Root remodeling is one form of valve-preserving root replacement for aortic regurgitation and root aneurysm, which we have employed consistently for more than 28 years.
MethodsBetween 10/95 and 7/2023 root remodeling was performed in 1285 patients (76% male, mean age 53±14 years). The aortic valve morphology was unicuspid in 34 (3%), bicuspid in 525 (41%) and tricuspid in 726 (56%) patients. Fifty-four patients (4%) had Marfan's syndrome. Measurement of valve configuration (effective height) was performed in 1075 (84%), and an external suture annuloplasty was added in 705 patients (55%). Cusp repair was performed in 1143 (89%) patients, most commonly for prolapse (n=1000; 82%). Mean follow-up was 6.7±5.5 years (1 month to 28 years). Follow-up was 95% complete (8026 patient-years).
ResultsSurvival was 71% at 20 years, freedom from cardiac death 80%. Freedom from aortic regurgitation ≥2 was 77% at 15 years. Freedom from reoperation was 89%, higher in tricuspid (94%) compared to bicuspid (84%) and unicuspid valves (p<0.001). With a suture annuloplasty, freedom from reoperation was 94% at 12 years. The difference with (94%) or without annuloplasty (91%) was not significant (p=0.949).
ConclusionRoot remodeling is a viable option in valve-preserving root replacement. Concomitant cusp prolapse is frequent and can be corrected reproducibly by intraoperative measurement of effective height. The long-term stability of the aortic valve depends primarily on the underlying morphology. Up to 15 years postoperatively, the addition of an annuloplasty had a limited positive effect on residual regurgitation, but (as yet) no effect on freedom from reoperation.
La remodelación de la raíz es una forma de reemplazo de la raíz con preservación de la válvula, debido a regurgitación aórtica y aneurisma de la raíz, que hemos utilizado consistentemente durante más de 28 años.
MétodosRealizamos entre 10/95 y 7/2023 remodelaciones de la raíz en 1.285 pacientes (76% varones, edad media 53 ± 14 años). La morfología de la raíz aórtica fue unicúspide en 34 (3%) pacientes, bicúspide en 525 (41%) y tricúspide en 726 (56%). Cincuenta y cuatro pacientes (4%) tenían síndrome de Marfan. Se realizó medida de la configuración de la válvula (altura efectiva) en 1.075 (84%) pacientes, y se añadió anuloplastia externa a la sutura en 705 pacientes (55%). Se reparó la cúspide en 1.143 (89%) pacientes, debido normalmente a prolapso (n = 1000; 82%). El seguimiento medio fue de 6,7 ± 5,5 años (de 1 mes a 28 años). Dicho seguimiento tuvo una compleción del 95% (8.026 pacientes-años).
ResultadosLa supervivencia fue del 71% a 20 años, con ausencia de muerte cardiaca en un 80%. La ausencia de regurgitación aórtica ≥2 fue del 77% a los 15 años. La ausencia de reintervención fue del 89%, siendo más alta en las válvulas tricúspides (94%) en comparación con las bicúspides (84%) y unicúspides (p < 0,001). Con anuloplastia de sutura, la ausencia de reintervención fue del 94% a 12 años. La diferencia entre la presencia (94%) o ausencia de anuloplastia (91%) no fue significativa (p = 0,949).
ConclusiónLa remodelación de la raíz es una opción viable en términos de reemplazo de la raíz con preservación de la válvula. Es frecuente el prolapso de cúspide concomitante, pudiendo ser corregido reproduciblemente mediante medida intraoperatoria de la altura efectiva. La estabilidad a largo plazo de la válvula aórtica depende principalmente de la morfología subyacente. Hasta un periodo de 15 postoperatorios, la adición de anuloplastia tuvo un efecto positivo limitado en la regurgitación residual, pero (hasta la fecha) ningún efecto en la ausencia de reintervención.
Root remodeling was designed to be an alternative to combined valve and root replacement in treating aortic regurgitation (AR) in the presence of root aneurysm.1 The original hypothesis was that AR was due to aortic dilatation, and normalizing root dimensions should lead to normal aortic valve function. While early results were good,2 late results of the original series showed a relevant proportion of patients requiring reoperation for recurrent AR.2 This was assumed to be related to the lack of annular stabilization,3,4 questioning the value of the technique.
