | 5 | 5 | 0 | Marfan | 142 | No | None |
| 4 | 59 | 4 | 3 | 0 | 0 | Marfan | 117 | Si (T9) | None |
| 5 | 64 | 4 | 5 | 0 | 0 | Loeys-Dietz | 213 | Si (T10) | None |
| 6 | 66 | 3 | 0 | 0 | 0 | Marfan | 116 | No | Paraplegia |
ECC: extracorporeal circulation.
Regarding previous surgical history, all 6 patients had undergone aortic valve and/or mitral valve repair or replacement surgeries. Additionally, they all had prior aortic arch replacement with an elephant trunk procedure.
Concerning the management of embolized intercostal arteries, in one patient, the T9 intercostal artery was reanastomosed, as it was considered to be of significant importance in the Adamkiewicz blood flow, according to angiography. In two other patients, the T9 and T10 intercostal arteries were reimplanted, respectively, as they had considerably larger diameters compared to the other embolized intercostals.
The surgery was performed conventionally as previously mentioned. In all cases, it was facilitated by a reduction in blood volume loss and surgical time during the intercostal management phase, which, unfortunately, couldn’t be quantified (due to the lack of previous records) but was clearly observed qualitatively.
The average duration of extracorporeal circulation was 156min (ranging from 116 to 213min). All patients woke up the day after the surgery and underwent a neurological assessment. In all cases, they exhibited complete mobility and sensation upon awakening.
During the postoperative period, special care was taken in monitoring cerebrospinal fluid drainage, maintaining a drainage rate of approximately 1–2cc/h to keep cerebrospinal fluid pressure (ICP) between 11 and 12mmHg (less than 10mmHg with a maximum withdrawal of 15cc/h, automated by Liquogard), and ensuring spinal cord pressure remained above 75mmHg. The catheter was removed after an average of 4 days.
Due to pulmonary issues, 5 out of 6 patients had extended ventilatory support, primarily due to right-sided pneumonia. In terms of renal function, 2 patients required temporary hemodialysis.
One patient passed away due to sepsis and multiorgan failure after 20 days in the ICU.
Regarding neurological assessment, no patient showed signs of spinal cord ischemia during the initial postoperative window, including the patient who later passed away. One patient, who had severe pneumonia and required vasoconstrictor support, developed paraplegia 6 days after the surgery, even with the cerebrospinal fluid drainage catheter removed. The use of a new drainage was not considered. Despite improvements in other vital functions and successful extubation, the patient did not recover from paraplegia, with a spinal cord injury at the T9 level confirmed by a magnetic resonance imaging scan. This patient had not undergone any intercostal artery reimplantation.
Upon discharge, four patients had full lower limb mobility, while one was left paraplegic. After an 8-month follow-up, none of the four patients with mobility issues in their lower limbs had experienced further neurological complications.
DiscussionThe largest series of patients undergoing open thoracoabdominal aortic surgery was presented by Coselli in 2016, involving 3309 patients (with an average age of 66 years), and included 762 patients with Crawford type II classification. In this subgroup, spinal cord injury was observed in up to 48% of patients.14,15 Therefore, it is recommended to reimplant at least one intercostal artery, whenever possible, to reduce the likelihood of spinal cord injury.
After observing improved outcomes, particularly regarding spinal cord injury, with stent-based treatment, the possibility of preconditioning the spinal circulation through selective preoperative embolization of intercostal arteries has been raised.16 This technique, known as MISACE, was described by Etz et al.11,12 In a series of 57 patients in Leipzig,17 no patient developed neurological complications after endovascular repair of thoracoabdominal aneurysms. They have also reported two patients (one treated percutaneously and one with open surgery) with positive results following prior embolization of their intercostal arteries. Currently, they are leading a prospective multicenter study for open surgery.18
Based on these preliminary results, it is understood that embolization reduces the “struggle time” against the intercostal arteries in the second stage of the surgery, thereby reducing surgical time and intraoperative bleeding. However, it is important to note that paraplegia in the case of patient 6 can be explained by reduced spinal cord irrigation due to the surgery, embolization, and the use of vasopressors in the postoperative period, considering the patient's septic shock.
Therefore, it is believed that complete embolization of intercostal arteries could jeopardize spinal cord irrigation in a significant percentage of patients.
Based on the observed results, our strategy is to prioritize the reimplantation of intercostal arteries angiographically identified between T8 and T12, as well as larger lumbar arteries, while embolizing the remaining ones. This approach is based on the Collateral Network theory, favoring the “border arteries” of the spinal cord, avoiding blood steal during the intervention, and stimulating, as much as possible, the development or reinforcement of the collateral network.
We do not believe that indiscriminate embolization of intercostal and lumbar arteries is a prudent alternative, as it reduces the chances of reimplanting a potential source for the Adamkiewicz network. In our experience, no patient experienced immediate spinal cord issues after surgery. However, the reduction in the vascular supply reserve likely contributed to the paraplegia in the patient who required vasoconstrictor drugs during the postoperative period.
The selective study and embolization of intercostal and lumbar arteries before open surgery, we believe, provide valuable information and will be a powerful tool if used judiciously. We are also aware of the possibility of inducing iatrogenic type B dissection in these patients, especially in patients with Marfan syndrome or Ehler-Danlos syndrome.
We think that further studies should be conducted to elucidate the most appropriate strategy for managing spinal cord perfusion in patients undergoing thoracoabdominal aortic replacement.
Conflict of interestsNo conflict of interest.