After we started to explore the concept of root remodeling as valve-preserving surgery (VPS) 28 years ago,5 we encountered apparent cusp prolapse in the presence of root aneurysm; intuitively we added cusp repair to the root procedure.6 This approach did not compromise valve function,7 it rather became obvious that concomitant cusp repair improved the functional results. In an in-vitro study, we found more physiologic cusp motion with root remodeling compared to valve reimplantation,8 which encouraged us to continue with the concept. We subsequently modified the procedure to accommodate the characteristics of the bicuspid valve (BAV9), and later also the unicuspid aortic valve (UAV10). We found equivalent mid-term valve function with root remodeling and valve reimplantation.11
Initial valve assessment relied on visual inspection. The analysis of failed valves stimulated us to analyze aortic valve configuration in more detail. We developed the concept of effective height (eH) as a cusp configuration parameter.12,13 In order to define the amount of cusp tissue, we introduced the measurement of geometric height (gH).14 The measurement of eH facilitated the creation of predictable valve configuration, also in the experience of others.15,16
Lansac propagated the addition of an annuloplasty to improve annular stabilization, which had been considered the Achilles heel of root remodeling.17 The improved results, however, were most likely due to the intraoperative measurement of eH, which was introduced together with the annuloplasty. Stimulated by the efficacy of a suture annuloplasty in isolated BAV repair, we also added an annuloplasty to root remodeling to determine whether it would indeed further improve valve function and stability.18
Over the past 28 years, we have employed root remodeling as a standardized procedure based on geometric principles. The objective of the current analysis was to review the long-term results of root remodeling with a special focus on the effect of an annuloplasty and the influence of the underlying valve morphology.
Patients and methodsPatientsWe conducted a retrospective analysis of 1285 patients who underwent root remodeling at Saarland University Medical Center between October 1995 and June 2023. The investigation was approved by the Saarland Regional Ethics Committee (CEP 202/19, CEP 203/19), and individual patient consent was waived for the analysis and publication in anonymized fashion.
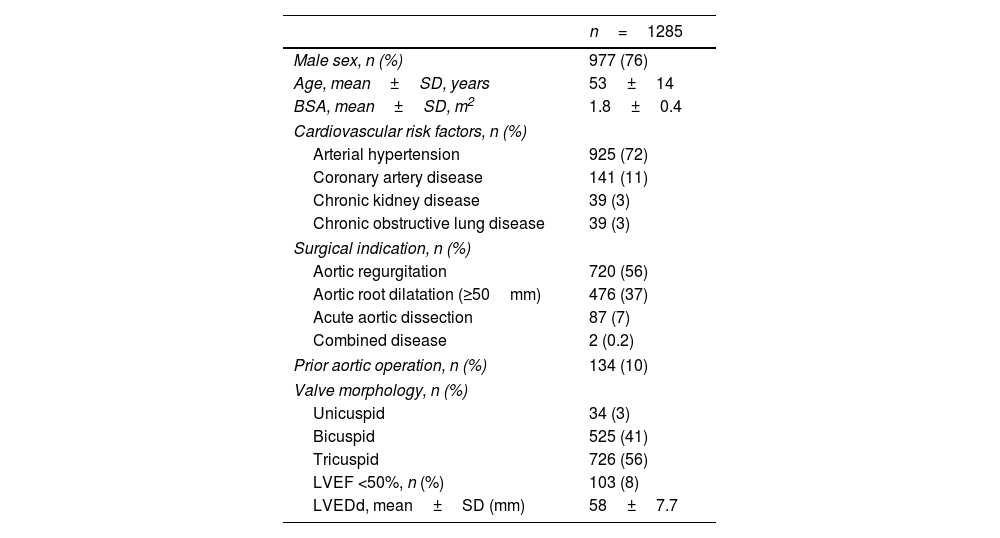
Of all patients, 76% were male with a mean age of 53±14 years (range 2–86 years; Table 1). The original aortic valve morphology was unicuspid in 34 (3%), bicuspid in 525 (41%), and tricuspid in 726 (56%) patients. Fifty-six patients (4%) had confirmed connective tissue disease, in the majority of cases Marfan's syndrome. Prior to the index procedure, 134 patients (10%) had undergone at least one cardiac operation. The primary indications for surgery were severe and symptomatic aortic regurgitation (n=720, 56%) and aortic root dilatation (sinus ≥50mm, n=476, 37%). Eighty-seven patients underwent remodeling for acute aortic dissection (7%; Table 1).
Preoperative patient characteristics.
| n=1285 | |
|---|---|
| Male sex, n (%) | 977 (76) |
| Age, mean±SD, years | 53±14 |
| BSA, mean±SD, m2 | 1.8±0.4 |
| Cardiovascular risk factors, n (%) | |
| Arterial hypertension | 925 (72) |
| Coronary artery disease | 141 (11) |
| Chronic kidney disease | 39 (3) |
| Chronic obstructive lung disease | 39 (3) |
| Surgical indication, n (%) | |
| Aortic regurgitation | 720 (56) |
| Aortic root dilatation (≥50mm) | 476 (37) |
| Acute aortic dissection | 87 (7) |
| Combined disease | 2 (0.2) |
| Prior aortic operation, n (%) | 134 (10) |
| Valve morphology, n (%) | |
| Unicuspid | 34 (3) |
| Bicuspid | 525 (41) |
| Tricuspid | 726 (56) |
| LVEF <50%, n (%) | 103 (8) |
| LVEDd, mean±SD (mm) | 58±7.7 |
n: number; SD: standard deviation; BSA: body surface area; LVEF: left ventricular ejection fraction: LVEDd: left ventricular end-diastolic diameter.
Intraoperative transesophageal echocardiography (TEE) was performed for analysis of root dimensions and cusp pathology. The surgical technique depended on the valve morphology and cusp pathology encountered, including cusp repair and suture annuloplasty as needed. The technique and its modification for bicuspid and unicuspid valves have been described in detail previously.9,10,19
Briefly, the operations were performed via a median sternotomy using aortic and right atrial cannulation; in acute dissection, the right axillary artery was used for arterial inflow. Antegrade blood cardioplegia was given directly into the coronary ostia. Cusp size was determined before deciding in favor of valve preservation. In tricuspid valves, a gH ≥18mm was the minimum for preservation. In bicuspid valves, a gH of ≥20mm of the non-fused cusp, and in unicuspid valves, a gH of >20mm of the left and non-coronary cusps was a prerequisite for repair. A decision for valve replacement was made generally for cusp calcification, active endocarditis, and retraction in bicuspid valves. Tricuspid aortic valves (TAVs) were replaced for cusp retraction, multiple fenestrations or calcification, UAVs for calcification beyond the limits of the right cusp.
After root mobilization and excision of the sinus wall, a tubular graft was tailored to accommodate the configuration of the aortic root and sutured to the cusp insertion lines. For TAV, three tongues were created. For asymmetric BAV, the commissures of the non-fused cusp were placed at a 160° orientation in the first 119 patients. In all subsequent patients, an orientation of approximately 180° was chosen with two symmetric tongues for symmetric and asymmetric BAV (n=309) as well as for UAV. In very asymmetric BAVs, three tongues were created in analogy to TAVs (n=44). The length of the tongues was adjusted according to the height of the native commissures, i.e. 1–1.5cm longer than the native commissural height.
Initially, remodeling was used for patients with an annular diameter of <30mm, and the graft size was chosen 1–2mm smaller than the basal diameter. Later, all root morphologies were included, and graft size was chosen according to the body surface area of the patient (24mm for <1.8m2, 26mm for 1.9–2.2m2, and 28mm for 2.3m2 and larger). In TAVs with a geometric height <20mm, a smaller graft (one size less) was taken.
Valve configuration was visually assessed after completing the root procedure (only visual: n=243; 23%). Since 2004, eH of each cusp was measured using a caliper (Fehling Instruments, Karlstein am Main, Germany; n=1075; 84%).12 Cusp prolapse was defined as eH <9mm (in BAV of the non-fused cusp) and corrected by central plication until an eH of 9–10mm was reached (TAV, n=726; BAV, n=525; UAV, n=34). Fenestrations were accepted if they were not involved in prolapse. Perforations and larger fenestrations with prolapse were closed with a pericardial patch (autologous pericardium, n=26; heterologous pericardium, n=6).
In unicuspid valves, the left/non-coronary commissure was used as a reference for commissural height. A new commissure was created opposite of this normal commissure for symmetric orientation.20 Using triangular patches, the gaps between preserved left or non-coronary cusp tissue and the new commissure were closed. In seven patients, cusp nadir relocation was performed without the use of a patch.21
An external annuloplasty was added after 2008 if the annulus measured >26mm. In most instances (n=716), an expanded polytetrafluoroethylene suture (Gore-TexCV-0, W.L. Gore & Assoc., Munich, Germany) was used. The suture was tied around a Hegar dilator (<1.8m2: 21mm, 1.8–2.0m2: 23mm, >2.0m2: 25mm). Of the 726 TAV patients, 404 were treated with an annuloplasty, in BAV 284 of 525, and in UAV 28 of 34.
All patients underwent intraoperative transesophageal echocardiography. They also underwent transthoracic echocardiography (TTE) before discharge, at three months, at one year and biannually thereafter. Mean and peak systolic gradients were measured, and AR was analyzed by color Doppler and classified as absent, mild, moderate or severe.
Follow-upAll patients were followed prospectively both clinically and echocardiographically (at discharge, 3 months, 1 year and yearly thereafter). For this study, the echocardiograms from our institution and referring cardiologists were reviewed. Systolic gradients were measured using continuous wave Doppler. AR was determined using color Doppler according to European guidelines.
Median and mean follow-up were six years (range one month to 28 years) and 6.7±5.5 years. Follow-up was 95% complete (7700 patient-years).
Statistical analysisNon-normally distributed continuous variables are presented as median (interquartile range), and the Mann–Whitney U test was used for between-group comparisons. Normally distributed continuous variables are presented as mean±SD and were compared using the t-test. Categorical variables are expressed as frequency (%). Time-dependent data were analyzed using the Kaplan–Meier method. Differences were assessed using the log-rank test. Survival and freedom from reintervention were calculated at one, five, ten, 15 and 20 years. All statistical tests were 2-sided, and p-values <0.05 were considered statistically significant for all analyses. Statistical analyses were performed using SPSS 28.0 (Version 28.0, IBM, Amrock, NY).
ResultsEarlyCusp pathology requiring correction included cusp prolapse (n=972; 82%), fenestrations (n=34), retraction (n=6) and perforations (n=5). Cusp repair was performed in 1143 (89%) patients (UAV n=34/34, BAV n=510/525, TAV n=716/726). A patch was used in 92 patients (7%).
Mean myocardial ischemia time was 85±20min with concomitant procedures and 68±14min without additional procedures (p<0.001). There was no myocardial infarction and two patients developed neurological complications. One patient required a permanent pacemaker implantation after ablation for persistent atrial fibrillation; no atrioventricular block was observed in patients with sinus rhythm. There were no early reoperations; re-exploration for bleeding was necessary in 30 patients (2.3%). Hospital mortality was 1.4% (n=18/1285). Of these, seven were cardiac deaths (cardiac failure, n=6; arrhythmia, n=1).
Aortic regurgitation at dischargeWith the introduction of eH measurement, the proportion of patients with a competent valve at discharge was higher (n=675; 65%) compared to only visual assessment (n=73; 30%; p=0.04). With suture annuloplasty, a higher proportion of patients had no AR at discharge (n=643/716; 91%) than without suture annuloplasty (n=464/569; 80%; p<0.001). This differed between the aortic valve morphologies (with annuloplasty TAV n=340/404; 84%; BAV n=202/284; 71%; UAV n=14/28; 50%; without annuloplasty TAV n=239/322; 74%; BAV n=152/241; 63%; UAV n=4/6; 67%).
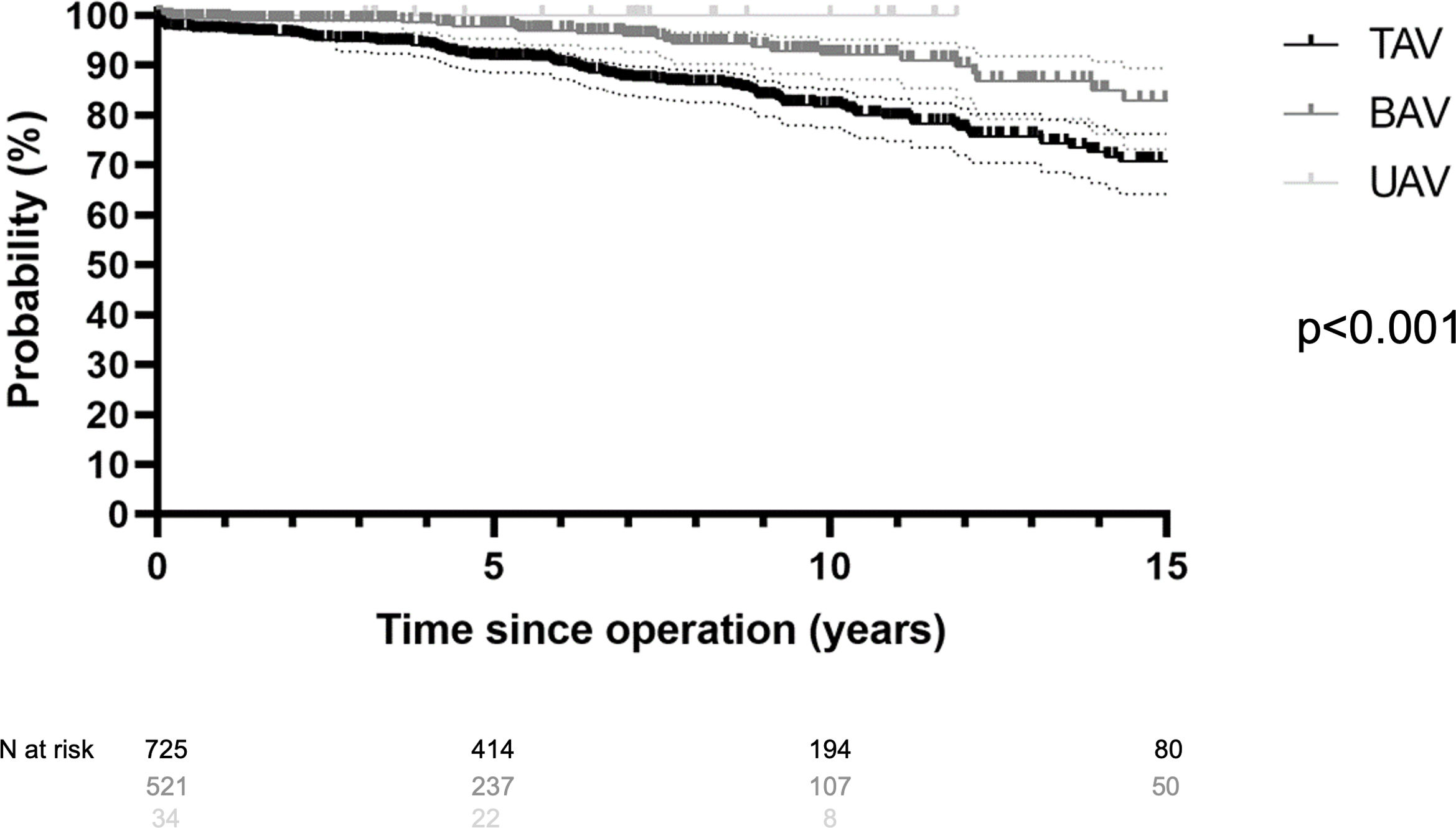
Late survivalLate postoperatively, 141 (12%) patients died between 1.1 months and 23 years. Of these, 62% (n=87) died of a cardiac cause for a cardiac survival of 80% at 20 years (n=1198). It was 80% for TAV (n=640), 95% for BAV (n=520), and 100% for UAV (n=34) (p<0.001; Fig. 1). Survival at 15 years was significantly better in patients who underwent elective surgery (76%) compared to acute dissection (58%) (p<0.001). It was superior at 15 years in patients without concomitant CABG procedure (79%) compared to those with CABG (54%) (p<0.001). Survival at 10 years was 81% without suture annuloplasty compared to 91% with suture annuloplasty (p<0.001).
Freedom from recurrent AR ≥2Valve function has remained stable in the majority of patients. Over time, however, 104 patients (8%) developed AR ≥2. Freedom from AR ≥2 was 91% at 10 years (TAV 91%; BAV 92%) and 80% at 15 years (TAV 81%; BAV 78%; p=0.102). Freedom from AR ≥2 in UAVs was 88% at 10 years. At 10 years, there was a trend towards a better freedom from AR ≥2 with the addition of a suture annuloplasty (92%) compared to patients without a suture annuloplasty (87%; p=0.07). At 10 years in patients with TAV, freedom from AR ≥2 was 93% with the addition of a suture annuloplasty compared to patients without a suture annuloplasty (88%; p=0.173). In BAV, freedom from AR ≥2 at 10 years was 92% with the addition of a suture annuloplasty compared to patients without a suture annuloplasty (90%; p=0.787). The limited number of UAV patients did not allow for a reasonable comparison.
GradientsIn patients with TAV, normal systolic gradients (mean 4±3mmHg) remained throughout the follow-up in almost all cases. With BAV, the mean gradient at last follow-up was 7±6mmHg; it was 10±8mmHg with asymmetric orientation compared to 6±5mmHg when symmetric repair was performed (p=0.03). With UAV, the mean gradient at last follow-up was 15±7mmHg. In all valve morphologies, gradients were not higher with compared to without annuloplasty (with annuloplasty: TAV 4±5mmHg, BAV 7±5mmHg, UAV 9±5mmHg; without: TAV 6±6mmHg, BAV 8±7mmHg, UAV 18±12mmHg).
ReoperationSixty-nine patients required aortic valve reoperation between one month and 21 years postoperatively (median 6 years). The main indications for reoperation included recurrent AR ≥2 (n=40), active endocarditis (n=11), and aortic stenosis (BAV n=6, TAV n=3). Reoperations consisted of valve replacement (n=37), valve repair (n=21), root replacement (n=6) and pulmonary autograft replacement (n=5).
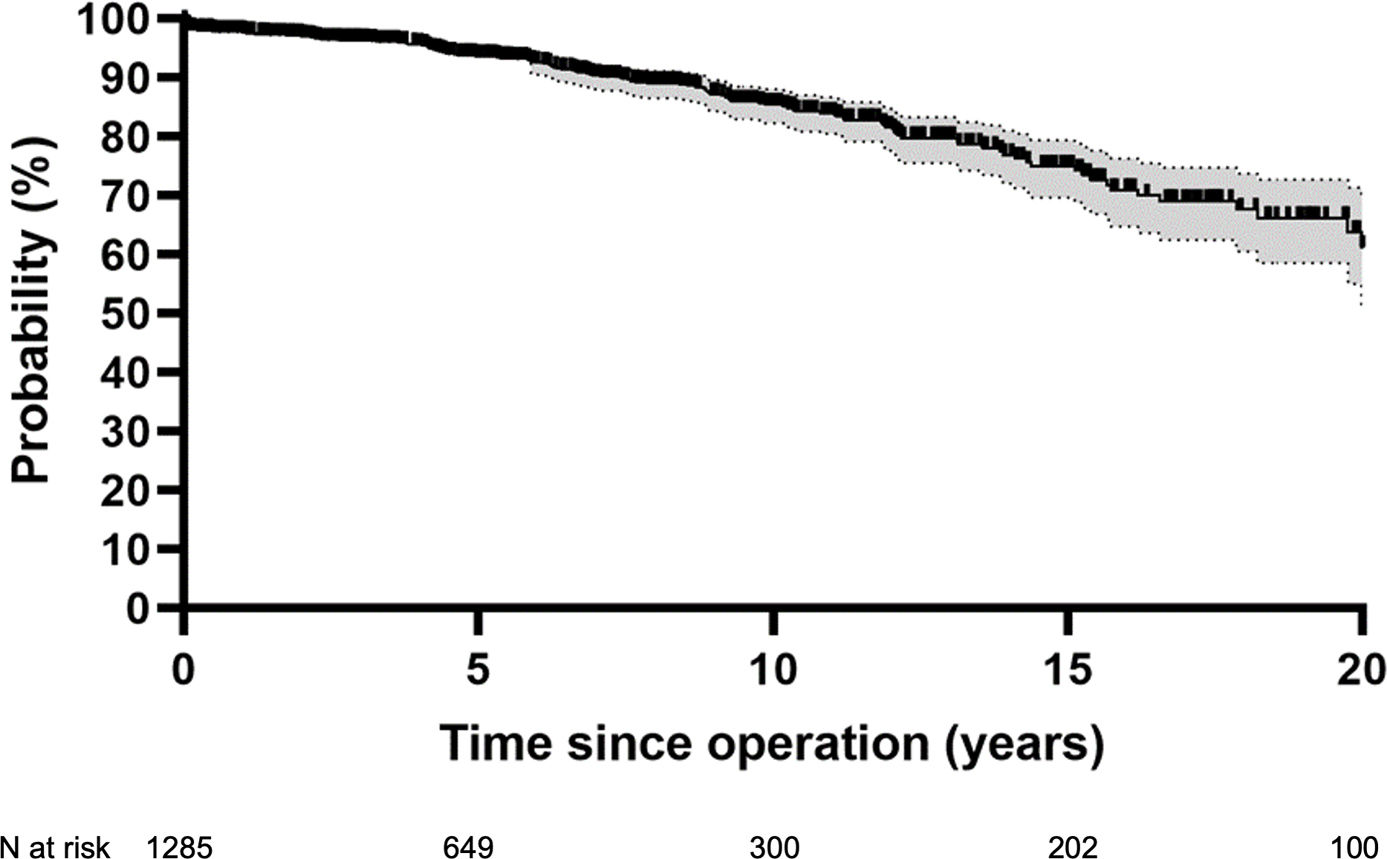
Overall freedom from reoperation was 78% at 20 years (Fig. 2). Freedom from reoperation at 15 years was best in TAV (94%) compared to BAV (84%) (p<0.001). Freedom from reoperation for UAV at ten years was 64% (Fig. 3). The introduction of eH measurement had no effect on freedom from reoperation at 15 years (92% with and 87% without; p=0.275). It was 92% without and 97% with eH measurement in tricuspid valves (p=0.043), and 83% without and 85% with eH measurement in bicuspid valves (p=0.524).
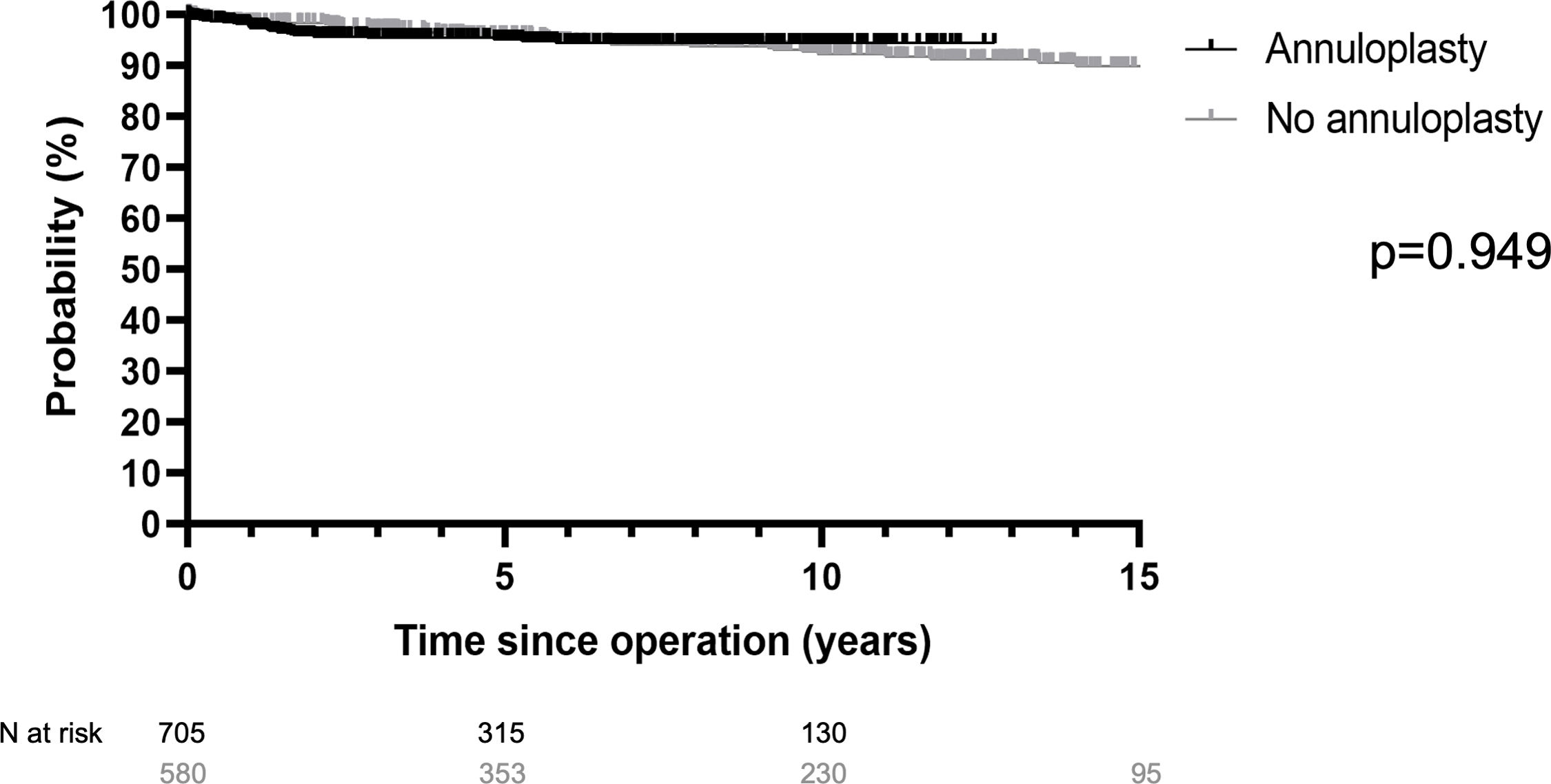
Freedom from reoperation at 12 years was 95% with the addition of a suture annuloplasty and 91% without (p=0.949; Fig. 4). It was 94% without and 97% with annuloplasty in TAV (p=0.209), and 88% and 92% with annuloplasty in BAV (p=0.488). In UAV, 5-year freedom from reoperation was 75% without and 85% with an annuloplasty (p=0.573).
DiscussionAfter almost three decades of root remodeling, the basic principle of the operation has remained generally unchanged. Compared to the original description,1 only minor details were modified and then kept constant throughout our practice: the length of the Dacron tongues was not predetermined but adjusted to exceed the height of the native commissures. In order to facilitate the procedure, we have started suturing in the sinus nadir. Our only conceptual modifications have been 1. the addition of cusp repair, 2. the introduction of systematic measurement of effective height to standardize detection and correction of cusp prolapse and 3. the addition of an annuloplasty.
We were positively impressed by the more physiologic cusp motion and systolic gradients in in-vitro experiments.8,22 There is lesser need for aggressive basal dissection compared to aortic valve reimplantation. This results in shorter ischemic times23 and fewer instances of atrioventricular block, which is in the range of 5% with reimplantation.24 In this context, the absence of atrioventricular block in our current series is noteworthy. We subsequently modified the original procedure to accommodate the anatomy of a BAV19 and UAV.10
Based on the analysis of the normal form of an aortic valve, we hypothesized that the height difference between annular plane and cusp margins in diastole – effective height – could be used as a configuration parameter for the aortic valve.12 We found a close correlation between eH and patient size,13 with 9–10mm being ideal for normal-sized adults.13,25 Normalized eH was associated with better durability in aortic valve repair,26 and we have systematically measured eH with a caliper since 2004.
Both in the pioneer series of root remodeling and others, a relevant proportion of residual and recurrent AR was observed2,4 with consecutive need for reoperation. The precise reason for these valve failures was not clear but it was attributed to a lack of annular stabilization.3,4 Consequently, reimplantation of the aortic valve became the preferred form of VPS for many surgeons, based on the excellent results of the pioneer series4 and the assumedly better annular stabilization. Interestingly, however, we were not as successful as the original pioneer.5 Also in the hands of others, a relevant proportion of patients developed postoperative AR.27,28 In retrospect, these failures were likely due to unrecognized prolapse in the absence of measuring eH. These findings raise the question whether annular stabilization is as important as it has been assumed, or whether patient selection and better control of valve configuration are the more important determinants of postoperative valve function.
Patient selection may lead to exclusion of patients with more pronounced cusp stretching. Very few – if any – reports indicate the proportion of patients with root aneurysm that undergo VPS. In the best-known international referral center, probably less than 40% have been treated by VPS.29 We have performed VPS in 90% of all cases,30 probably accepting more cusp deformation than others. This has not yet been associated with an increase in the need for reoperation.30
Patient selection also includes aortic valve morphology. Most series focus on TAV, some include a limited proportion of BAV, and we are not aware of any series including UAV. The natural history of BAV and UAV, however, suggests that they should take a different course from TAV. Indeed, our experience and the current data show that valve morphology has a strong impact on the late results. There is a certain degree of attrition in preserved TAVs over time; it is present in the current series and others with reimplantation.31 BAVs take a different course, with fibrosis and calcification occurring more frequently. In BAVs, the use of pericardial patches or other substitutes for cusp repair has been associated with a higher degree of failure compared to TAVs.32 Both mechanisms have been responsible for the majority of reoperations also in our current series. UAVs will need a cusp substitute in most instances to create a functioning valve design,20 and expectedly, even more attrition of UAVs was observed with root remodeling. Interestingly, in some instances root remodeling allows for UAV repair without patch material.21
In order to achieve annular stabilization, a ring annuloplasty was proposed by Lansac33; its use apparently resulted in drastic improvement of valve competence and durability.17 Interestingly, this was in contrast to our experience.34 Even without any annuloplasty, annular size reduction was observed,35 and valve durability in our hands was markedly better than that of the initial results of Lansac.33 Thus, the described improvement17 was likely due to introducing the measurement of eH intraoperatively, allowing for better control of valve configuration. We had systematically applied the intraoperative measurement to correct prolapse since 2004.
We therefore decided to explore the additional value of an annuloplasty while the other operative details remained constant, and also added the concept of annuloplasty to root remodeling. Of the different options,36 we used a suture annuloplasty for ease of application. Early results were promising, and the proportion of competent aortic valves at discharge increased significantly.18 We have, however, not yet seen an improvement in valve durability with the addition of annuloplasty when BAVs were treated by root remodeling.37,38 This is confirmed by the current series. In a TAV anatomy, the addition of an annuloplasty has not yet shown a significant effect on freedom from reoperation, contradicting our expectations and the experience of others.39 As seen in the current analysis, the competence of the aortic valves was improved significantly; up to 14 years, freedom from reoperation is unchanged. A possible explanation for this observation could be the eH-driven and thus aggressive strategy of cusp repair. In doing so, we were even able to treat a relevant number of root aneurysms with prolapse of all three cusps. The avoidance of symmetrical prolapse could perceivably reduce the stress at the level of the ring and thus contribute to annular size reduction.35 It remains to be seen whether a difference in freedom from reoperation may be observed in the second and third postoperative decade.
In assessing the current long-term experience, it has become increasingly clear that both adequate postoperative valve configuration and the original aortic valve anatomy (i.e. tricuspid, bicuspid or unicuspid) are important. This is confirmed by the current data. While visual assessment of adequate valve form is seemingly easier in BAV than in TAV, valves may still fail due to symmetric prolapse.30 With experience and longer follow-up, a limited but increasing proportion of non-TAVs fails in the second decade due to calcification. The highest probability of failure is to be expected if pericardial patches have been used for cusp repair.37,38 In UAVs, failure occurs even earlier than with BAVs and generally affects the pericardium used for cusp repair. It is noteworthy that remodeling can be utilized to modify UAV anatomy in such a way that no patch material is necessary.21 Further follow-up will be required to judge the long-term value of this approach.
Cusp pathology was the main reason for failure in most instances after remodeling39; persistent/recurrent cusp prolapse or degeneration of patch material used for cusp repair were the main pathologies in some BAV and all UAV. Persistent or recurrent prolapse has been a predictor of failure previously34; the majority of cases with postoperative cusp prolapse in our experience had been performed prior to the introduction of intraoperative measurement of eH. In addition, in TAV anatomy, prolapse and secondary retraction were the most frequent mechanisms in the current analysis. Valve calcification (2.5%; most had a BAV) and cusp retraction (0.65%) have been relatively rare.39 In TAVs, the results were comparable when one, two or three cusps were repaired, in both survival and freedom from reoperation. Patients with prolapse repair of three cusps fared somewhat worse only regarding freedom from AR ≥2 at 10 years.
The current series includes different indications, i.e. aneurysm, severe AR, acute aortic dissection, and connective tissue disease. Early mortality was low (1.5%) despite the inclusion of patients with acute aortic dissection40 as well as morbidity. Only 2.5% required surgical reintervention for hemorrhage, indicating that the procedure is as hemostatic as other procedures4,41; this has been confirmed by a multi-center analysis.41 We did not observe postoperative atrioventricular block requiring pacemaker implantation, most likely due to less basal dissection and the difference in suturing. No relevant difference was observed between patients with aneurysm as the primary indication versus those with AR. The incidence of valve-related complications was very low, with need for reoperation being the most frequent, confirming other studies.37,42
Interestingly, isolated annular dilatation was not identified as a reason for failure.39 While we have observed a few instances of annular dilatation in conjunction with cusp prolapse. The presence of cusp prolapse may have a negative effect on annular stress distribution and its absence contributes to root stabilization. While we have found a clear stabilizing effect of a suture annuloplasty in isolated BAV repair,43 this positive effect was not observed with remodeling for BAV38 and now for TAV. These findings correlate with a previous study in that even without annuloplasty, a size reduction of the annulus was observed.35 Nevertheless, an annuloplasty improves early valve competence; it may improve late durability of valve repair beyond the first 15 years and is thus probably a useful adjunct.
ConclusionRoot remodeling is a viable option in valve-preserving root replacement, both for tricuspid and bicuspid valve morphologies. If combined with objective assessment of cusp configuration and aggressive cusp repair, reproducible and durable restoration of aortic valve function can be achieved. It is thus a good and up-to-date option for patients with aortic root aneurysm, independent of the preoperative degree of aortic regurgitation.
Ethical disclosureSaarland Regional Ethics Committee (CEP 202/19, CEP 203/19) approved the research.
FundingThe authors received no funding.
Conflict of interestThe authors have no conflict of interest to report.